
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 27 April 2023
Sec. Headache and Neurogenic Pain
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1177879
 Bosi Dong1
Bosi Dong1 Shuming Ji2
Shuming Ji2 Yajiao Li3
Yajiao Li3 Hua Li1
Hua Li1 Ruiqi Yang1
Ruiqi Yang1 Na Yang2
Na Yang2 Zhu Liu1
Zhu Liu1 Chenxing Zhu2
Chenxing Zhu2 Hui Wang3
Hui Wang3 Yusha Tang1
Yusha Tang1 Anjiao Peng1
Anjiao Peng1 Lei Chen1*
Lei Chen1*Background: Hypersensitivity to light is a common symptom associated with dysfunction of the occipital region. Earlier studies also suggested that clinically significant right-to-left shunt (RLS) could increase occipital cortical excitability associated with the occurrence of migraine. The aim of this study was to investigate the relationship between RLS and photosensitivity.
Methods: This cross-sectional observational study included the residents aged 18–55 years living in the Mianzhu community between November 2021 and October 2022. Photosensitivity was evaluated using the Photosensitivity Assessment Questionnaire along with baseline clinical data through face-to-face interviews. After the interviews, contrast-transthoracic echocardiography (cTTE) was performed to detect RLS. Inverse probability weighting (IPW) was used to reduce selection bias. Photosensitivity score was compared between individuals with and without significant RLS using multivariable linear regression based on IPW.
Results: A total of 829 participants containing 759 healthy controls and 70 migraineurs were finally included in the analysis. Multivariable linear regression analysis showed that migraine (β = 0.422; 95% CI: 0.086–0.759; p = 0.014) and clinically significant RLS (β = 1.115; 95% CI: 0.760–1.470; p < 0.001) were related to higher photosensitivity score. Subgroup analysis revealed that clinically significant RLS had a positive effect on hypersensitivity to light in the healthy population (β = 0.763; 95% CI: 0.332–1.195; p < 0.001) or migraineurs (β = 1.459; 95% CI: 0.271–2.647; p = 0.010). There was also a significant interaction between RLS and migraine for the association with photophobia (pinteraction = 0.009).
Conclusion: RLS is associated with photosensitivity independently and might exacerbate photophobia in migraineurs. Future studies with RLS closure are needed to validate the findings.
Trial registration: This study was registered at the Chinese Clinical Trial Register, Natural Population Cohort Study of West China Hospital of Sichuan University, ID: ChiCTR1900024623, URL: https://www.chictr.org.cn/showproj.html?proj=40590.
Photophobia, a high sensitivity to light (photosensitivity), is a common sensory disturbance seen in several neurological conditions, especially migraine which is the third most prevalent disease worldwide (1, 2). There are ~70–80% of migraineurs experiencing photophobia (3). Current analyses show that photosensitivity of migraine is the most bothersome non-headache symptom, and it still exists during the interictal period (4). It has also been reported that history of abuse, trauma, meningitis, and intracranial tumors are associated with photosensitivity (5, 6). However, these factors cannot fully account for the development of photophobia, and the mechanism of interictal photosensitivity in migraine remains unclear.
Meanwhile, the incidence of a right-to-left shunt (RLS) is ~50–60% in migraineurs, which is similar to that of photophobia (7, 8). RLS allows micro-emboli, air emboli, and partial unoxygenated venous blood to enter the systemic circulation directly, which can trigger cortical spreading depression (CSD) (9, 10). CSD is a phenomenon that extends from the initial site of excessive neuronal excitability to the surrounding tissues and further causes the continuous inhibition of cortical electrical activity (11). CSD, which often initially occurs in the visual cortex, provides the most likely explanation for visual symptoms in migraine (12). The occipital cortex and connected regions are also hyperexcitable in subjects with photosensitivity, including migraine with interictal photosensitivity, idiopathic generalized epilepsy, anxiety, and depression (13–15). Moreover, the induction of CSD can also increase the activity of trigeminovascular neurons, contributing to the formation of visual phenomena (16). Photophobia is also associated with dysfunction of the trigeminovascular pathway which could alter the responsiveness to visual stimuli (17). Basic studies have also verified that the performance of photophobia may be due to an enhanced interaction of trigeminal and visual inputs through the neuronal pannexin-1 channel opened by the spread of CSD (18). Furthermore, RLS could cause circulation disorders involved with autonomic dysfunction which is also associated with photosensitivity (19). The clinical evidence suggests that RLS-induced embolic events might be linked to the clinical manifestation of cutaneous vasculitis and photosensitivity (20). Nevertheless, the relationship between RLS and photosensitivity has never been explored directly. Thus, we designed a community-based cross-sectional study with the primary aim to investigate the correlation between clinically significant RLS and photosensitivity.
This cross-sectional study was part of the ongoing community-based Mianzhu study, a cohort study designed to investigate the risk factors and burden of chronic diseases in Chinese community-dwelling adults. All inhabitants over the age of 18 who had lived in communities for more than 6 months in Mianzhu (a rural district located 50 miles from urban area of Chengdu) were invited by text message recruitment letters and banner advertisements. From November 2021 to October 2022, 1,533 individuals aged 18–55 years participated, and their standard baseline assessments were undertaken. All participants were also invited for contrast-transthoracic echocardiography (cTTE) examination and photosensitivity assessment. Participant exclusion criteria for this analysis were as follows: (1) self-reported history of ophthalmological conditions; (2) self-reported history of heart disease except RLS, distinct respiratory, neurological or psychiatric diseases, and/or use of psychotropic medication (present and past); (3) 9-item Patient Health Questionnaire (PHQ9) score > or = 15 (21); (4) 7-item General Anxiety Disorder Scale (GAD7) score > or = 10 (22); (5) cTTE not completed; and (6) unwillingness to take cTTE or photosensitivity assessment.
All baseline data were collected from clinician-entered information via face-to-face interviews based on the standard structured questionnaire. The following information was assessed: age, gender, educational level, body mass index (BMI), smoking, alcohol drinking, and regular coffee intake. Educational levels were divided into low (≤ 9 years) and high (more than 9 years). Smoking was defined as having at least one cigarette per day for more than 1 year. Alcohol drinking was defined as having at least one drink a week for more than half a year. Regular coffee was defined as having coffee at least three times a week for more than half a year. Migraine was diagnosed by two specialists independently based on the guidelines of the International Classification of Headache Disorders (ICHD-3 beta) (23). As for migraineurs, approximate years lived with headache and headache frequency (calculated by self-reported headache days per month on average over the last 3 months) were recorded (6). The 6-item Headache Impact Test (HIT6) rating the severity of the underlying migraine disorder (24) was also assessed.
