- Department of Neurology, Headache Center, The First Hospital of Shanxi Medical University, Taiyuan, Shanxi, China
Introduction: Hypnic headache (HH) is a rare primary headache that is characterized by strict sleep-related attacks. However, the pathophysiology of HH remains unclear. The nocturnal nature of this activity suggests a hypothalamic involvement. The pathogenesis of HH may involve the brain structure that regulates circadian rhythms and is related to an imbalance between hormones, such as melatonin and serotonin. Currently, evidence-based medicine for HH pharmacotherapy is lacking. Acute and prophylactic treatment of HH is based on only a few case reports. Here, we report a case study in which agomelatine showed desirable responsiveness for the prophylactic treatment of HH for the first time.
Case description: We present the case of a 58-year-old woman with a 3-year history of nocturnal left temporal pain that awakened her during the wee hours. Brain magnetic resonance imaging did not reveal any midline structural abnormalities associated with circadian rhythms. Polysomnography revealed headache-related awakening at approximately 5:40 am, after the last rapid eye movement phase. No sleep apnea-hypopnea events were observed, without oxygen saturation or blood pressure abnormalities. The patient was prescribed agomelatine 25 mg at bedtime as a prophylactic treatment. In the following month, the frequency and severity of the headaches decreased by 80%. After 3 months, the patient’s headache completely resolved, and the medication was discontinued.
Conclusion: HH only occurs during sleep in the real world, leading to substantial sleep disturbances in older populations. Headache center neurologists need to focus on the prophylactic treatment of patients before bedtime to avoid nocturnal awakening. Agomelatine is a potential prophylactic treatment option for patients with HH.
Introduction
Hypnic headache (HH) is a rare primary headache that was first described by Raskin in 1988 (1) and is characterized by strict sleep-related headache attacks. HH was once named clockwise or alarm clock headache because it only occurs during sleep and almost always at the same time (2). The International Headache Society suggests that the following criteria must be met for the headache to be diagnosed as HH (3): (1) it develops only during sleep and causes awakening, (2) it occurs for 10 days/month for >3 months, (3) it lasts from 15 min up to 4 h after awakening, and (4) there are no cranial autonomic symptoms or restlessness, according to the International Classification of Headache Disorders, 3rd edition (ICHD-3). The diagnosis of HH requires the exclusion of secondary causes of nocturnal headaches, such as nocturnal hypertension (4), hypoglycemia (5), auditory neuroma (6), and influenza A virus infection (7). Cervicogenic headaches can also awaken older adults from sleep. Headache may be caused by abnormal neck posture during sleep or degenerative cervical spine disease that compresses nerve roots (8). Obstructive sleep apnea syndrome (OSAS) is a common cause of nocturnal awakening and may be involved in HH (9). However, the presence of an OSAS does not necessarily exclude the possibility of HH (3).
HH is more frequent after the age of 50 years and is more common in women but may also occur in young adults and children (10). It usually occurs in the whole brain but may also occur bilaterally in the frontotemporal lobe and rarely in the occipital lobe as moderate to severe dull pain (11). However, epidemiological data regarding HH are limited. A recent Icelandic study suggested that the prevalence of HH is 0.22% (12). However, the pathophysiology of HH remains unclear. Previously, HH episodes were thought to occur strictly during rapid eye movement (REM) (13). However, subsequent reports have shown that many HHs occur during non-REM (14). The nocturnal nature of this activity suggests hypothalamic involvement (11, 15). The hypothalamus is considered a major integration center that regulates the neurological and endocrine systems. It has a reciprocal influence on pain control and sleep regulation owing to its close connection with the periaqueductal gray matter, locus coeruleus, and median raphe nucleus (16). The suprachiasmatic nucleus (SCN) in the anterior hypothalamus is the internal clock that regulates the sleep–wake state and is governed by the circadian cycle (17). With age, the hypothalamic-pineal axis, especially the SCN, becomes less functional, resulting in reduced melatonin secretion (18). This hypothesis is supported by the significant reduction in posterior hypothalamic gray matter volume in a voxel-based morphometric (VBM) study of patients with HH (15). Recently, an Indian study confirmed the loss of posterior hypothalamic gray matter using VBM-magnetic resonance imaging (MRI) (19). Moreover, fluctuations in melatonin levels alone do not seem to explain HH pathogenesis. Serum melatonin levels usually peak between 2:00 am and 5:00 am. In a clinical study, non-fasting serum was extracted at five different time points (12 noon, 4 pm, 7 pm, 10 pm, and 8 am). No significant changes in melatonin levels were detected between patients with HH and healthy controls (20). In summary, the pathogenesis of HH may involve the brain structure that regulates circadian rhythms and is related to an imbalance between hormones, such as melatonin and serotonin (10). Agomelatine is a selective melatonin receptor agonist (MT1/MT2) and a serotonin receptor antagonist (5-HT2C). In addition to its antidepressant effects, agomelatine is involved in the resynchronization of interrupted circadian rhythms, with beneficial effects on sleep architectures (21). Here, we report a case study in which agomelatine showed desirable responsiveness for the prophylactic treatment of HH for the first time.
