- 1Department of Poisoning and Occupational Diseases, Emergency Medicine, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, China
- 2Emergency Department, Affiliated the Jianhu Clinical Medical College of Yangzhou University, Yancheng, China
- 3Department of Emergency, Liaocheng People's Hospital, Liaocheng, Shandong, China
- 4School of Nursing and Rehabilitation, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, China
- 5Nursing Theory and Practice Innovation Research Center of Shandong University, Jinan, Shandong, China
- 6Department of Occupational and Environmental Health, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, China
- 7Department of Geriatric Medicine and Department of Nursing, Qilu Hospital, Nursing Theory Innovation and Research Center of Shandong University, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, China
- 8Department of Nursing, Qilu Hospital of Shandong University Dezhou Hospital, Nursing Theory Innovation and Research Center of Shandong University, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, China
Diquat (DQ), chemically known as 1,1 ‘-ethylene-2,2’ -bipyridine, is a non-selective herbicide for leaf removal and drying. It has toxic effects on central nervous system cells, and toxic neurological lesions include axonal degeneration and pontine myelolysis. At the same time, DQ can also affect the activity of dopaminergic nerve cells through oxidative stress, causing degeneration and reducing dopamine uptake. With the increasing application of DQ in agricultural production, the clinical reports of neurotoxicity caused by acute DQ poisoning are also increasing. At present, DQ rapid-phase-related toxic encephalopathy mainly involves the pons, midbrain, basal ganglia, thalamus and other brain regions. However, this case is unusual in that the lesion mainly involved the splenium of the corpus callosum. It is also the first time to be reported.
Introduction
Reversible splenial lesion syndrome(RESLES) is a clinical imaging syndrome. It was proposed by Garcia-Monco et al. based on imaging features (1). Imaging examination revealed that the characteristic change of this disease was a reversible lesion in the splenium of the corpus callosum. RESLES and mild encephalitis with a reversible splenial lesion of the corpus callosum (MERS) are different terms for the same clinical disease. There are a wide range of causes for RESLES. But there are few reports of pesticide poisoning causing RESLES so far. Diquat has not been reported in the literature to cause RESLES.