All the participants provided written informed consent. This study complied with the Declaration of Helsinki. The study was approved by the Ethics Committee of Sichuan University (No. 2020145) and registered at the Chinese Clinical Trial Register (ChiCTR1900024623).
Photosensitivity was measured using the Photosensitivity Assessment Questionnaire, which could not only specifically address changes in light sensitivity caused by headache but also assess general photosensitivity (25). The thirteen items regarding photophobia and the 10 items regarding the photophilia score ranging from 0 to 1 were separately calculated from responses. The mean photophobia score and the photophilia score were reported at 4.99 (SD 2.64) and 4.15 (SD 1.98) in healthy people, respectively (25). The questionnaire was developed and validated in an Italian population, while it had never been used in studies with the Chinese population. Translation for the scales was performed by two certified translation specialists. To ensure that the versions in Chinese were the same as those in Italian, a back-to-back translation strategy was used. After translation, we also adapted the scale to account for Chinese cultural characteristics. We had multiple face-to-face meetings to discuss the rating and wording of statements for finalizing the draft scale. After we agreed on the content validity of the statements in the Photosensitivity Assessment Questionnaire, the scale was released to the participants (see Supplementary Table 1). For migraineurs, all of them completed the questionnaire in the interictal state. All obtained data were used to evaluate the reliability and validity of the Photosensitivity Assessment Questionnaire. Raw Cronbach's alpha of our Photosensitivity Assessment Questionnaire was 0.816, and the standardized alpha was 0.818 for this study. The overall Comparative Fit Index (CIF) was good at 0.768. Only the photophobia score of the Photosensitivity Assessment Questionnaire refers to “photosensitivity” and has been analyzed in previous studies (6, 13); thus, we evaluated only questions relating to photophobia, which we will continue to refer to within this article as “photosensitivity”.
Participants were invited to undergo cTTE after collecting clinical characteristics and finishing the Photosensitivity Assessment Questionnaire. First, transthoracic echocardiography (TTE) was performed using 1–5 MHz or 3–8 MHz multiplane transducers in a Philips IE33 for each participant. In this step, participants would be excluded if other cardiac diseases were identified by two experienced sonographers. Next, sonographers analyzed RLS by cTTE. A contrast medium was made by shaking the solution mixing 1 ml of blood and 1 ml of air with 8 ml of saline and then injected into the antecubital veins for increased sensitivity (26). RLS was assessed at rest, during a Valsalva maneuver and coughing, and the highest number of microbubbles in the left atrium observed was recorded, either spontaneously or after provocative maneuvers. For semiquantitative analysis, the degree of RLS assessed by cTTE was divided into four groups based on the International Consensus Criteria as follows: Grade 0, no occurrence of microbubbles; Grade 1, 1–10 microbubbles; Grade 2, 10–30 microbubbles; and Grade 3, > 30 microbubbles or left atrium nearly filled with microbubbles or left atrial opacity (27). Grades 1 and 0 were considered as insignificant or no RLS group, and Grades 2 and 3 were considered as clinically significant shunt group.
Categorical variables were described as frequencies and percentages, and continuous variables were described as mean ± SD. For comparison between healthy controls and migraineurs, the χ2 test (or Fisher's exact test when any expected cell count was <5 for a 2 × 2 table) was used for categorical variables, and the independent Student's t-test was used for continuous variables. Inverse probability weighting (IPW) was used to adjust for differences in baseline characteristics of healthy controls and migraineurs. A standardized mean difference (SMD) after IPW <0.1 was acceptable. Significance was tested using the regression models weighted by the same weights. Multivariate linear regression models were used to determine whether RLS was independently associated with photophobia in all participants and subgroups of healthy controls and migraineurs, respectively. We also used the function “visreg” from the visreg package to visualize the contrast plot of the RLS and migraine interaction effects in photophobia. A p-value of < 0.05 was considered to be statistically significant. All statistical analyses were conducted in R 4.0.1.
Finally, 829 participants were included in the analysis, including 759 healthy controls and 70 migraineurs. The flowchart of the study inclusion process is shown in Figure 1. The mean age (SD) of the participants was 47.81 (6.61) years, and the ratio of female participants was 80.1% (664/829). Clinically significant RLS was observed in 25.3% of all participants (210/829), and none of the participants had atrial septal aneurysm. As for migraineurs, 12.86% of them (9/70) were diagnosed with chronic migraine according to ICHD-3 beta, and only one patient had migraine with medication overuse (characteristics of different types of migraine are shown in Supplementary Table 2). The characteristics of the migraineurs and healthy controls are presented in Table 1. Migraineurs had a higher incidence of significant RLS (37.1% vs. 24.2%, p = 0.026) and a higher photosensitivity score than healthy controls (5.39 vs. 4.57, p = 0.013). To account for selection bias, observed differences in baseline characteristics between the healthy controls and migraineurs were controlled with IPW-adjusted analyses (Figure 2). After the inverse probability of weighting, the SMD of age was 0.135, and the absolute SMD of other variables was all <0.1, indicating that the weighted cohorts were comparable. The results of all remaining analyses were based on IPW-adjusted analyses.
