
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
STUDY PROTOCOL article
Front. Neurol. , 30 May 2023
Sec. Stroke
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1177500
 Vijay K. Sharma1,2*
Vijay K. Sharma1,2* Anil Gopinathan3
Anil Gopinathan3 Benjamin Y. Q. Tan1
Benjamin Y. Q. Tan1 Poay Huan Loh4
Poay Huan Loh4 Jennifer Hung1
Jennifer Hung1 David Tang1
David Tang1 Christopher Chua1
Christopher Chua1 Amanda C. Y. Chan1
Amanda C. Y. Chan1 Jonathan J. Y. Ong1
Jonathan J. Y. Ong1 Amanda Chin1
Amanda Chin1 Mingxue Jing1
Mingxue Jing1 Yihui Goh1
Yihui Goh1 Sibi Sunny1
Sibi Sunny1 Chin Howe Keat1
Chin Howe Keat1 Zhang Ka1
Zhang Ka1 Shivani Pandya1
Shivani Pandya1 Lily Y. H. Wong1
Lily Y. H. Wong1 Jin Tao Chen1
Jin Tao Chen1 Leonard L. L. Yeo1,2
Leonard L. L. Yeo1,2 Bernard P. L. Chan1
Bernard P. L. Chan1 Hock Luen Teoh1
Hock Luen Teoh1 Arvind K. Sinha3
Arvind K. Sinha3Intracranial stenosis is prevalent among Asians and constitutes a common cause of cerebral ischemia. While the best medical therapy carries stroke recurrence rates in excess of 10% per year, trials with intracranial stenting have been associated with unacceptable peri-procedural ischemic events. Cerebral ischemic events are strongly related to the severity of intracranial stenosis, which is high in patients with severe intracranial stenosis with poor vasodilatory reserve. Enhanced External Counter Pulsation (EECP) therapy is known to improve myocardial perfusion by facilitating the development of collateral blood vessels in the heart. In this randomized clinical trial, we evaluate whether EECP therapy may be useful in patients with severe stenosis of intracranial internal carotid (ICA) or middle cerebral artery (MCA). The review of literature, methods of evaluation, status of currently used therapeutic approaches, and trial protocol have been presented.
Clinical trial registration: ClinicalTrials.gov, Identifier: NCT03921827.
Atherosclerosis of cerebral vessels is a common cause of ischemic stroke. Among Asian stroke patients, intracranial atherosclerotic disease (ICAD) is more common and accounts for ~30–50% of all strokes as compared to only 5–10% of strokes in their Caucasian counterparts (1–3). This difference is believed to be most likely related to genetic susceptibility, different lifestyles, and a risk factor profile (2, 4, 5).
After an acute ischemic stroke (AIS) or transient ischemic attack (TIA), the risk of recurrent cerebral ischemia within 90 days is ~10% (6–8). Among patients with significant ICAD, this risk increases to more than 20% in 1 year despite the best medical treatment (9). The rate of recurrence of cerebral ischemia in ICAD is determined by the degree of stenosis as well as the presence of collaterals. In warfarin–aspirin symptomatic intracranial disease (WASID) study, patients with more than 70% stenosis of an intracranial artery had a greater risk of a recurrent event when compared to those with lesser (50–69%) degree of stenosis (9). Interestingly, patients with more than 70% intracranial stenosis with good collaterals carry a lower risk for subsequent cerebral ischemic events (10, 11).
The two main mechanisms of ischemic stroke in ICAD patients are thromboembolism and cerebral hemodynamic insufficiency (12–15). While transcranial Doppler (TCD) monitoring can establish the thrombo-embolic phenomenon by identifying the spontaneous micro-embolic signals in the arterial segments distal to the site of stenosis (16–18) and help in planning appropriate antithrombotic treatment (19), assessment of cerebral hemodynamic insufficiency remains a complex issue and may serve as a potential target for improving the outcomes in patients with severe intracranial stenosis.
Anti-platelet agents are the mainstay of secondary prevention of stroke (20, 21). Earlier studies suggested warfarin to be better than aspirin for ICAD (22). However, the WASID trial did not show any significant difference in stroke recurrence between warfarin and aspirin. Furthermore, aspirin was found to be safer as compared to warfarin (23).