Case description
We present the case of a 58-year-old woman with a 3-year history of nocturnal left temporal pain that awakened her during the wee hours. Headaches were dull and not accompanied by migraine-related symptoms, such as nausea, vomiting, photophobia, phonophobia, blurred vision, flashing light, amaurosis, diplopia, tinnitus, and numbness or weakness of the body limbs. There were no cranial autonomic symptoms, such as conjunctival congestion, tearing, nasal congestion, runny nose, sweating, or ptosis, which are helpful in distinguishing between the types or subtypes of trigeminal autonomic cephalalgias, especially cluster headache. The headache lasted for approximately 2 h, even after the patient was awake, and the frequency of attacks was >15 days per month. The patient did not snore and denied a family history of headaches. The patient had a medical history of asthma and gastric ulcer.
On admission, her blood pressure was normal. No signs of nervous system impairment were observed. Serological analysis results, including hematology, electrolytes, fasting blood sugar, glycosylated hemoglobin, erythrocyte sedimentation rate, hepatic function, and renal function, were within normal limits. Brain MRI did not reveal any midline structural abnormalities associated with circadian rhythms (Figure 1). To explore the possible causes of secondary headaches, such as sleep apnea, nocturnal hypertension (22), and awakening from sleep, we performed polysomnography and ambulatory blood pressure monitoring (Figure 2). The patient was awakened by a headache at approximately 5:40 am after the last REM phase. No sleep apnea-hypopnea events were observed. The oxygen saturation and blood pressure were normal. The patient was diagnosed with HH according to the ICHD-3 guidelines and was prescribed agomelatine 25 mg at bedtime as a prophylactic treatment. In the following month, the frequency and severity of headaches decreased by 80%. After 3 months, the patient’s headache completely resolved, and the medication was discontinued.