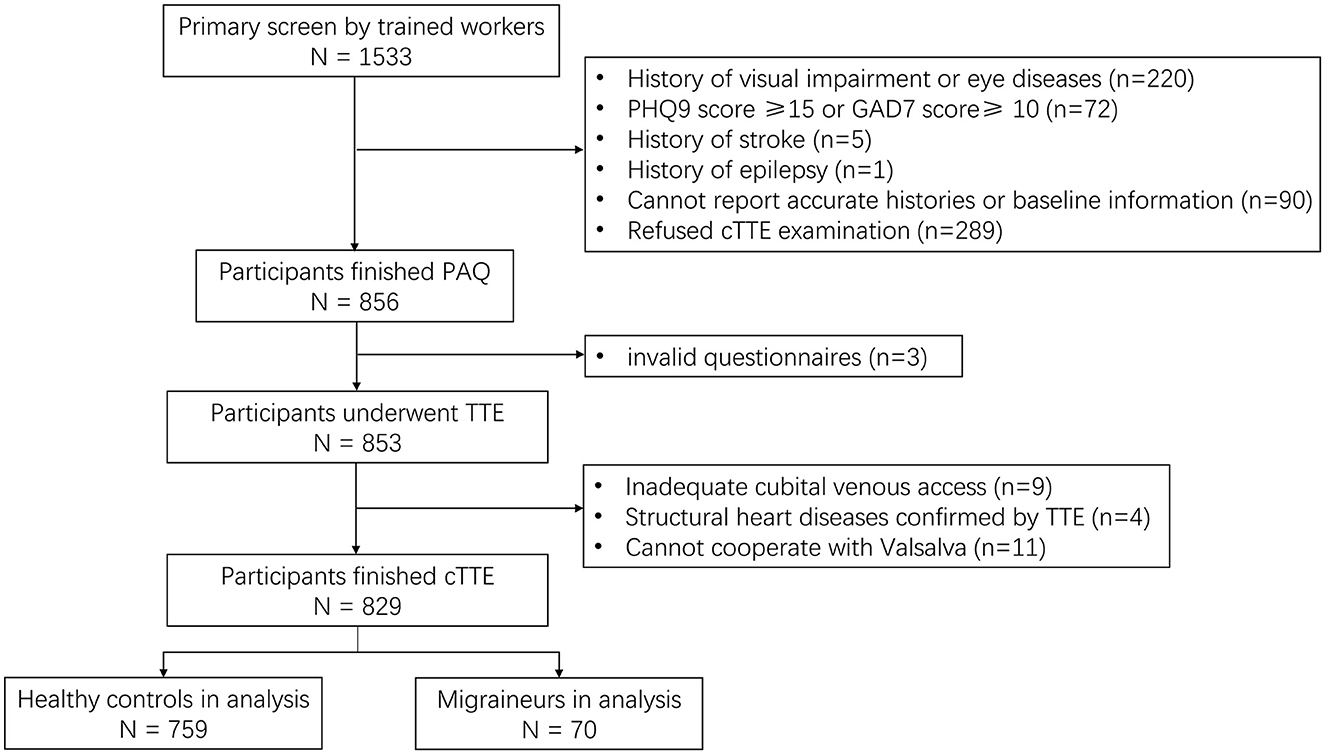
Figure 1. Flowchart of patient recruitment. TTE, transthoracic echocardiography; cTTE, contrast medium transthoracic echocardiography; PAQ, Photosensitivity Assessment Questionnaire; PHQ, Patient Health Questionnaire; GAD, General Anxiety Disorder.
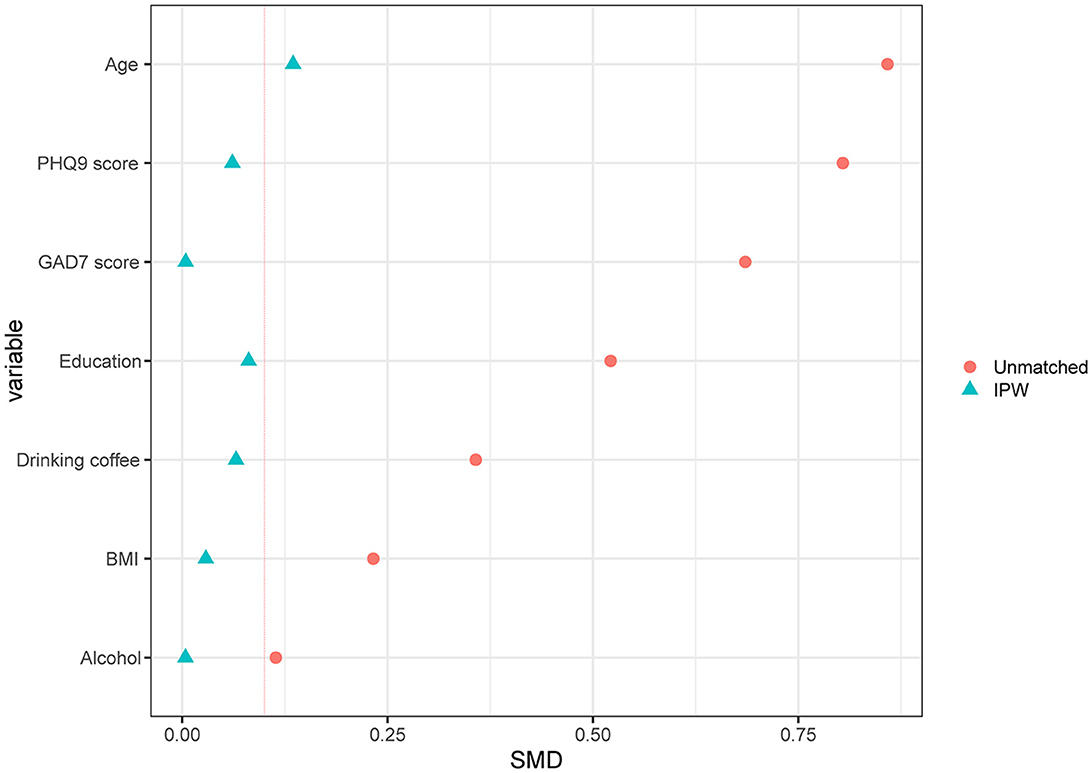
Figure 2. Standardized differences shown for each confounder variable of RLS. The X-axis represents the standardized differences value, and the Y-axis represents baseline variables. The red vertical line signifies the standardized differences cutoffs of >0.1. RLS, right-to-left shunt; IPW, inverse probability weighted; SMD, standardized mean difference.
We noticed that all the participants with significant RLS had a higher score of photosensitivity (5.21 ± 2.54) compared to those without significant RLS (4.45 ± 2.64, p < 0.001) (Table 2). Univariate analysis of photosensitivity score revealed that older age (β = 0.041; p < 0.01), larger weight (β = 0.125; p < 0.001), lower education (β = −1.151; p < 0.001), migraine (β = 0.711; p < 0.001), and clinically significant RLS (β = 1.362; p < 0.001) were associated with greater photosensitivity (Supplementary Table 3). After controlling for the effects of all baseline variates, migraine (β = 0.422; 95% CI: 0.086–0.759; p = 0.014) and clinically significant RLS (β = 1.115; 95% CI: 0.760–1.470; p < 0.001) were still independently related to greater photosensitivity in the multivariate linear regression (Table 3). In the subgroup of healthy controls, individuals with clinically significant RLS had a higher photosensitivity than those without significant RLS (5.05 vs. 4.42, p = 0.004) (Table 2), and RLS was still associated with higher photosensitivity score after considering all variates (β = 0.763; 95% CI: 0.332–1.195; p < 0.001) (Table 3). As for migraineurs, those with significant shunt also had a higher photosensitivity score than those with insignificant or no shunt (4.82 vs. 6.35, p = 0.015) (Table 2). The association of greater photosensitivity and significant shunt remained significant in migraineurs after controlling baseline variates (β = 1.459; 95% CI: 0.271–2.647; p = 0.017). We furtherly controlled the effects of headache characteristics, and it was also evident that RLS was significantly correlated with photosensitivity independently (β = 1.560; 95% CI: 0.373–2.746; p = 0.010). There was an insignificant relationship between photosensitivity and years lived with headache, headache frequency, aura, chronic migraine, or HIT6 in multivariate linear regression analysis (Table 3).