The use of short-term double antiplatelet therapy (DAPT) is effective in reducing stroke recurrence risk in patients with minor stroke or TIA (22, 24). In a TCD study, patients treated with short-term clopidogrel plus aspirin demonstrated a significant reduction in the number of spontaneous emboli, as compared to aspirin alone, in the relevant intracranial artery in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR) (25). The use of DAPT in intracranial stenosis was also supported by the stenting vs. aggressive medical therapy for intracranial arterial stenosis (SAMMPRIS) trial (26).
Extracranial to intracranial (EC-IC) bypass has been compared with medical management in patients with intracranial stenosis, and it did not lower the risk of recurrent ischemic stroke. Furthermore, it was associated with worse outcomes as compared to medical management (27, 28). However, in a recent prospective observational study, we demonstrated that superficial temporal artery-middle cerebral artery (STA-MCA) bypass surgery in carefully selected patients with severe intracranial stenosis of the intracranial internal carotid artery (ICA) or middle cerebral artery (MCA) results in significant improvement in cerebral hemodynamic parameters as well as led to absolute reduction (32% during 34 months follow up) in stroke recurrence (29). Interestingly, the benefits in stroke recurrence persisted during an extended follow-up of more than 6 years (30).
The self-expanding Wingspan stent was approved for medically refractory patients with 50–99% stenosis of a major intracranial artery (31). In total, two multicenter registries showed the feasibility of angioplasty with a Wingspan stent (32, 33). However, the SAMMPRIS trial was stopped prematurely as the angioplasty and stenting group showed 14.7% event rate (stroke or death) at 30 days as compared to the medical management arm (5.8% event rate) (26). Similarly the Vitesse Intracranial Stent Study for Ischemic Stroke Therapy (VISSIT) trial was terminated prematurely due to a significant increase in peri-procedural complications (34). However, a Chinese trial found that intracranial stenting could be a safe and efficient treatment for carefully selected patients with MCA stenosis (35). Similarly improved safety of intracranial stenting was reported in the Wingspan Stent System Post Market Surveillance (WEAVE) trial (36). Recent studies with drug-eluting stents have shown promise in satisfactory recanalization, coupled with lesser chances of restenosis as well as other peri-procedural complications (37). With the advent of rapid improvements in stent technology, stenting for ICAD is expected to become a standard of care in future if safety and efficacy could be established in adequately powered randomized clinical trials.
EECP has been used as a non-invasive therapy for treating patients with angina, congestive heart failure, acute myocardial infarction, and cardiogenic shock (38–40). It uses ECG-triggered pressure application during ventricular diastole delivered by air-filled cuffs placed around the lower limbs to improve systemic hemodynamics (Figure 1). EECP leads to a nearly 25% improvement in arterial flow volume in carotid, renal, and hepatic arteries along with a 20–40% improvement in coronary artery blood flow and a 12% increase in left ventricular (LV) stroke volume (41). EECP improves cardiac functional class, decreased anginal episodes, and reduced nitroglycerin use in patients with refractory angina (42). Although it is non-invasive, EECP produces similar hemodynamic effects as the intra-aortic balloon pump (43).
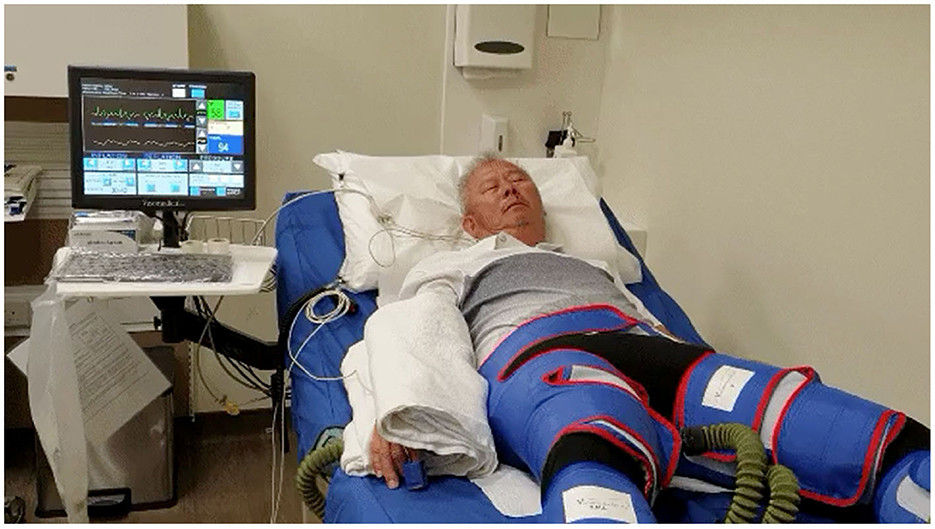
Figure 1. Set-up for enhanced external counter pulsation (EECP) therapy equipment. This figure shows a patient with cuffs over both lower limbs as well the monitor, which shows patient ECG as well as continuous finger plethysmography.