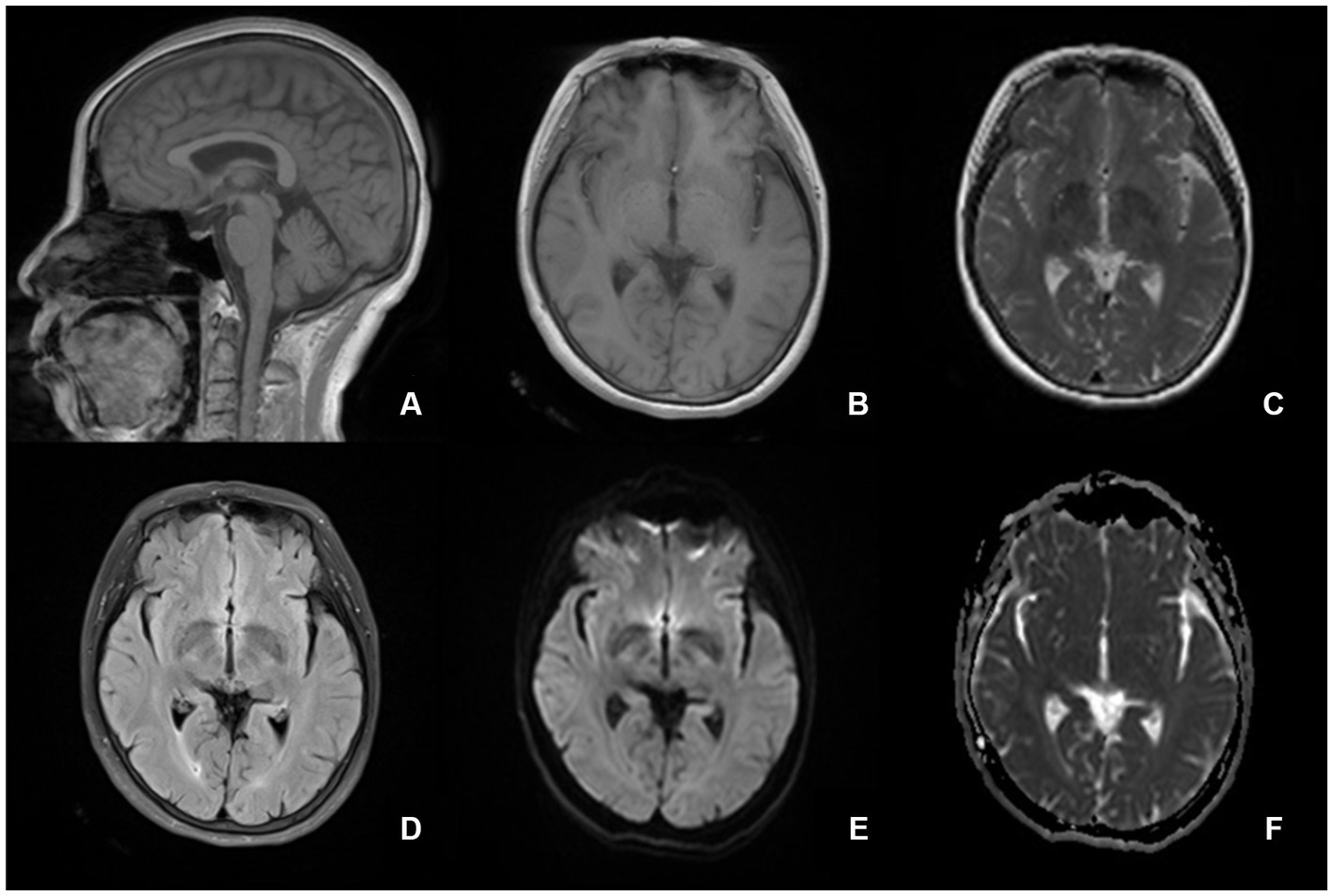
Figure 1. Brain magetic resonance imaging suggests normal structures. There are no midline structural abnormalities associated with circadian rhythms. (A) sagittal T1-weighted image, (B) axial T1-weighted image, (C) T2-weighted image, (D) fluid-attenuated inversion recovery, (E) diffusion-weighted imaging, and (F) apparent diffusion coefficient.
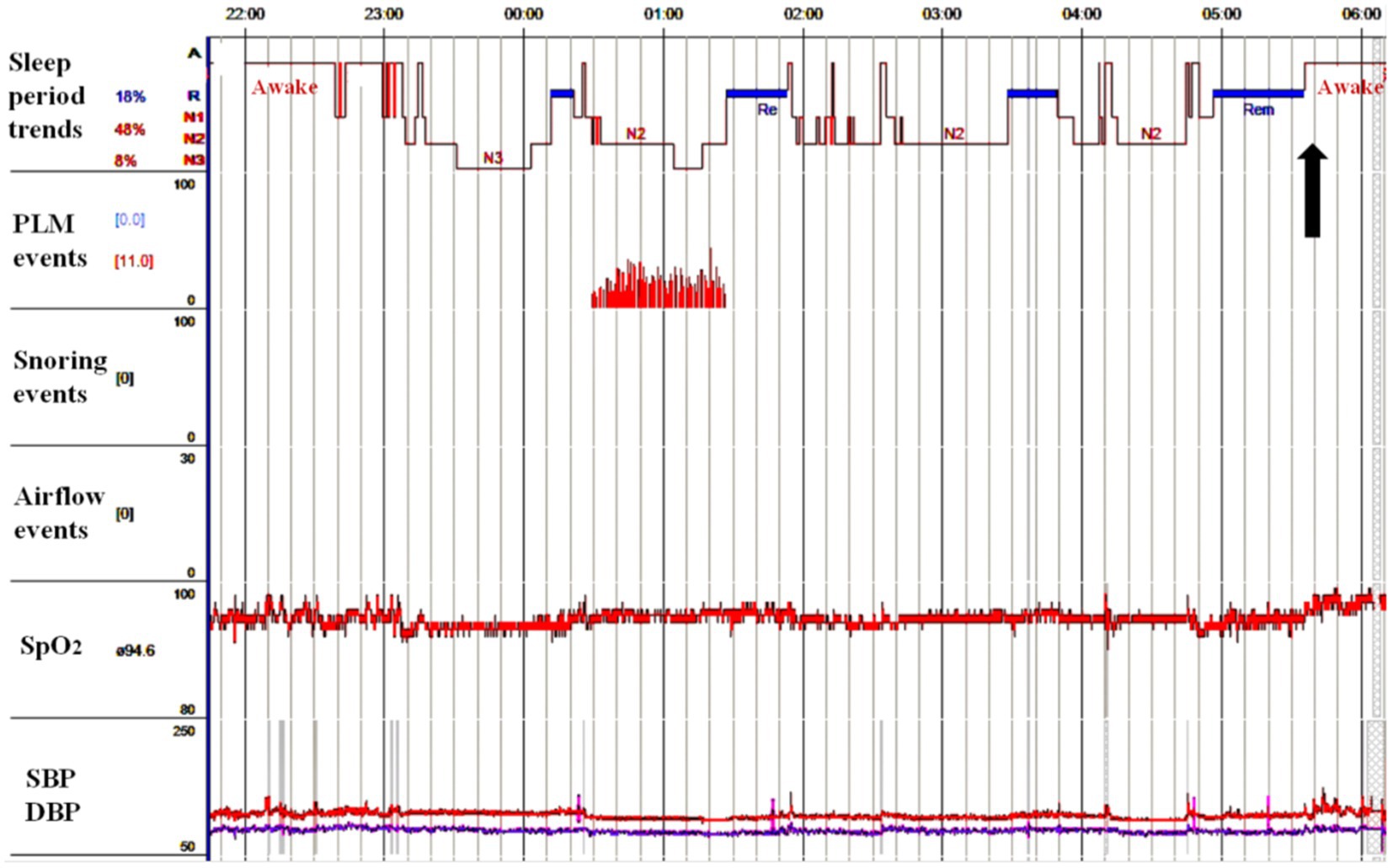
Figure 2. The sleep structure was approximately normal, with an increased proportion of N1 stage, a normal proportion of N2 stage, a decreased proportion of N3 stage, and a normal proportion of REM stage. Patient sat up with headache after the last REM sleep period at approximately 5:40 am (black arrow) without sleep apnea-hypopnea event observed. Meanwhile, the patient’s oxygen saturation and blood pressure were normal. PLM, periodic limb movement; SpO2, saturation of pulse oxygen; SBP, systolic blood pressure; DBP, diastolic blood pressure; REM, rapid eye movement.
Discussion
Currently, evidence-based medicine for HH pharmacotherapy is lacking. Acute and prophylactic treatment of HH is based on only a few case reports. Caffeine, lithium, and indomethacin are the three most reported effective drugs for HH (8, 10, 11, 23, 24). A cup of strong coffee before bedtime appears to be a safe option for acute treatment. Caffeine is an antagonist of adenosine A1, A2A, and A2B receptors, and the cerebral A2 receptor is a key signaling molecule for sleep induction (25). In addition to drinking coffee at bedtime for HH prophylaxis, its use at night when waking up from pain can also shorten