Case description
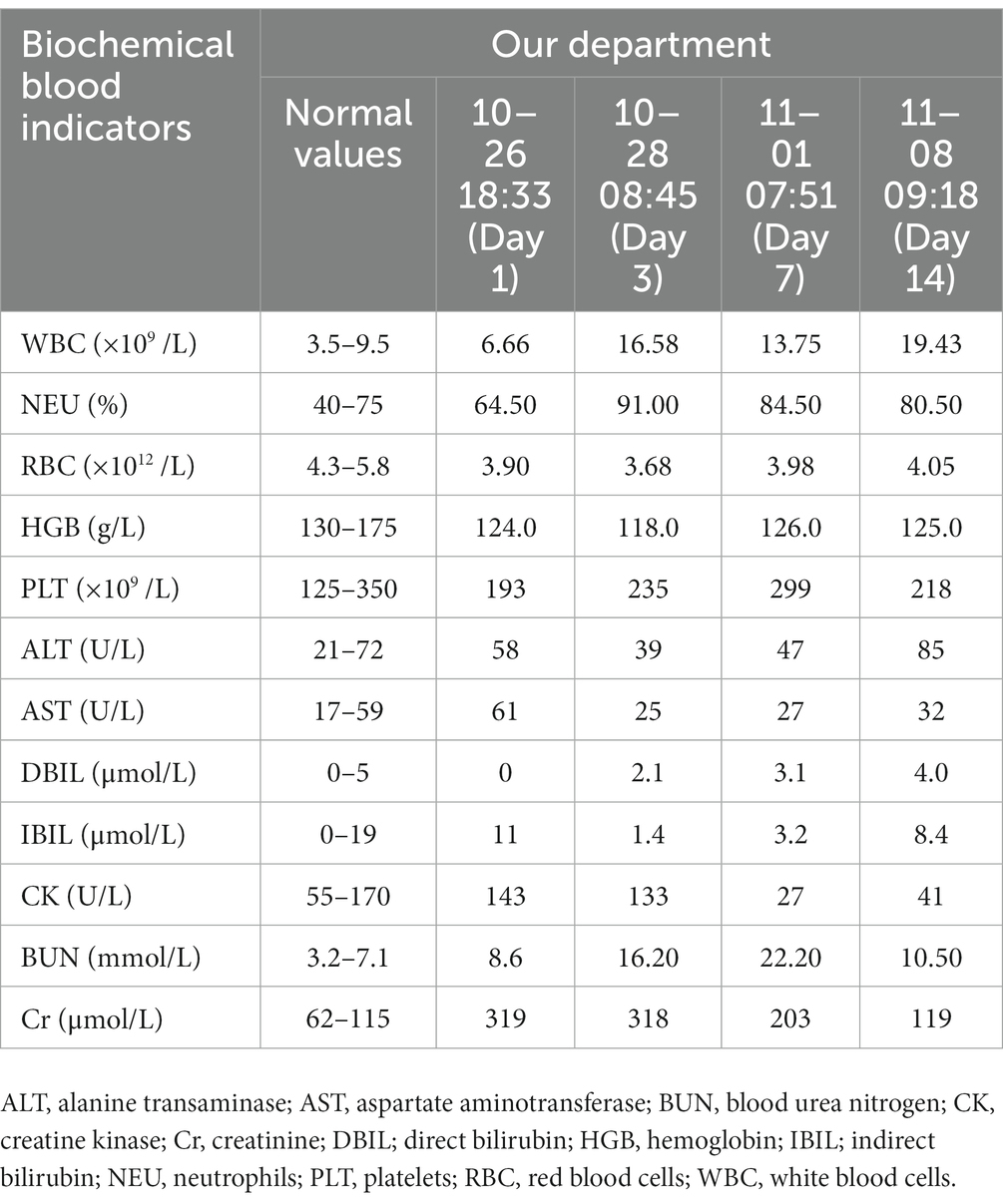
A 25-year-old male patient was admitted to a local hospital with oral diquat 9 days ago. There, he was treated with gastric lavage, hemoperfusion, organ protection, and nutritional support. The renal function gradually deteriorated during the treatment, so he was transferred to our hospital on October 26. On admission, the patient was conscious and had a normal urine output. His vital signs were: body temperature 36.8°C, heart rate 87 beats /min, respiration 18 beats /min, blood pressure 126/72 mmHg, and physical examination was normal except for mild oedema of the lower extremities. Blood results were as follows: D-dimer at admission: 2.36 μg/mL (normal value, <0.5ug/ml), blood urea nitrogen (BUN):8.6 mmol/L (normal value, 3.2–7.1 mmol/L), creatinine (Cr):319 umol/L (normal value, 62–115 umol/L);Other laboratory test results are shown in Table 1. The magnetic resonance imaging (MRI) examination of the head suggested that the lesions of the splenium of the corpus callosum, combined with the medical history, could be consistent with the MR manifestations of toxic encephalopathy (Figure 1). The patient was given betamethasone (8 mg, once a day), torasemide (20 mg, twice a day), alanylglutamine (10 g, once a day), lansoprazole (30 mg, twice a day), reduced glutathione (1.8 gram once a day) and nutritional support. On the 7th day of treatment, brain MRI showed that the abnormal signal of the splenium of the corpus callosum might be considered as RESLES (Figure 2). At this time blood tests showed: white blood cell count:13.75×109/L(normal value, 3.5–9.5 ×109/L), neutrophils:82.50%; d-dimer: 1.99 μ g/mL; BUN:22.20 mmol/L, Cr:203 umol/L. The patient’s renal function improved and lansoprazole, torasemide, betamethasone were discontinued. On day 10, his renal function had improved, and he was discharged from the hospital. On day 14, the splenial lesion of the corpus callosum is no longer visible on computed tomography scan (Figure 3). The patient’s laboratory test results are shown in Table 1.