EECP may help in the recovery of stroke patients with large artery occlusive disease (44). The hemodynamic alterations induced during EECP therapy may be monitored in real time by continuous finger plethysmography as well as TCD (Figure 2). In a Hong Kong study of 155 patients with ICAD, EECP treatment was found to be a significant factor for predictor of good outcomes at 3 months (45). In another preliminary study, EECP performed on healthy volunteers induced marked changes in cerebral arterial waveforms and augmented peak diastolic and mean MCA flow velocities on TCD (46). Given its low-risk complication profile, EECP may offer an alternative therapeutic approach for improving cerebral hemodynamics in patients with severe ICAD.

Figure 2. Changes in finger plethysmography and transcranial Doppler spectra from middle cerebral artery during EECP therapy. (A) Shows the ECG and finger plethysmography at rest while the change in the waveform after applying an augmentation pressure of 160 mmHg is shown in (B). Note the second peak during diastole. Corresponding changes on TCD at rest and during EECP therapy are shown in (C, D), respectively.
The study aimed to evaluate whether EECP therapy that would lead to an improvement in cerebral vasodilatory reserve (CVR) in patients with severe and recently symptomatic stenosis of intracranial carotid (ICA) or middle cerebral artery (MCA).
1. To evaluate the impact of EECP on the recurrence of a cerebral ischemic event in patients with severe and recently symptomatic stenosis of ICA or MCA.
2. To evaluate the impact of EECP on neurocognitive performance in patients with severe and recently symptomatic stenosis of ICA or MCA.
This is a prospective, randomized, controlled, observer-blinded phase III trial. The study will be conducted at the National University Hospital, Singapore. The study would recruit a total of 130 participants. The primary objective will be assessed at 4 months after the initiation of treatment. Details of patient workflow are summarized in Figure 3.
Consecutive patients with symptomatic and severe ICAD involving intra-cranial ICA or MCA would be included in the study. We define “symptomatic” ICAD as the identification of a severe stenosis or occlusion of the index artery (by TCD and contrast CT angiography or digital subtraction angiography) in patients who present with symptoms attributable to that particular artery within the previous 3 months.
1. Patients with recent stroke/TIA and severe stenosis of intracranial ICA or MCA and impaired CVR within the previous 3 months (but not before 3 weeks) after acute stroke or TIA. This is to differentiate between patients with long-standing fixed stenosis from patients with the partially recanalized intracranial artery (masquerading as severe stenosis).
2. Age > 21 years
3. A modified Rankin score (MRS) of 3 or less.
1. Patients with atrial fibrillation/arrhythmias.
2. Within 2 weeks of cardiac catheterization or arterial puncture at the femoral puncture site.
3. Decompensated heart failure (NYHA class 3 or 4).
4. Left ventricular ejection fraction (EF) < 30%.
5. Moderate or severe aortic regurgitation.
6. Persistent and uncontrolled hypertension (BP persistently > 160/100 mmHg).
7. Bleeding diathesis.
8. Active thrombophlebitis/venous disease of lower limbs.
9. Severe lower extremity vaso-occlusive disease.
10. Presence of a documented aortic aneurysm/dissection requiring surgical repair.
11. Pregnancy.
Patients eligible for the study will be evaluated for their neurological deficits and functional status by using the National Institute of Health Scale Score (NIHSS) and MRS, respectively.