the duration and intensity of headaches (8). However, many people hesitate to use replacement therapies for coffee-induced insomnia. Most older individuals in Asia are not accustomed to drinking coffee (24). Lithium was the first drug reported for the prophylactic treatment of HH (1). Lithium regulates the biological clock by increasing the expression of the transcription factor BMAL1, which may help restore healthy circadian rhythms in patients (26). In addition, lithium may interact with NO and N-methyl-D-aspartate receptor signaling in the brain’s injury processing system (27). Lithium also downregulates serotonin receptors and increases serotonin release and transmission in the central nervous system (28) while indirectly increasing serum melatonin levels (29). A case series reported that lithium treatment was effective in >70.0% of the patients (30). A dose of 300 mg at bedtime resulted in a response in 90% of patients (8). However, adverse reactions to lithium are common and require monitoring of blood levels. Furthermore, titration to adequate plasma concentrations is difficult, and many patients discontinue treatment owing to side effects (24, 31). Indomethacin is an effective prophylactic agent for HH. Prophylactic doses of indomethacin between 25 and 150 mg/day have shown favorable results in approximately 70% of the patients with HH (32). There was an absolute response to indomethacin by day 3 at a dose of 25 mg, three times daily (23). It has been hypothesized that HH is associated with changes in cerebrospinal fluid (CSF) pressure and that indomethacin exerts its therapeutic effect by regulating the CSF pressure (33, 34). The patient declined the above three treatment options owing to concerns about coffee-induced insomnia, lithium-related adverse effects, and aggravation of ulcers by indomethacin.