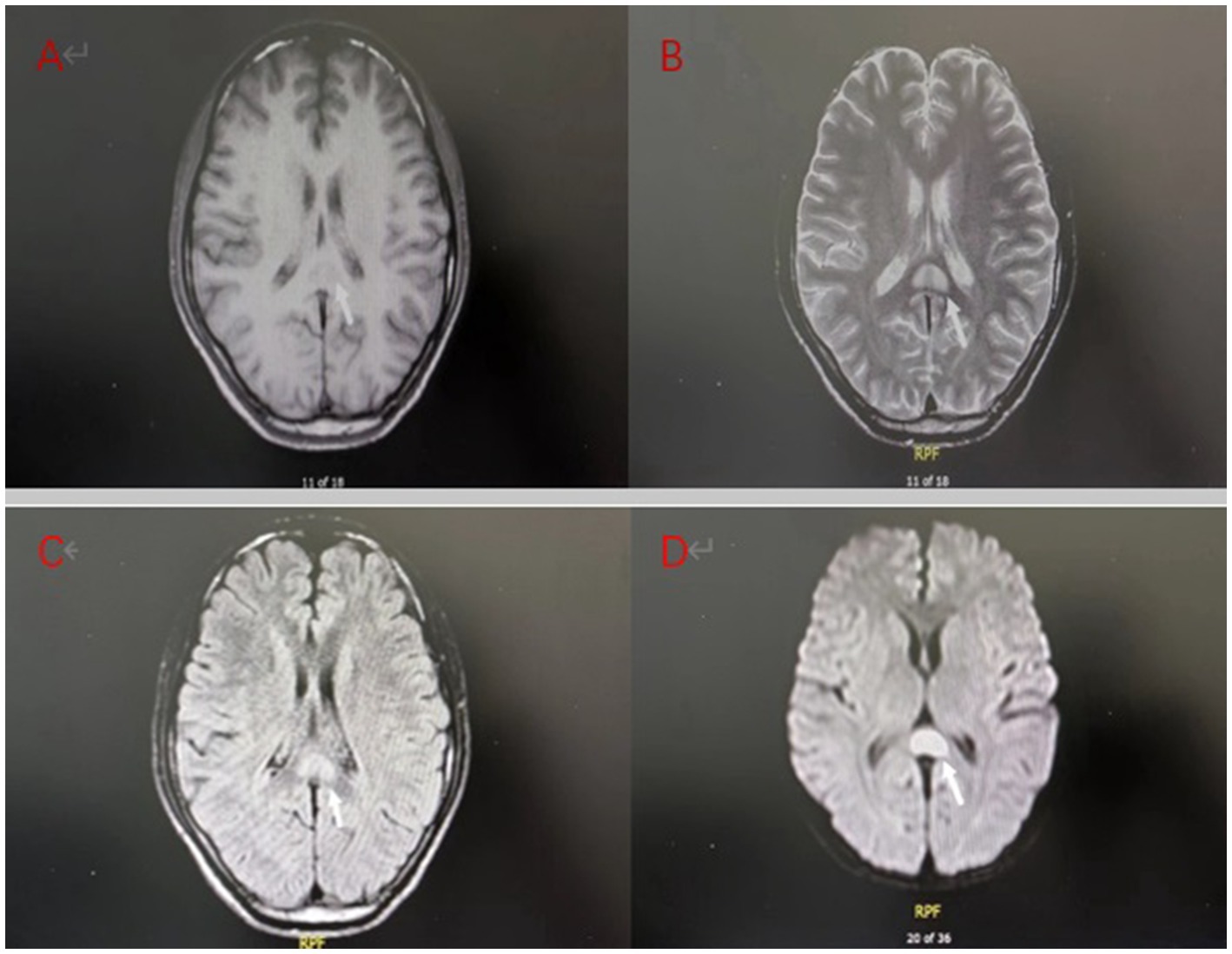
Figure 1. Brain MRI on admission of the patient: (A) T1 weighted imaging (T1WI) showed low signal intensity; (B) T2 weighted imaging (T2WI) showed high signal intensity; (C) Fluid attenuated inversion recovery (FLAIR) showed a high signal; (D) Diffusion weighted imaging (DWI) showed more pronounced hyperintensity than T2WI.