Complete TCD evaluation would be performed with a 2 MHz pulse wave Doppler probe using the commercially available TCD machine. Intracranial stenosis would be diagnosed according to the established as well as locally validated criteria (47, 48). ICAD would be considered severe (>70%) according to the velocity criteria and presence of “blunted” flow spectra in the arterial segment distal to the stenosis (49). Tandem lesions in a single arterial tree (intracranial ICA and MCA, proximal MCA and distal MCA, extracranial ICA and ipsilateral intracranial ICA, or extracranial ICA and ipsilateral MCA) if associated with blunted flow spectra in distal MCA would also qualify the criteria for >70% stenosis.
All patients would undergo continuous TCD monitoring for 40 min to detect spontaneous microemboli in the index intracranial artery distal to the stenosis. It would be performed using Spencer's TCD head frame.
Cerebral autoregulation enables a constant cerebral blood flow (CBF) over a wide range of systemic blood pressure by varying the diameter of the intracranial arterioles (50). This may fail in some patients with severe ICAD. TCD can measure vasomotor reactivity (VMR) by assessment of flow velocities in response to increasing carbon dioxide levels. This can be performed by a simple breath-holding test and calculating the so-called breath holding index (BHI) (51). BHI is calculated as the relative increase in the MFV during breath holding divided by the time of apnea in seconds. In normal persons, the BHI amounts to 1.2 ± 0.6. An impaired BHI can help to identify patients at higher risk of recurrent cerebral ischemia (29, 52). This test will also identify patients with intracranial steal phenomenon (reversed Robin Hood syndrome) (53). This method has been validated in our previous study for selecting patients eligible for revascularization among patients with severe and symptomatic ICAD (29, 54). Based upon our previous experience, we will be using 0.69 as the cutoff for TCD VMR. A value of <0.69 would define impaired CVR. We will calculate the steal magnitude according to the previously published criteria (29).
All patients would undergo the standard MRI of the brain to exclude a new ischemic stroke (diffusion and absolute diffusion coefficient), subacute stroke (fluid-attenuated inversion recovery-FLAIR), and intracranial hemorrhage (gradient echo). Time-of-flight MR angiography would be performed to document ICAD. In addition, all MRI scans would include arterial spin labeling (ASL) to assess regional CBF. ASL is a well-established MRI method for assessing cerebral perfusion in a quantitative manner, yielding values in ml blood/100 g tissue/min (55). ASL offers the benefit as it enables longitudinal assessment of perfusion in a vascular territory. It does not require the use of gadolinium-based contrast agents. This makes ASL an attractive candidate for monitoring disease progression or treatment response in various cerebrovascular disorders (56, 57). We will be using selective pseudocontinuous ASL with a circular labeling spot to evaluate regional CBF.
Acetazolamide is a potent cerebral vasodilator and is increasingly being used to assess the hemodynamic reserve in the brain (58). Intravenously administered acetazolamide induces vasodilatation of cerebral vasculature, increasing both CBF and cerebral blood volume (CBV). The maximum response is noted ~25 min after intravenous injection. Decreased reactivity to acetazolamide represents a reduced CVR. Patients with severe arterial stenosis who show little or no improvement in CBF in response to acetazolamide are known to carry a high clinical risk of recurrent cerebral ischemia or infarction after arterial occlusion, which is believed to be due to an insufficient supply of physiologic collateral vessels (15, 59, 60).
SPECT is used for the assessment of CVR by comparing the images before and after the infusion of acetazolamide. CVR is preserved and blood flow generally increases in response to acetazolamide in most cases where the perfusion pressure is normal and sufficient collateral vessels are established. However, when the collaterals are inadequate, the CVR decreases, and blood flow does not increase or may even decrease after administration of acetazolamide. Thus, brain SPECT with acetazolamide challenge can identify patients with reduced CVR. Hirano et al. (61) reported that patients with reduced CVR had significantly lower CBF values and higher CBV/CBF ratios compared to patients with normal acetazolamide reactivity. Reduced CVR on SPECT corresponds to enhanced oxygen extraction fraction (OEF) and represents stage II hemodynamic failure as determined with positive emission tomography (PET) studies (58).