The orexins are two neuropeptides that are derived from prepro-orexin via proteolytic cleavage. They are synthesized in the lateral, posterior, and periventricular hypothalamic nuclei (35). The hypothalamic orexinergic system may be a crucial pathway in circadian rhythm disorders like narcolepsy, cluster headache, and HH. Daridorexant, a recently developed dual orexin receptor antagonist, proved advantageous in improving wake after sleep onset and latency to persistent sleep in elderly individuals with insomnia disorder (36). The recent randomized, double-blind, placebo-controlled phase 3 trials further confirmed the safety and efficacy of daridorexant in insomnia disorder patients (37). However, there are currently no reported cases of using orexins for prophylactic treatment of HH.
Melatonin (MT) is considered beneficial for circadian rhythm disorders and may be beneficial for HH treatment (8, 10, 20, 23, 24, 38). It is the only prophylactic drug reported for the treatment of HH in children (39). Melatonin is an indole heterocyclic compound with a short half-life (≤20 min). It is produced only at night by the pineal gland, and its biological clock properties are mediated by two G protein-coupled melatonin receptors associated with different signaling mechanisms in the SCN, including driving the sleep–wake cycle (40). MT1 receptors inhibit the SCN and induce sleep, whereas MT2 receptors induce changes in SCN signaling that convert the sleep–wake cycle into a light–dark cycle. Melatonin receptors resynchronize irregular circadian rhythms and are beneficial for sleep architectures (41). 5-hydroxytryptamine (5-HT)2C receptors are the only serotonin receptors involved in circadian rhythms. 5-HT2C receptors agonists can simulate the effects of light on the SCN and suppress the production of melatonin (42). They can inhibit melatonin release by mediating light information in the SCN during early night via a post-synaptic mechanism (43–45). Therefore, both 5-HT and melatonin can regulate circadian rhythmicity. Ramelteon is a selective MT1/MT2 agonist approved by the Food and Drug Administration as a hypnotic. Ramelteon has an elimination half-life of approximately 1–2 h, which is much longer than that of melatonin (38). Ramelteon has been reported to yield good results in a patient with HH. This suggests that ramelteon is a potential prophylactic agent for HH (38). However, melatonin and ramelteon are not available as prescription medications in Mainland China. Agomelatine possesses both melatonergic agonist and complementary 5-HT2C antagonist properties (46, 47). It has a stronger affinity for MT1 and MT2 receptors and a longer half-life than melatonin (48). The effects of agomelatine on sleep–wake rhythms in rats have also been studied (49). Agomelatin administration before dark exposure enhanced REM and slow-wave sleep in rats. The decrease in wakefulness induced by agomelatine was different from that induced by melatonin and ramelteon, which may reflect an interaction between melatonin and the 5-HT2C receptor. These findings provide a rationale for HH treatment using agomelatine.
Conclusion
In the real world, HH only occurs during sleep, leading to substantial sleep disturbances in older populations. Headache center neurologists need to focus on the prophylactic treatment of patients before bedtime to avoid nocturnal awakenings. Agomelatine may be a potential prophylactic treatment option for HH, particularly in patients with caffeine-induced insomnia, lithium-related adverse effects, or a history of ulcers.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of the First Hospital of Shanxi Medical University. Written informed consent was obtained from the patient for the publication of this case report.
Author contributions
LL, W-xS, and J-yS conducted the study. S-yX and LL wrote the first draft. S-yX and C-xL conceptualized the study and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from Doctoral Fund of the First Hospital of Shanxi Medical University (YB161706, BS03201631, and SD2215); Shanxi Applied Basic Research Program (201801D221426, and 20210302124404).