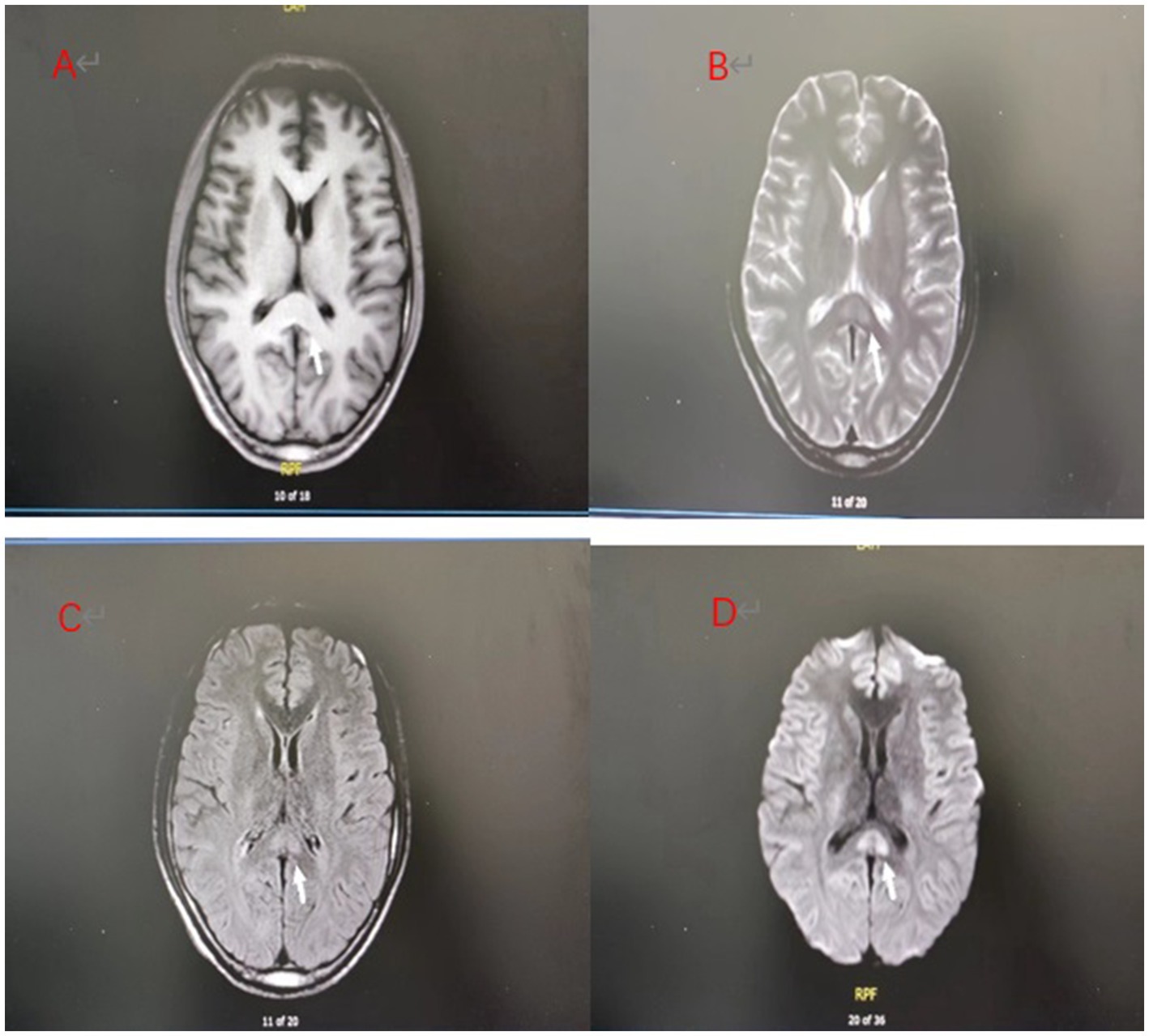
Figure 2. (A–D) Day 7 brain MRI:The splenial lesion of the corpus callosum had improved and might be considered as RESLES.
Figure 3. Day 14 brain CT: The splenial lesion of the corpus callosum was no longer visible on computed tomography scan.