Positron emission tomography (PET) is considered the gold standard for estimating CVR. Using oxygen-15 (O-15) labeled radiotracers, PET is able to give estimates of cerebral perfusion and hemodynamic parameters and allows the quantitative determination of the degree of hemodynamic compromise in patients with occlusive cerebral arterial disease. It was used to select patients for STA-MCA bypass surgery in the COSS trial (28). However, PET cannot be applied routinely to stroke patients because of its cost and limited availability. Blood flow reserve assessed by brain perfusion SPECT correlates well with the OEF measured by PET. Furthermore, SPECT is widely available. Thus, we chose to do acetazolamide-challenged SPECT for evaluating brain perfusion and blood flow reserve. Based upon our previous experience, we will be using a net CVR magnitude of 6% or more (difference between baseline and acetazolamide-challenged SPECT) as the definition of vasodilatory failure on SPECT (29).
Vascular cognitive impairment (VCI) is one of the major sequelae of stroke and transient ischemic attack (TIA) with negative functional impact and an elevated risk for institutionalization, dependency, and death. Post-stroke VCI or dementia was reported in 24% of patients in a recent meta-analysis (62). Similarly, highly prevalent VCI (43%) has also been reported in Singaporean patients with non-disabling ischemic stroke (63). Of this population, 40% of patients had cognitive impairment without dementia, and a further 4% were diagnosed with dementia within 6 months of the index event. Interestingly, we demonstrated the impact of STA-MCA bypass surgery on neurocognitive parameters in patients with severe steno-occlusive disease of the intracranial internal carotid artery or MCA (64). We hypothesize that by improving cerebral hemodynamics, EECP treatment would improve cerebral hemodynamics as well as cognition. We will administer cognitive measures among patients recruited in this study at the baseline and follow-up. Additionally, to evaluate whether the effects of EECP are sustained, we will assess cognitive functions 1 year after completing EECP sessions. The measures would include brief cognitive screening tests (Montreal Cognitive Assessment and Mini-Mental State Examination) as well as a 30-min protocol for neuropsychological evaluation [Symbol-Digit Modalities Test (SDMT), Trail Making Test, Controlled Oral Word Association Test, Animal Naming, and the Hopkins Verbal Learning Test-Revised]. The formal neuropsychological battery employed in this study has been validated locally for Singaporean elderly people (65).
Patients would be randomized (1:1) by picking the lot from a box, prepared in advance. The included subjects (n = 130) will be randomly divided into EECP plus best medical therapy (n = 65) and best medical therapy (n = 65) groups according to a random draw of lots.
Patients randomized to the EECP plus best medical therapy will undergo 35 sessions (1 h per day, 5 days a week for 7 weeks), while the best medical therapy arm will receive the treatment according to the current treatment guidelines (66).
All patients would be followed up for a period of 1 year. An overview of the follow-up plan is represented in Figure 1. Briefly, the evaluations performed at visit 2 and visit 3 would be as explained as follows.
Visit- 2 would be after 4 ± 2 months from the time of randomization. Cerebral ischemic events, if any, would be recorded. In addition to the clinical evaluation, all study participants would undergo TCD vasodilatory reserve, MRI (with ASL), acetazolamide-challenged SPECT, and a detailed neuropsychological assessment.
Visit-3 would be after 12 ± 2 months after the randomization. In addition to the clinical evaluation, all study participants would undergo TCD vasodilatory reserve and detailed neuropsychological assessment.
A 52-year-old Chinese man presented with recurrent and transient episodes of left-sided weakness for the past 3 months. Each episode lasted for nearly 15 min. His cardiovascular risk factors included hypertension and dyslipidemia. Neurological examination revealed mild left-sided residual weakness. MRI of the brain revealed old infarcts in both anterior and posterior watershed regions. MR angiography revealed severe stenosis of right MCA (Figure 4A). TCD showed blunted Doppler spectra in the distal right MCA and an exhausted vasodilatory reserve during the hypercapnoeic challenge. An impaired vasodilatory reserve SPECT (net perfusion deficit 11.46%) in the right MCA territory was noted on acetazolamide-challenged SPECT (Figure 4B). The MMSE score was 19 points. He underwent uneventful 35 sessions of EECP therapy. At 2 months after completion of EECP therapy, his TCD showed normal vasodilatory reserve on TCD as well as acetazolamide-challenged SPECT (Figure 4C). Interestingly, his MMSE score improved to 24 points. He has remained asymptomatic during the past 1 year.