Acknowledgments
The authors would like to thank Editage (www.editage.com) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
5-HT, 5-hydroxytryptamine; CSF, Cerebrospinal fluid; HH, Hypnic headache; ICHD-3, International Classification of Headache Disorders, 3rd edition; MT, Melatonin; MRI, Magnetic resonance imaging; OSAS, Obstructive sleep apnea syndrome; REM, Rapid eye movement; SCN, Suprachiasmatic nucleus; VBM, Voxel-based morphometric.
References
1. Raskin, NH. The Hypnic headache syndrome. Headache. (1988) 28:534–6. doi: 10.1111/j.1526-4610.1988.hed2808534.x
2. Al Khalili, Y, and Chopra, P. Hypnic headache. Statpearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC (2022).
3. Headache classification committee of the international headache society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia. (2018) 38:1–211. doi: 10.1177/0333102417738202
4. Caminero, AB, Martín, J, Sánchez, DEL, and Río, M. Secondary Hypnic headache or symptomatic nocturnal hypertension? Two case reports. Cephalalgia. (2010) 30:1137–9. doi: 10.1111/j.1468-2982.2009.02011.x
5. Silva-Néto, RP, Soares, AA, and Peres, MFP. Hypnic headache due to hypoglycemia: a case report. Headache. (2019b) 59:1370–3. doi: 10.1111/head.13627
6. Ceronie, B, Green, F, and Cockerell, OC. Acoustic neuroma presenting as a Hypnic headache. BMJ Case Rep. (2021) 14:e235830. doi: 10.1136/bcr-2020-235830
7. Pérez Hernández, A, and Gómez Ontañón, E. Influenza a virus: a possible trigger factor for Hypnic headache? Neurologia. (2017) 32:67–8. doi: 10.1016/j.nrl.2015.03.003
8. Tariq, N, Estemalik, E, Vij, B, Kriegler, JS, Tepper, SJ, and Stillman, MJ. Long-term outcomes and clinical characteristics of Hypnic headache syndrome: 40 patients series from a tertiary referral center. Headache. (2016) 56:717–24. doi: 10.1111/head.12796
9. Bender, SD. An unusual case of Hypnic headache ameliorated utilizing a mandibular advancement oral appliance. Sleep Breath. (2012) 16:599–602. doi: 10.1007/s11325-011-0562-5
10. Silva-Néto, RP, Santos, P, and Peres, MFP. Hypnic headache: a review of 348 cases published from 1988 to 2018. J Neurol Sci. (2019a) 401:103–9. doi: 10.1016/j.jns.2019.04.028
11. Kesserwani, H. Hypnic headache responds to Topiramate: a case report and a review of mechanisms of action of therapeutic agents. Cureus. (2021) 13:E13790. doi: 10.7759/cureus.13790
12. Eliasson, JH, Scher, AI, Buse, DC, Tietjen, G, Lipton, RB, Launer, LJ, et al. The prevalence of Hypnic headache in Iceland. Cephalalgia. (2020) 40:863–5. doi: 10.1177/0333102420911209
13. Pinessi, L, Rainero, I, Cicolin, A, Zibetti, M, Gentile, S, and Mutani, R. Hypnic headache syndrome: association of the attacks with REM sleep. Cephalalgia. (2003) 23:150–4. doi: 10.1046/j.1468-2982.2003.00472.x
14. Holle, D, Naegel, S, and Obermann, M. Hypnic headache. Cephalalgia. (2013) 33:1349–57. doi: 10.1177/0333102413495967
15. Holle, D, Naegel, S, Krebs, S, Gaul, C, Gizewski, E, Diener, HC, et al. Hypothalamic gray matter volume loss in Hypnic headache. Ann Neurol. (2011a) 69:533–9. doi: 10.1002/ana.22188
16. Montagna, P. Hypothalamus, sleep and headaches. Neurol Sci. (2006) 27:S138–43. doi: 10.1007/s10072-006-0589-8
17. Froy, O. The circadian clock and metabolism. Clin Sci (Lond). (2011) 120:65–72. doi: 10.1042/CS20100327
18. Lisotto, C, Rossi, P, Tassorelli, C, Ferrante, E, and Nappi, G. Focus on therapy of Hypnic headache. J Headache Pain. (2010) 11:349–54. doi: 10.1007/s10194-010-0227-y