Discussion
The characteristic findings of RESLES on brain MRI include lesions that are mostly confined to the splenium of the corpus callosum with clear boundaries; a few can involve the white matter area outside the splenium of the corpus callosum, without obvious oedema and mass effect around the lesions. T1-weighted imaging (T1WI) shows equal or slightly low signal intensity, T2-weighted imaging (T2WI) shows slightly high signal intensity, diffusion weighted imaging (DWI) shows more obvious high signal intensity than T2WI and apparent diffusion coefficient(ADC) shows low values, and most lesions disappear within 2 weeks. They can be divided into three categories: (a) small round or oval lesions at the midline of the splenium of the corpus callosum; (b) extention laterally through the fibers of the corpus callosum, showing the ‘boomerang sign’; (c) extension from the splenium of the corpus callosum to the anterior part of the corpus callosum and the deep white matter (2). The etiology is complex and diverse. In addition to seizures and withdrawal of antiepileptic drugs (3–5), the use of drugs, such as metronidazole (6), olanzapine (7), glucocorticoids (8), cisplatin, carboplatin, 5-fluorouracil and other antitumour drugs (1), and anorexic sympathetic weight loss drugs (1) can also cause RESLES. Other causes include influenza A (9), rotavirus infection (10), streptococcus pneumoniae (11), meningococcal meningitis (12), metabolic conditions such as hypoglycemia or hypernatremia (13, 14), glycophosate poisoning (15), and other diseases. These include vitamin B12 deficiency (16), malnutrition (17), high plateau brain oedema (18), systemic lupus erythematosus (SLE) (19), Kawasaki disease (20), anti-volt-gated potassium channel (VGKC) autoantibody syndrome (21), malignant tumours (14), cerebral venous sinus thrombosis (22), preeclampsia (23), cranial brain trauma (24) and renal failure (8).
The clinical manifestations of RESLES patients are diverse and non-specific. Disturbance of consciousness and headache are common symptoms. It can also manifest as fever, dizziness, vomiting, disturbance of consciousness, convulsions, delirium, blurred vision and paraesthesia. (25). Pathogenesis is unclear; the characteristics of the focal reversible change suggest that the pathogenesis is transient damage of the blood–brain barrier, reversible demyelination, oedema, cytotoxic oedema of myelin sheath, and arginine vasopressin (AVP) release. It may also be associated with short inflammatory reaction, elevated inflammatory cytokines and genetic factors (8, 26). Due to the complex etiology, RESLES may be the result of multiple mechanisms.
After admission, the patient’s brain MRI showed abnormal signal at the splenium of the corpus callosum.Splenic infarction of the corpus callosum was excluded because it is relatively rare for young patients to have splenic infarction (27), and there were no suggestive symptoms such as headache, dizziness or visual impairment. Apart from renal function, routine blood examination was normal, and neurological physical examination was negative. Therefore, no specific drugs for encephalitis or encephalopathy were used in the treatment process, and only 8 mg of betamethasone was given. After reexamination, abnormal signals were still found in the splenium of corpus callosum on brain MRI, but the range of high signal intensity on DWI was reduced compared with that on admission, and the signal intensity was also weakened; this was considered to be RESLES. Combined with the medical history, it was considered that it might have been caused by diquat poisoning. However, it could not be ruled out that it was caused by renal insufficiency and inflammatory reaction after diquat poisoning. It might also have been related to the use of glucocorticoids in the treatment of the diquat poisoning.
Conclusion