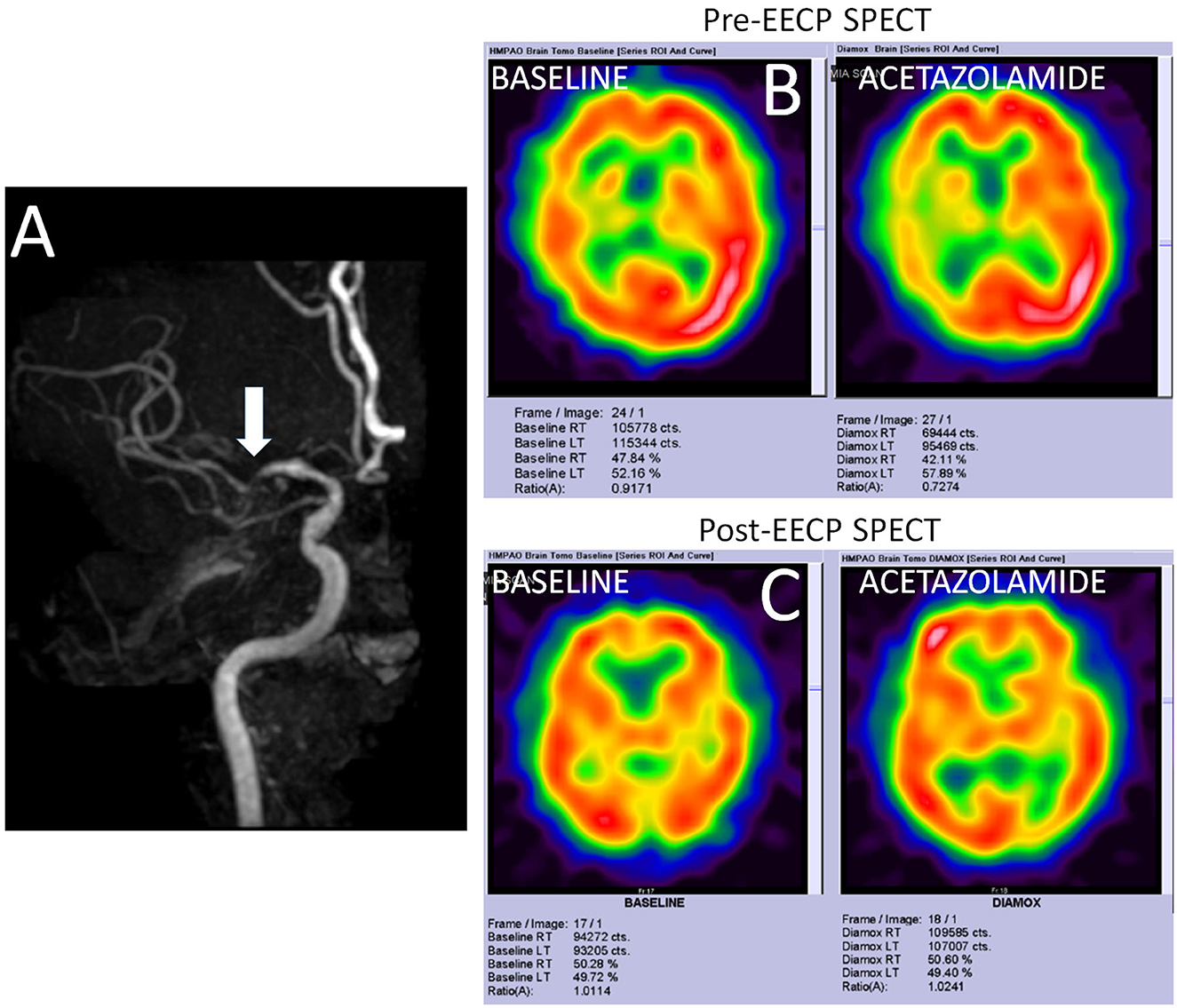
Figure 4. Imaging findings in a study participant. (A) Shows the time-of-flight MR angiography in a patient with severe stenosis of right middle cerebral artery (MCA). (B) Shows the SPECT findings at the baseline and after acetazolamide challenge. While only mildly reduced metabolic perfusion is noted in the right MCA territory at rest, acetazolamide challenge reveals a significantly impaired vasodilatory reserve (net perfusion deficit 11.46%). (C) Shows normalization of the metabolic perfusion at the baseline as well as after acetazolamide challenged SPECT.