19. Rammohan, K, Shyma, MM, Das, S, and Shaji, CV. Hypnic headache: a rare primary headache syndrome in an Indian population with a Mini review of literature. Neurol India. (2021) 69:1277–81. doi: 10.4103/0028-3886.329541
20. Naegel, S, Huhn, JI, Gaul, C, Diener, HC, Obermann, M, and Holle, D. No pattern alteration in single nocturnal melatonin secretion in patients with Hypnic headache: a case-control study. Headache. (2017) 57:648–53. doi: 10.1111/head.12983
21. Savino, R, Polito, AN, Marsala, G, Ventriglio, A, Di Salvatore, M, De Stefano, MI, et al. Agomelatine: a potential multitarget compound for neurodevelopmental disorders. Brain Sci. (2023) 13:734. doi: 10.3390/brainsci13050734
22. Silva-Néto, RP, and Bernardino, SN. Ambulatory blood pressure monitoring in patient with Hypnic headache: a case study. Headache. (2013) 53:1157–8. doi: 10.1111/head.12066
23. Dissanayake, KP, Wanniarachchi, DP, and Ranawaka, UK. Case report of Hypnic headache: a rare headache disorder with nocturnal symptoms. BMC Res Notes. (2017) 10:318. doi: 10.1186/s13104-017-2641-6
24. Liang, JF, and Wang, SJ. Hypnic headache: a review of clinical features, therapeutic options and outcomes. Cephalalgia. (2014) 34:795–805. doi: 10.1177/0333102414537914
25. Holle, D, and Obermann, M. Hypnic headache and caffeine. Expert Rev Neurother. (2012) 12:1125–32. doi: 10.1586/ern.12.100
26. Yin, L, Wang, J, Klein, PS, and Lazar, MA. Nuclear receptor rev-Erbalpha is a critical lithium-sensitive component of the circadian clock. Science. (2006) 311:1002–5. doi: 10.1126/science.1121613
27. Ghasemi, M, and Dehpour, AR. The NMDA receptor/nitric oxide pathway: a target for the therapeutic and toxic effects of Lithium. Trends Pharmacol Sci. (2011) 32:420–34. doi: 10.1016/j.tips.2011.03.006
28. Treiser, SL, Cascio, CS, O'donohue, TL, Thoa, NB, Jacobowitz, DM, and Kellar, KJ. Lithium increases serotonin release and decreases serotonin receptors in the Hippocampus. Science. (1981) 213:1529–31. doi: 10.1126/science.6269180
29. Pablos, MI, Santaolaya, MJ, Agapito, MT, and Recio, JM. Influence of lithium salts on chick pineal gland melatonin secretion. Neurosci Lett. (1994) 174:55–7. doi: 10.1016/0304-3940(94)90117-1
30. Silva-Néto, RP, and Almeida, KJ. Hypnic headache: a descriptive study of 25 new cases in Brazil. J Neurol Sci. (2014) 338:166–8. doi: 10.1016/j.jns.2013.12.042
31. Holle, D, Naegel, S, Krebs, S, Katsarava, Z, Diener, HC, Gaul, C, et al. Clinical characteristics and therapeutic options in Hypnic headache. Cephalalgia. (2010) 30:1435–42. doi: 10.1177/0333102410375727
32. Summ, O, and Evers, S. Mechanism of action of indomethacin in indomethacin-responsive headaches. Curr Pain Headache Rep. (2013) 17:327. doi: 10.1007/s11916-013-0327-x
33. Holle, D, Wessendorf, TE, Zaremba, S, Naegel, S, Diener, HC, Katsarava, Z, et al. Serial Polysomnography in Hypnic headache. Cephalalgia. (2011b) 31:286–90. doi: 10.1177/0333102410381146
34. Rasmussen, M. Treatment of elevated intracranial pressure with indomethacin: friend or foe? Acta Anaesthesiol Scand. (2005) 49:341–50. doi: 10.1111/j.1399-6576.2005.00647.x
35. Holland, PR, and Goadsby, PJ. Cluster headache, hypothalamus, and Orexin. Curr Pain Headache Rep. (2009) 13:147–54. doi: 10.1007/s11916-009-0025-x
36. Zammit, G, Dauvilliers, Y, Pain, S, Sebök Kinter, D, Mansour, Y, and Kunz, D. Daridorexant, a new dual Orexin receptor antagonist, in elderly subjects with insomnia disorder. Neurology. (2020) 94:E2222–32. doi: 10.1212/WNL.0000000000009475