Here we report for the first time a case of RESLES associated with diquat. Although the specific mechanism is unknown, based on the history and symptom analysis, we believe that it is related to diquat poisoning. RESLES has no specific clinical manifestations, mild symptoms and a good prognosis, but no neurological sequelae.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
PD and JS investigated the description of the incident and conceived the study and drafted the manuscript. PD, JS, ZY, and TZ supervised data collection. PD, JS, BK, BZ, and XJ take responsibility for the paper as a whole. PD, JS, ZY, TZ, ZW, TJ, LG, AG, BK, BZ, and XJ contributed substantially to its revision. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Garcia-Monco, JC, Cortina, IE, Ferreira, E, Martínez, A, Ruiz, L, Cabrera, A, et al. Reversible splenial lesion syndrome (RESLES): what’s in a name? J Neuroimaging. (2011) 21:e1–e14. doi: 10.1111/j.1552-6569.2008.00279.x
2. Starkey, J, Kobayashi, N, Numaguchi, Y, and Moritani, T. Cytotoxic lesions of the corpus callosum that show restricted diffusion: mechanisms, causes, and manifestations. Radiographics. (2017) 37:562–76. doi: 10.1148/rg.2017160085
3. Polster, T, Hoppe, M, and Ebner, A. Transient lesion in the splenium of the corpus callosum: three further cases in epileptic patients and a pathophysiological hypothesis. J Neurol Neurosurg Psychiatry. (2001) 70:459–63. doi: 10.1136/jnnp.70.4.459
4. Mirsattari, SM, Lee, DH, Jones, MW, and Blume, WT. Transient lesion in the splenium of the corpus callosum in an epileptic patient. Neurology. (2003) 60:1838–41. doi: 10.1212/01.wnl.0000058754.99940.11
5. Jing, C, Sun, L, Wang, Z, Chu, C, and Lin, W. Reversible splenial lesion syndrome due to oxcarbazepine withdrawal: case report and literature review. J Int Med Res. (2018) 46:1277–81. doi: 10.1177/0300060517736452
6. Neshige, S, Kanaya, Y, Takeshima, S, Yoshimoto, T, Tanaka, A, and Kuriyama, M. Reversible changes on MR images in a patient with metronidazole-induced encephalopathy. Rinsho Shinkeigaku. (2015) 55:174–7. doi: 10.5692/clinicalneurol.55.174
7. Kaino, K, Kumagai, R, Furukawa, S, Isono, M, Muramatsu, A, Fujii, M, et al. Reversible splenial lesion syndrome with a hyperosmolar hyperglycemic state and neuroleptic malignant syndrome caused by olanzapine. J Diabetes Investig. (2017) 8:392–4. doi: 10.1111/jdi.12597
8. Aksu, B, Kurtcan, S, Alkan, A, Aralasmak, A, and Oktem, F. Reversible corpus callosum splenial lesion due to steroid therapy. J Neuroimaging. (2015) 25:501–4. doi: 10.1111/jon.12128
9. Takatsu, H, Ishimaru, N, Ito, M, and Kinami, S. Mild encephalitis/encephalopathy with a reversible splenial lesion in an adult patient with influenza. Intern Med. (2017) 56:3093–5. doi: 10.2169/internalmedicine.8997-17
10. Karampatsas, K, Spyridou, C, Morrison, IR, Tong, CY, and Prendergast, AJ. Rotavirus-associated mild encephalopathy with a reversible splenial lesion (MERS)-case report and review of the literature. BMC Infect Dis. (2015) 15:446. doi: 10.1186/s12879-015-1192-5
11. Avcu, G, Kilinc, MA, Eraslan, C, Karapinar, B, and Vardar, F. Mild encephalitis/encephalopathy with reversible splenial lesion (MERS) associated with Streptococcus pneumoniae bacteraemia. J Infect Public Health. (2017) 10:479–82. doi: 10.1016/j.jiph.2016.08.019
12. Hayashi, Y, Yasunishi, M, Hayashi, M, Asano, T, Kimura, A, and Inuzuka, T. Reversible splenial lesion of the corpus callosum associated with meningococcal meningitis. J Neurol Sci. (2017) 373:81–2. doi: 10.1016/j.jns.2016.12.035
13. Takanashi, J, Tada, H, Maeda, M, Suzuki, M, Terada, H, and Barkovich, AJ. Encephalopathy with a reversible splenial lesion is associated with hyponatremia. Brain and Development. (2009) 31:217–20. doi: 10.1016/j.braindev.2008.04.002
14. Maeda, M, Tsukahara, H, Terada, H, Nakaji, S, Nakamura, H, Oba, H, et al. Reversible splenial lesion with restricted diffusion in a wide spectrum of diseases and conditions. J Neuroradiol. (2006) 33:229–36. doi: 10.1016/s0150-9861(06)77268-6