Our previous study on the role of STA-MCA bypass in patients with inclusion criteria similar to the current proposal revealed that the surgical revascularization resulted in improving metabolic perfusion (on SPECT) by 6 points (SD 7.2) (29). This resulted in a nearly 32% absolute reduction in stroke recurrence during 27 months of follow-up. However, the bypass surgery is invasive, not applicable to all cases, and has surgery-related complications. We presume that short-term EECP would have a milder improvement in cerebral hemodynamics (at least an improvement of 4 points (SD 6.5). We expect that this improvement in cerebral hemodynamics by EECP would translate into at least a 10% absolute reduction in stroke recurrence (on top of the best medical therapy). For sample size calculation, a sample size of 60 in each group (120 in total) is sufficient to detect a mean difference of 4 units (with SD 6.5 units) in CVR improvement from the baseline between EECP and medical management group with 80% power and 5% significant level. We decided to recruit 65 patients per group (130 in total) to allow for treatment dropouts, withdrawal, and lack of follow-up.
Statistical analysis of all study endpoints was carried out on an intention-to-treat basis. In the event of the loss of follow-up, patients were still included in the analysis for the duration in which they are observed.
All the demographic and baseline characteristics were analyzed descriptively. Frequency tables are presented for categorical variables, while mean with SD or median with range, whichever is more appropriate, is presented for numerical variables.
A two-sample t-test was used to compare the CVR improvement from the baseline between the EECP group and the medical management group if the normality assumption got satisfied for the CVR improvement; otherwise, the Mann–Whitney U-test was used. Linear regression will be carried out for relevant covariates.
The recurrence of a cerebral ischemic event was compared by the chi-square test or Fisher's Exact test, whichever is appropriate, between the EECP group and the medical management group.
For evaluating the effect of EECP on cognitive performance, various cognitive domain scores (z-scores) were calculated.
Using the mean and SD of the baseline values of the whole sample, controlling the significant baseline characteristic factors, analysis of covariance was employed to compare the mean difference of the change scores from the baseline to follow-up of the cognitive domains and individual subtest scores between real EECP and sham-EECP patients. In addition, paired t-tests were conducted to compare the mean within-group difference in cognition before and after EECP treatment.
We present the baseline characteristic of the 83 patients recruited in the study (Table 1).
Currently, the best medical therapy remains the most evidence-based therapeutic option for secondary prevention of cerebral ischemic events for symptomatic patients with severe steno-occlusive disease of intracranial ICA or MCA. Although intracranial stenosis and STA-MCA bypass surgery are performed in carefully selected patients, both modalities are associated with unacceptable peri-procedural risks. In this clinical trial, we aimed to evaluate the impact of EECP treatment on improving CVR (and reducing stroke recurrence) in patients with severe steno-occlusive disease of ICA or MCA. This non-invasive novel therapeutic intervention may help a larger number of patients to prevent ischemic events and reduce the stroke burden.