37. Mignot, E, Mayleben, D, Fietze, I, Leger, D, Zammit, G, Bassetti, CLA, et al. Safety and efficacy of Daridorexant in patients with insomnia disorder: results from two multicentre, randomised, double-blind, placebo-controlled, phase 3 trials. Lancet Neurol. (2022) 21:125–39. doi: 10.1016/S1474-4422(21)00436-1
38. Arai, M. A case of unilateral Hypnic headache: rapid response to Ramelteon, a selective melatonin MT1/MT2 receptor agonist. Headache. (2015) 55:1010–1. doi: 10.1111/head.12616
39. Silva-Néto, RP, and Almeida, KJ. Hypnic headache in childhood: a literature review. J Neurol Sci. (2015) 356:45–8. doi: 10.1016/j.jns.2015.06.048
40. Samanta, S. Physiological and pharmacological perspectives of melatonin. Arch Physiol Biochem. (2022) 128:1346–67. doi: 10.1080/13813455.2020.1770799
41. Naveed, M, Li, LD, Sheng, G, Du, ZW, Zhou, YP, Nan, S, et al. Agomelatine: an astounding sui-generis antidepressant? Curr Mol Pharmacol. (2022) 15:943–61. doi: 10.2174/1874467214666211209142546
42. Kennaway, DJ, and Moyer, RW. Serotonin 5-HT2c agonists mimic the effect of light pulses on circadian rhythms. Brain Res. (1998) 806:257–70. doi: 10.1016/S0006-8993(98)00746-X
43. Fuchs, E, Simon, M, and Schmelting, B. Pharmacology of a new antidepressant: benefit of the implication of the Melatonergic system. Int Clin Psychopharmacol. (2006) 21:S17–20. doi: 10.1097/01.yic.0000199456.39552.c7
44. Racagni, G, Riva, MA, and Popoli, M. The interaction between the internal clock and antidepressant efficacy. Int Clin Psychopharmacol. (2007) 22:S9–S14. doi: 10.1097/01.yic.0000277957.75852.c7
45. San, L, and Arranz, B. Agomelatine: a novel mechanism of antidepressant action involving the Melatonergic and the serotonergic system. Eur Psychiatry. (2008) 23:396–402. doi: 10.1016/j.eurpsy.2008.04.002
46. De Bodinat, C, Guardiola-Lemaitre, B, Mocaër, E, Renard, P, Muñoz, C, and Millan, MJ. Agomelatine, the first Melatonergic antidepressant: discovery, characterization, and development. Nat Rev Drug Discov. (2010) 9:628–42. doi: 10.1038/nrd3140
47. Millan, MJ. Agomelatine for the treatment of generalized anxiety disorder: focus on its distinctive mechanism of action. Ther Adv Psychopharmacol. (2022) 12:20451253221105128. doi: 10.1177/20451253221105128
48. Konstantakopoulos, G, Dimitrakopoulos, S, and Michalopoulou, PG. The preclinical discovery and development of Agomelatine for the treatment of depression. Expert Opin Drug Discov. (2020) 15:1121–32. doi: 10.1080/17460441.2020.1781087
49. Descamps, A, Rousset, C, Millan, MJ, Spedding, M, Delagrange, P, and Cespuglio, R. Influence of the novel antidepressant and melatonin agonist/Serotonin2c receptor antagonist, Agomelatine, on the rat sleep-wake cycle architecture. Psychopharmacology. (2009) 205:93–106. doi: 10.1007/s00213-009-1519-2
Keywords: Hypnic headache, circadian rhythm, agomelatine, melatonin, serotonin
Citation: Xu S-y, Li L, Sun W-x, Shen J-y and Li C-x (2023) Case report: Hypnic headache responds to agomelatine–a potential prophylactic treatment option. Front. Neurol. 14:1179391. doi: 10.3389/fneur.2023.1179391
Edited by:
Raffaele Ornello, University of L'Aquila, ItalyReviewed by:
Carlo Baraldi, University of Modena and Reggio Emilia, ItalyZhao Dong, Chinese PLA General Hospital, China
Copyright © 2023 Xu, Li, Sun, Shen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chang-xin Li, bGljaGFuZ3hpbjAzNTFAc2luYS5jb20=
†These authors have contributed equally to this work
 Sui-yi Xu†
Sui-yi Xu† Chang-xin Li
Chang-xin Li