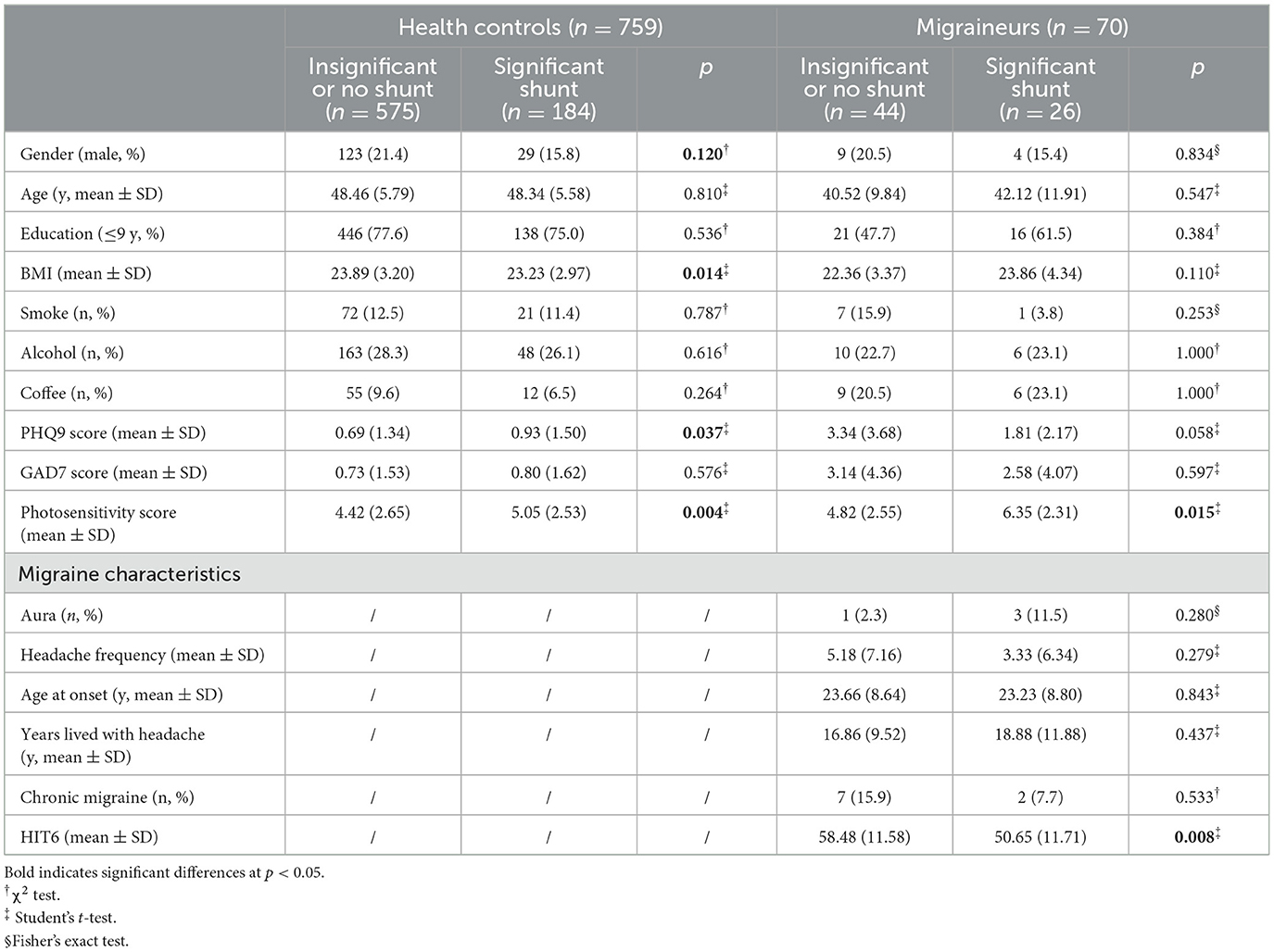
Table 2. High photosensitivity score in participants with significant RLS in the group of healthy controls or group of migraineurs.