15. Jeong TOYoon, JC, Lee, JB, Jin, YH, and Hwang, SB. Reversible splenial lesion syndrome (RESLES) following glufosinate ammonium poisoning. J Neuroimaging. (2015) 25:1050–2. doi: 10.1111/jon.12216
16. Theeler, BJ, Wilson, DJ, Crawford, CM, and Grazko, M. Optic neuropathy and a reversible splenial lesion after gastric bypass: shared pathophysiology? J Neurol Sci. (2010) 291:92–4. doi: 10.1016/j.jns.2010.01.015
17. Kosugi, T, Isoda, H, Imai, M, and Sakahara, H. Reversible focal splenial lesion of the corpus callosum on MR images in a patient with malnutrition. Magn Reson Med Sci. (2004) 3:211–4. doi: 10.2463/mrms.3.211
18. Pan, JJ, Zhao, YY, Lu, C, Hu, YH, and Yang, Y. Mild encephalitis/encephalopathy with a reversible splenial lesion: five cases and a literature review. Neurol Sci. (2015) 36:2043–51. doi: 10.1007/s10072-015-2302-2
19. Soon, GS, Rodan, LH, Laughlin, S, Laxer, RM, Benseler, S, and Silverman, ED. Reversible splenial lesion syndrome in pediatric systemic lupus erythematosus. J Rheumatol. (2012) 39:1698–9. doi: 10.3899/jrheum.120390
20. Itamura, S, Kamada, M, and Nakagawa, N. Kawasaki disease complicated with reversible splenial lesion and acute myocarditis. Pediatr Cardiol. (2011) 32:696–9. doi: 10.1007/s00246-011-9937-4
21. Gilder, TR, Hawley, JS, and Theeler, BJ. Association of reversible splenial lesion syndrome (RESLES) with anti-VGKC autoantibody syndrome: a case report. Neurol Sci. (2016) 37:817–9. doi: 10.1007/s10072-015-2464-y
22. Liu, J, Liu, D, Yang, B, Yan, J, Pu, Y, Zhang, J, et al. Reversible splenial lesion syndrome (RESLES) coinciding with cerebral venous thrombosis: a report of two cases. Ther Adv Neurol Disord. (2017) 10:375–9. doi: 10.1177/1756285617727978
23. Chen, Z, Xu, M, Shang, D, and Luo, B. A case of reversible splenial lesions in late postpartum preeclampsia. Intern Med. (2012) 51:787–90. doi: 10.2169/internalmedicine.51.6500
24. Al Brashdi, YH, and Albayram, MS. Reversible restricted-diffusion lesion representing transient intramyelinic cytotoxic edema in a patient with traumatic brain injury. Neuroradiol J. (2015) 28:409–12. doi: 10.1177/1971400915598071
25. Lu, PL, Hodes, JF, Zheng, X, and Hu, XY. Reversible splenial lesion syndrome with some novel causes and clinical manifestations. Intern Med. (2020) 59:2471–80. doi: 10.2169/internalmedicine.4516-20
26. Morichi, S, Kawashima, H, Ioi, H, Yamanaka, G, Kashiwagi, Y, and Hoshika, A. High production of interleukin-10 and interferon-γ in influenza-associated MERS in the early phase. Pediatr Int. (2012) 54:536–8. doi: 10.1111/j.1442-200X.2011.03483.x
27. Hirono, S, Kawauchi, D, Kobayashi, M, Orimoto, R, Ikegami, S, Horiguchi, K, et al. Mechanism of corpus callosum infarction associated with acute hydrocephalus: clinical, surgical, and radiological evaluations for pathophysiology. World Neurosurg. (2019) 127:e873–80. doi: 10.1016/j.wneu.2019.03.288
Keywords: diquat, acute kidney injury, toxic encephalopathy, reversible splenial lesion syndrome, MRI
Citation: Dai P, Sun J, Yu Z, Zhang T, Wen Z, Jian T, Guo L, Genjiafu A, Kan B, Zhang B and Jian X (2023) Case report: Reversible splenial lesion syndrome caused by diquat poisoning. Front. Neurol. 14:1178272. doi: 10.3389/fneur.2023.1178272
Edited by:
Fahim Mohamed, University of Peradeniya, Sri LankaReviewed by:
Yuto Uchida, Johns Hopkins Medicine, United StatesRonald Antulov, University Hospital of Southern Denmark, Denmark
Copyright © 2023 Dai, Sun, Yu, Zhang, Wen, Jian, Guo, Genjiafu, Kan, Zhang and Jian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Binbin Zhang, emhhbmdiYkBzZHUuZWR1LmNu; Xiangdong Jian, amlhbnhpYW5nZG9uZ3ZpcEB2aXAuMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Ping Dai1†
Ping Dai1† Zixin Wen
Zixin Wen Lanlan Guo
Lanlan Guo Baotian Kan
Baotian Kan Binbin Zhang
Binbin Zhang Xiangdong Jian
Xiangdong Jian