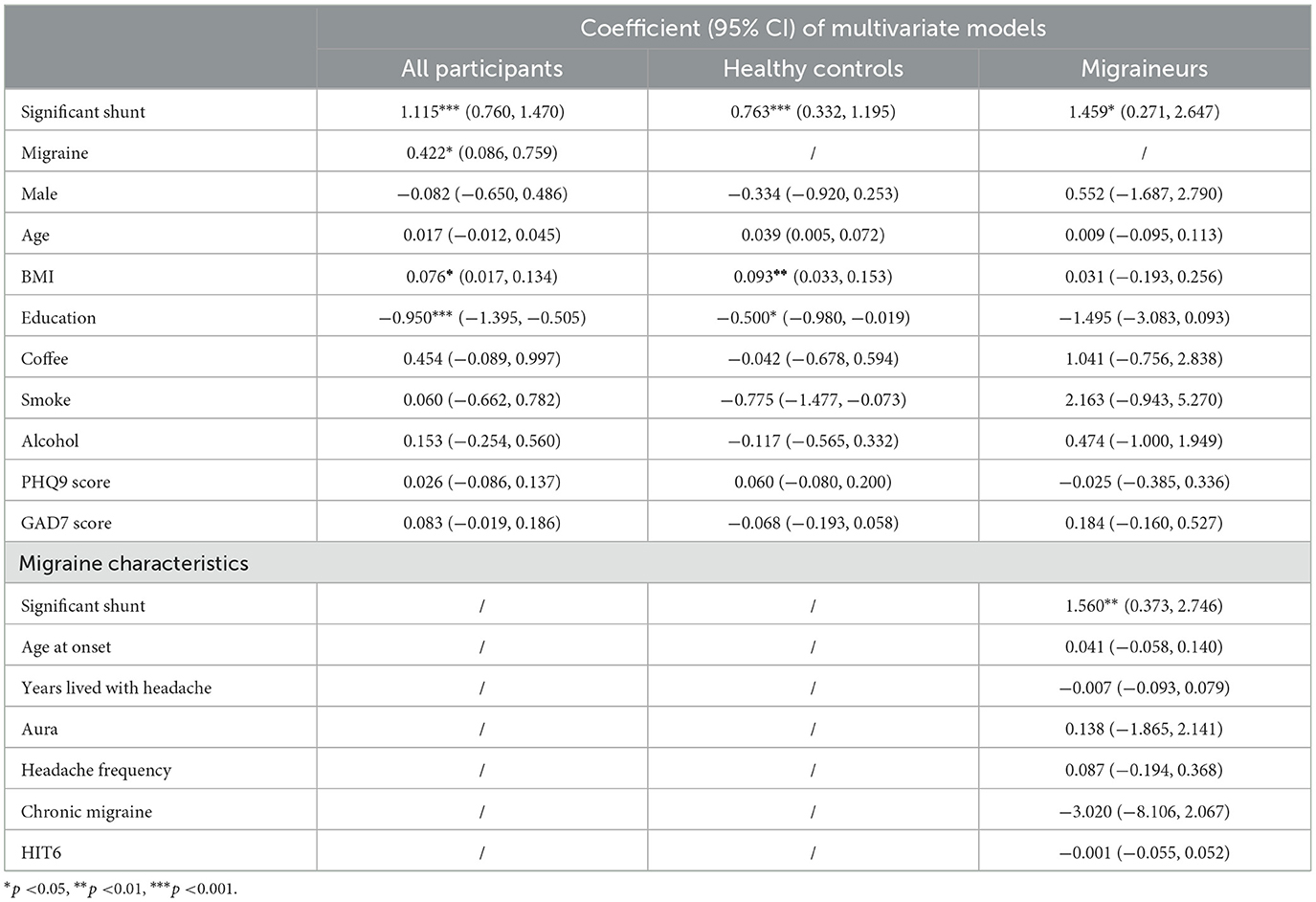
Table 3. Multivariate linear regression models of photosensitivity scores in healthy controls and migraineurs.
Additionally, an interaction term between significant RLS and migraine was also created and entered as a factor of photosensitivity score in the regression model. There was a significant interaction between clinically significant RLS and migraine for the association with greater photosensitivity (βinteraction = 0.957; 95% CI: 0.234–1.678; pinteraction = 0.009), and Figure 3 shows that RLS could increase photosensitivity scores in migraine.
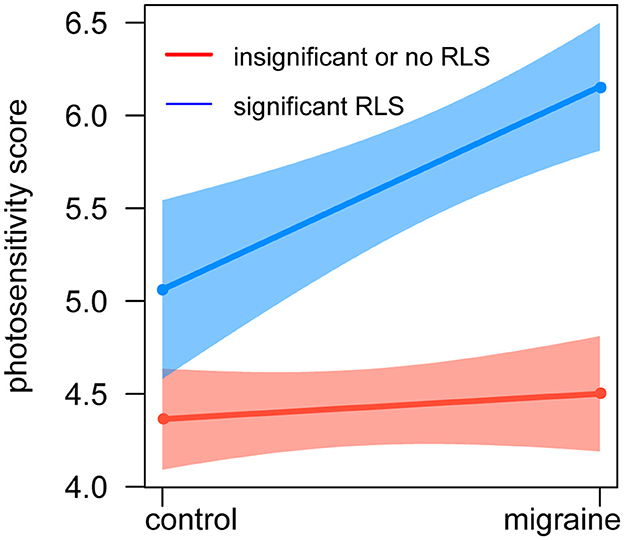
Figure 3. Relationship between photosensitivity, RLS, and migraine. The red line represents insignificant or no shunt, the blue line represents significant shunt, and the whiskers show a 95% CI. The X-axis represents the groups of healthy control and migraine, and the Y-axis represents the photosensitivity score. There was an interaction between shunt and migraine for the association with greater photosensitivity. CI, confidence interval.
In this study, participants from the community were assessed for photosensitivity by the adjusted and validated Photosensitivity Assessment Questionnaire, as well as for RLS by the cTTE. The results showed that (1) individuals with clinically significant RLS had a higher photosensitivity score compared with those without RLS; (2) RLS was an independent factor associated with hypersensitivity to light; and (3) there was an interaction between RLS and migraine for the association with photosensitivity, suggesting that RLS might worsen photophobia in migraineurs.
Photophobia is a light-induced phenomenon with underlying pathophysiologic mechanisms in visual, trigeminal, and autonomic systems so far; however, the factors of this increased photosensitivity are incompletely understood, especially in non-migraine (28). In this study, we tested the relationship between RLS and photosensitivity directly and found higher photosensitivity in the population with significant RLS and that RLS was an independent risk factor of hypersensitivity to light. The connection between RLS and photosensitivity may have several explanations. The generation of CSD induced by RLS was reported to be involved with the occipital cortex, which might underlie many visual symptoms, such as long-term contrast discrimination, visual evoked potential prolongation, and visual field sensitivity (29–31). Hypersensitivity to light was also associated with cortical hypersensitivity within the visual cortex (32). Animal experimental research verified that air micro-emboli could trigger CSD in the posterior region of the mice's brain and induce obvious photophobia in mice (10, 33). Moreover, functional magnetic resonance imaging (fMRI) studies described the activation of the trigeminothalamic pathway including central changes in individuals with high photosensitivity (34). There was also evidence that RLS-induced CSD mediated activation of trigeminal primary afferents (35). In addition to CSD, several studies suggested that RLS had an impact on hemodynamics in the anterior and posterior circulation through frequent transit of micro-emboli or vasoactive substances bypassing the deactivating pulmonary filters (36, 37). The posterior microcirculation abnormalities were related to the dysfunction of the autonomic system with the manifestation of photophobia (38, 39). In addition, prior clinical surveys proved that paradoxical embolism induced by RLS could damage both cerebral and peripheral microcirculation, leading to an alteration of serotonin, pro-inflammatory bradykinin, or neurotensin metabolism (40). These metabolic impairments also had an adverse effect on autonomic function, causing disturbances in the response to visual stimuli (41, 42).
Additionally, our results were consistent with the previous findings that the prevalence of migraine in the Asian population was nearly 9% and photophobia was a common interictal characteristic of migraine (43, 44). Our finding also showed that the interaction between RLS and migraine was significant in photophobia, suggesting that RLS could aggravate photosensitivity in migraine. On the one hand, interictal photophobia may be a symptom of a structural and functional disorder of the posterior head in migraine. Prior clinical studies had established a link between migraine and increased interictal activation of visual processing brain regions in response to a visual stimulus (45). Migraineurs performed hyperexcitability of the visual cortex with a wider photo-responsive area during interictal periods in fMRI studies (46). Transcranial static magnetic field stimulation of the visual cortex and greater occipital nerve block could improve photophobia in migraine (47, 48). On the other hand, RLS could be one of causal factors for the development of migraine, probably due to RLS-induced CSD in the calcarine cortex. Our result was in concordance with a higher incidence of RLS in migraineurs (49). Clinical research found that the paradoxical air micro-embolism bypass RLS could develop CSD or CSD-like bioelectrical abnormalities in occipital lobes and then occasionally induce headache (50). In addition, neuroimaging evidence showed micro-embolic origin located in the posterior vascular border zone territory in young migraineurs (51), and researchers concluded that the presence of RLS might be responsible for the development of migraine by affecting the integrity of the white matter in posterior circulation and the brain function of the posterior region (52). Thus, we speculated that RLS might contribute to cerebral hemodynamic disorders, particularly in the posterior head, which would further aggravate the symptom of hypersensitivity to light in migraineurs. In previous studies, the reduction of visual stimulation was suggested to be a useful tool for reducing migraine attacks (53). It has also been reported that there was a reduction in the frequency and severity of migraine attacks after RLS closure in migraineurs (54). Nevertheless, some negative studies proposed that patient selection for RLS closure should be careful, and indications for cessation of RLS in migraine remained challenging (55). We therefore considered that photosensitivity score needed to be evaluated in future RLS closure trials to validate the association and that photosensitivity might also be a factor for future evaluation of RLS closure in migraineurs.
Our study also had limitations. The sample size of migraine in the community was small, which probably led to the lack of significant correlation between photophobia and migraine with aura. The results should be further investigated in future large migraine case–control studies. Meanwhile, there was a mismatch in age between migraineurs and health controls, which might be due to the large age span and small sample size of the recruited migraineurs. Though the effect size of SMD of age <0.2 was considered negligible, care is still needed when generalizing the results. In addition, there was a relatively small sample size of male; however, migraine affects more women than men (56). In addition, ophthalmic and neurological diseases were mainly self-reported and rarely confirmed by imaging evaluations. The cross-sectional study design was inherently subject to bias, especially recall bias, as well as the effect of unmeasured confounders.
In conclusion, our study presents that RLS is associated with photosensitivity independently and that RLS could aggravate photosensitivity in migraineurs. Further investigation of a possible mechanistic link between RLS and photosensitivity is warranted, which might help in evaluating RLS closure operation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of Sichuan University. The patients/participants provided their written informed consent to participate in this study.
BD and LC: conceptualization. BD, SJ, and YL: methodology. BD, SJ, YL, HL, RY, NY, CZ, and HW: formal analysis and investigation. BD: writing—original draft preparation. BD, SJ, HL, and ZL: writing—review and editing. LC: funding acquisition and resources. YT and AP: supervision. All authors contributed to the article and approved the submitted version.
This research was supported by the National Natural Science Foundation of China [82271500].
The authors gratefully acknowledge Danxuan Yang, Zixuan Zhao, and Wei Zhao from the Tianjin Foreign Studies University for the translation and revision of the Photosensitivity Assessment Questionnaire. The authors also thank Siqi Jia from Durham University for the English revision.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1177879/full#supplementary-material
1. Katz BJ, Digre KB. Diagnosis, pathophysiology, and treatment of photophobia. Surv Ophthalmol. (2016) 61:466–77. doi: 10.1016/j.survophthal.2016.02.001
2. Li Z, Zeng F, Yin T, Lan L, Makris N, Jorgenson K, et al. Acupuncture modulates the abnormal brainstem activity in migraine without aura patients. Neuroimage Clin. (2017) 15:367–75. doi: 10.1016/j.nicl.2017.05.013
3. Rasmussen BK, Jensen R, Olesen J, A. population-based analysis of the diagnostic criteria of the International Headache Society. Cephalalgia. (1991) 11:129–34. doi: 10.1046/j.1468-2982.1991.1103129.x
4. Munjal S, Singh P, Reed ML, Fanning K, Schwedt TJ, Dodick DW, et al. Most Bothersome Symptom in Persons With Migraine: Results From the Migraine in America Symptoms and Treatment (MAST) Study. Headache. (2020) 60:416–29. doi: 10.1111/head.13708
5. Kim SK, Chong CD, Dumkrieger G, Ross K, Berisha V, Schwedt TJ. Clinical correlates of insomnia in patients with persistent post-traumatic headache compared with migraine. J Headache Pain. (2020) 21:33. doi: 10.1186/s10194-020-01103-8
6. Trivedi M, Dumkrieger G, Chong CD, Dodick DW, Schwedt TJ. Impact of abuse on migraine-related sensory hypersensitivity symptoms: Results from the American Registry for Migraine Research. Headache. (2021) 61:740–54. doi: 10.1111/head.14100
7. Koutroulou I, Tsivgoulis G, Karacostas D, Ikonomidis I, Grigoriadis N, Karapanayiotides T. Prevalence of patent foramen ovale in the Greek population is high and impacts on the interpretation of the risk of paradoxical embolism (RoPE) score. Ther Adv Neurol Disord. (2020) 13:1279186241. doi: 10.1177/1756286420964673
8. Schwedt TJ, Demaerschalk BM, Dodick DW. Patent foramen ovale and migraine: a quantitative systematic review. Cephalalgia. (2008) 28:531–40. doi: 10.1111/j.1468-2982.2008.01554.x
9. Cramer SC, Rordorf G, Maki JH, Kramer LA, Grotta JC, Burgin WS, et al. Increased pelvic vein thrombi in cryptogenic stroke: results of the Paradoxical Emboli from Large Veins in Ischemic Stroke (PELVIS) study. Stroke. (2004) 35:46–50. doi: 10.1161/01.STR.0000106137.42649.AB
10. Nozari A, Dilekoz E, Sukhotinsky I, Stein T, Eikermann-Haerter K, Liu C, et al. Microemboli may link spreading depression, migraine aura, and patent foramen ovale. Ann Neurol. (2010) 67:221–29. doi: 10.1002/ana.21871
11. Mancini V, Mastria G, Frantellizzi V, Troiani P, Zampatti S, Carboni S, et al. Migrainous Infarction in a Patient With Sporadic Hemiplegic Migraine and Cystic Fibrosis: A 99mTc-HMPAO Brain SPECT Study. Headache. (2019) 59:253–58. doi: 10.1111/head.13472
12. Hadjikhani N, Sanchez DRM, Wu O, Schwartz D, Bakker D, Fischl B, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S a. (2001) 98:4687–92. doi: 10.1073/pnas.071582498
13. Chong CD, Starling AJ, Schwedt TJ. Interictal photosensitivity associates with altered brain structure in patients with episodic migraine. Cephalalgia. (2016) 36:526–33. doi: 10.1177/0333102415606080
14. Brigo F, Bongiovanni LG, Nardone R, Trinka E, Tezzon F, Fiaschi A, et al. Visual cortex hyperexcitability in idiopathic generalized epilepsies with photosensitivity: a TMS pilot study. Epilepsy Behav. (2013) 27:301–06. doi: 10.1016/j.yebeh.2013.02.010
15. Wessa M, Lois G. Brain functional effects of psychopharmacological treatment in major depression: a focus on neural circuitry of affective processing. Curr Neuropharmacol. (2015) 13:466–79. doi: 10.2174/1570159X13666150416224801
16. Zhang X, Levy D, Kainz V, Noseda R, Jakubowski M, Burstein R. Activation of central trigeminovascular neurons by cortical spreading depression. Ann Neurol. (2011) 69:855–65. doi: 10.1002/ana.22329
17. Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci. (2010) 13:239–45. doi: 10.1038/nn.2475
19. Gurgun C, Ercan E, Ceyhan C, Yavuzgil O, Zoghi M, Aksu K, et al. Cardiovascular involvement in Behcet's disease. Jpn Heart J. (2002) 43:389–98. doi: 10.1536/jhj.43.389
20. Sargin G, Senturk T, Cildag S. Rhupus syndrome and Chiari's network. J Family Med Prim Care. (2018) 7:249–51. doi: 10.4103/jfmpc.jfmpc_197_17
21. O'Cleirigh C, Newcomb ME, Mayer KH, Skeer M, Traeger L, Safren SA. Moderate levels of depression predict sexual transmission risk in HIV-infected MSM: a longitudinal analysis of data from six sites involved in a “prevention for positives” study. AIDS Behav. (2013) 17:1764–69. doi: 10.1007/s10461-013-0462-8
22. Coyle RM, Lampe FC, Miltz AR, Sewell J, Anderson JF, Apea V, et al. Associations of depression and anxiety symptoms with sexual behaviour in women and heterosexual men attending sexual health clinics: a cross-sectional study. Sex Transm Infect. (2019) 95:254–61. doi: 10.1136/sextrans-2018-053689
23. The The International Classification of Headache Disorders 3rd edition (beta version). Cephalalgia. (2013) 33:629–808. doi: 10.1177/0333102413485658
24. Kosinski M, Bayliss MS, Bjorner JB, Ware JJ, Garber WH, Batenhorst A, et al. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res. (2003) 12:963–74. doi: 10.1023/A:1026119331193
25. F.Morana SICP. Sensibilità alla luce: confronto fra disturbo bipolare e disturbo di panico. J Psychopathol. (2001) 7:327–7. Available online at: https://old.jpsychopathol.it/article/sensibilita-alla-luce-confronto-fra-disturbo-bipolare-e-disturbo-di-panico/
26. Silvestry FE, Cohen MS, Armsby LB, Burkule NJ, Fleishman CE, Hijazi ZM, et al. Guidelines for the echocardiographic assessment of atrial septal defect and patent foramen Ovale: from the American society of echocardiography and society for cardiac angiography and interventions. J Am Soc Echocardiogr. (2015) 28:910–58. doi: 10.1016/j.echo.2015.05.015
27. Maffe S, Dellavesa P, Zenone F, Paino AM, Paffoni P, Perucca A, et al. Transthoracic second harmonic two- and three-dimensional echocardiography for detection of patent foramen ovale. Eur J Echocardiogr. (2010) 11:57–63. doi: 10.1093/ejechocard/jep165
28. Diel RJ, Mehra D, Kardon R, Buse DC, Moulton E, Galor A. Photophobia: shared pathophysiology underlying dry eye disease, migraine and traumatic brain injury leading to central neuroplasticity of the trigeminothalamic pathway. Br J Ophthalmol. (2021) 105:751–60. doi: 10.1136/bjophthalmol-2020-316417
29. Calic Z, Nham B, Bradshaw AP, Young AS, Bhaskar S, D'Souza M, et al. Separating posterior-circulation stroke from vestibular neuritis with quantitative vestibular testing. Clin Neurophysiol. (2020) 131:2047–55. doi: 10.1016/j.clinph.2020.04.173
30. Shatillo A, Koroleva K, Giniatullina R, Naumenko N, Slastnikova AA, Aliev RR, et al. Cortical spreading depression induces oxidative stress in the trigeminal nociceptive system. Neuroscience. (2013) 253:341–49. doi: 10.1016/j.neuroscience.2013.09.002
31. Yemisci M, Eikermann-Haerter K. Aura and Stroke: relationship and what we have learnt from preclinical models. J Headache Pain. (2019) 20:63. doi: 10.1186/s10194-019-1016-x
32. Pearl TA, Dumkrieger G, Chong CD, Dodick DW, Schwedt TJ. Sensory hypersensitivity symptoms in migraine with vs without aura: results from the American registry for migraine research. Headache. (2020) 60:506–14. doi: 10.1111/head.13745
33. Pi C, Tang W, Li Z, Liu Y, Jing Q, Dai W, et al. Cortical pain induced by optogenetic cortical spreading depression: from whole brain activity mapping. Mol Brain. (2022) 15:99. doi: 10.1186/s13041-022-00985-w
34. Moulton EA, Becerra L, Borsook D. An fMRI case report of photophobia: activation of the trigeminal nociceptive pathway. Pain. (2009) 145:358–63. doi: 10.1016/j.pain.2009.07.018
35. Akerman S, Romero-Reyes M. Targeting the central projection of the dural trigeminovascular system for migraine prophylaxis. J Cereb Blood Flow Metab. (2019) 39:704–17. doi: 10.1177/0271678X17729280
36. Altamura C, Paolucci M, Brunelli N, Cascio RA, Cecchi G, Assenza F, et al. Right-to-left shunts and hormonal therapy influence cerebral vasomotor reactivity in patients with migraine with aura. PLoS ONE. (2019) 14:e220637. doi: 10.1371/journal.pone.0220637
37. Gollion C, Nasr N, Fabre N, Barege M, Kermorgant M, Marquine L, et al. Cerebral autoregulation in migraine with aura: a case control study. Cephalalgia. (2019) 39:635–40. doi: 10.1177/0333102418806861
38. von Sarnowski B, Schminke U, Grittner U, Tanislav C, Bottcher T, Hennerici MG, et al. Posterior vs. anterior circulation stroke in young adults: a comparative study of stroke aetiologies and risk factors in stroke among young fabry patients (sifap1). Cerebrovasc Dis. (2017) 43:152–60. doi: 10.1159/000454840
39. Tanev KS, Federico LE, Sydnor VJ, Leveroni CL, Hassan K, Biffi A. Neuropsychiatric symptoms in a occipito-temporal infarction with remarkable long-term functional recovery. Cortex. (2021) 137:205–14. doi: 10.1016/j.cortex.2021.01.013
40. Alvarez-Fernandez JA, Alarcon-Fernandez O, Perez-Quintero R. Contribution of the patent foramen ovale to the etiopathogeny of the irritable bowel syndrome. Rev Clin Esp. (2009) 209:136–40. doi: 10.1016/s0014-2565(09)70879-3
41. Bouffard MA. The Pupil. Continuum (Minneap Minn). (2019) 25:1194–214. doi: 10.1212/CON.0000000000000771
42. Anyanwu E, Harding GF, Edson A. The involvement of serotonin (5-hydroxytryptamine) in photosensitive epilepsy. J Basic Clin Physiol Pharmacol. (1994) 5:179–206. doi: 10.1515/JBCPP.1994.5.3-4.179
43. Zele AJ, Dey A, Adhikari P, Feigl B. Melanopsin hypersensitivity dominates interictal photophobia in migraine. Cephalalgia. (2021) 41:217–26. doi: 10.1177/0333102420963850
44. Kim SK, Hong SM, Park IS, Choi HG. Association between migraine and benign paroxysmal positional vertigo among adults in South Korea. JAMA Otolaryngol Head Neck Surg. (2019) 145:307–12. doi: 10.1001/jamaoto.2018.4016
45. Pinheiro CF, Moraes R, Carvalho GF, Sestari L, Will-Lemos T, Bigal ME, et al. The influence of photophobia on postural control in patients with migraine. Headache. (2020) 60:1644–52. doi: 10.1111/head.13908
46. Martin H, Sanchez DRM, de Silanes CL, Alvarez-Linera J, Hernandez JA, Pareja JA. Photoreactivity of the occipital cortex measured by functional magnetic resonance imaging-blood oxygenation level dependent in migraine patients and healthy volunteers: pathophysiological implications. Headache. (2011) 51:1520–28. doi: 10.1111/j.1526-4610.2011.02013.x
47. Lozano-Soto E, Soto-Leon V, Sabbarese S, Ruiz-Alvarez L, Sanchez-Del-Rio M, Aguilar J, et al. Transcranial static magnetic field stimulation (tSMS) of the visual cortex decreases experimental photophobia. Cephalalgia. (2018) 38:1493–97. doi: 10.1177/0333102417736899
48. Membrilla JA, de Lorenzo I, Sanchez-Casado L, Sastre M, Diaz DTJ. Impact of greater occipital nerve block on photophobia levels in migraine patients. J Neuroophthalmol. (2022) 42:378–83. doi: 10.1097/WNO.0000000000001541
49. Eikermann-Haerter K. Spreading depolarization may link migraine and stroke. Headache. (2014) 54:1146–57. doi: 10.1111/head.12386
50. Sevgi EB, Erdener SE, Demirci M, Topcuoglu MA, Dalkara T. Paradoxical air microembolism induces cerebral bioelectrical abnormalities and occasionally headache in patent foramen ovale patients with migraine. J Am Heart Assoc. (2012) 1:e1735. doi: 10.1161/JAHA.112.001735
51. Kruit MC, Launer LJ, Ferrari MD, van Buchem MA. Infarcts in the posterior circulation territory in migraine. The population-based MRI CAMERA study. Brain. (2005) 128:2068–77. doi: 10.1093/brain/awh542
52. Cao W, Shen Y, Zhong J, Chen Z, Wang N, Yang J. The patent foramen ovale and migraine: associated mechanisms and perspectives from MRI evidence. Brain Sci. (2022) 12:941. doi: 10.3390/brainsci12070941
53. Tatsumoto M, Suzuki E, Nagata M, Suzuki K, Hirata K. Prophylactic treatment for patients with migraine using blue cut for night glass. Intern Med. (2022). doi: 10.2169/internalmedicine.0132-22
54. Vigna C, Marchese N, Inchingolo V, Giannatempo GM, Pacilli MA, Di Viesti P, et al. Improvement of migraine after patent foramen ovale percutaneous closure in patients with subclinical brain lesions: a case-control study. Jacc Cardiovasc Interv. (2009) 2:107–13. doi: 10.1016/j.jcin.2008.10.011
55. Rundek T, Elkind MS, Di Tullio MR, Carrera E, Jin Z, Sacco RL, et al. Patent foramen ovale and migraine: a cross-sectional study from the Northern Manhattan Study (NOMAS). Circulation. (2008) 118:1419–24. doi: 10.1161/CIRCULATIONAHA.108.771303
Keywords: right-to-left shunt, photosensitivity, migraine, community-based study, cross-sectional study
Citation: Dong B, Ji S, Li Y, Li H, Yang R, Yang N, Liu Z, Zhu C, Wang H, Tang Y, Peng A and Chen L (2023) Connection between right-to-left shunt and photosensitivity: a community-based cross-sectional study. Front. Neurol. 14:1177879. doi: 10.3389/fneur.2023.1177879
Received: 02 March 2023; Accepted: 03 April 2023;
Published: 27 April 2023.
Edited by:
Raffaele Ornello, University of L'Aquila, ItalyReviewed by:
Claudia Altamura, Fondazione Policlinico Campus Bio-Medico, ItalyCopyright © 2023 Dong, Ji, Li, Li, Yang, Yang, Liu, Zhu, Wang, Tang, Peng and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Chen, bGVpbGVpXzI1QDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.