The studies involving human participants were reviewed and approved by the Domain Specific Review Board (DSRB; Ref. 2018/00040). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
VS, AS, HT, PL, and AG contributed to the conception and design of the study. LW, JC, and VS organized the database. BT, VS, and LY performed the statistical analysis. VS and AS wrote the first draft of the manuscript. BC, JH, DT, MJ, YG, SS, CK, ZK, SP, and HT contributed toward patient recruitment and performed a critical review of this manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This study was funded by the National Medical Research Council, Singapore via Clinician Scientist Award (Senior Investigator) to VS (NMRC/CSA-SI/0019/2017).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. (2008) 39:2396–9. doi: 10.1161/STROKEAHA.107.505776
2. Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. (2006) 1:158–9. doi: 10.1111/j.1747-4949.2006.00045.x
3. Wityk RJ, Lehman D, Klag M, Coresh J, Ahn H, Litt B. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke. (1996) 27:1974–80. doi: 10.1161/01.STR.27.11.1974
4. Feldmann E, Daneault N, Kwan E, Ho KJ, Pessin MS, Langenberg P, et al. Chinese-white differences in the distribution of occlusive cerebrovascular disease. Neurology. (1990) 40:1541–5. doi: 10.1212/WNL.40.10.1540
5. Arenillas JF, Molina CA, Chacón P, Rovira A, Montaner J, Coscojuela P, et al. High lipoprotein (a), diabetes, and the extent of symptomatic intracranial atherosclerosis. Neurology. (2004) 63:27–32. doi: 10.1212/01.WNL.0000132637.30287.B4
6. Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA. (2000) 284:2901–6. doi: 10.1001/jama.284.22.2901
7. Coull AJ, Lovett JK, Rothwell PM. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ. (2004) 328:326. doi: 10.1136/bmj.37991.635266.44
8. Hill MD, Yiannakoulias N, Jeerakathil T, Tu JV, Svenson LW, Schop- flocher DP. The high risk of stroke immediately after transient ischemic attack: a population-based study. Neurology. (2004) 62:2015–20. doi: 10.1212/01.WNL.0000129482.70315.2F
9. Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. (2006) 113:555–63. doi: 10.1161/CIRCULATIONAHA.105.578229
10. Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Turan TN, Cloft HJ, et al. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol. (2011) 69:963–74. doi: 10.1002/ana.22354
11. Lau AY, Wong EH, Wong A, Mok VC, Leung TW, Wong KS. Significance of good collateral compensation in symptomatic intracranial atherosclerosis. Cerebrovasc Dis. (2012) 33:517–24. doi: 10.1159/000337332
12. Gibbs JM, Wise RJS, Leenders KL, Jones T. Evaluation of cerebral perfusion reserve in patients with carotid artery occlusion. Lancet. (1984) 1:310–4. doi: 10.1016/S0140-6736(84)90361-1
13. Kistler JP, Ropper AH, Heros RC. Therapy of ischemic cerebral vascular disease due to atherothrombosis. N Engl J Med. (1984) 311:27–34. doi: 10.1056/NEJM198407053110105
14. Powers WJ, Press GA, Grubb RL, Gado M, Raichle ME. The effect of hemodynamically significant carotid artery disease on the hemodynamic status of the cerebral circulation. Ann Intern Med. (1987) 106:27–35. doi: 10.7326/0003-4819-106-1-27
15. Schroeder T. Cerebrovascular reactivity to acetazolamide in carotid artery disease: enhancement of side-to-side CBF asymmetry indicates critically reduced perfusion pressure. Neurol Res. (1986) 8:231–6. doi: 10.1080/01616412.1986.11739760
16. Gaunt M, Naylor AR, Lennard N, Smith JL, Bell PR. Transcranial Doppler detected cerebral microembolism following carotid endarterectomy. High microembolic signal loads predict postoperative cerebral ischaemia. Brain. (1997) 120:621–9. doi: 10.1093/brain/120.4.621
17. Ringelstein EB, Droste DW, Babikian VL, Evans DH, Grosset DG, Kaps M, et al. Consensus on microembolus detection by TCD. Int Cons Group Microemb Detect Stroke. (1998) 29:725–9. doi: 10.1161/01.STR.29.3.725
18. Markus HS, MacKinnon A. Asymptomatic embolization detected by Doppler ultrasound predicts stroke risk in symptomatic carotid artery stenosis. Stroke. (2005) 36:971–5. doi: 10.1161/01.STR.0000162717.62684.40
19. Markus HS, Droste DW, Kaps M, Larrue V, Lees KR, Siebler M, et al. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using doppler embolic signal detection: The Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial. Circulation. (2005) 111:2233–40. doi: 10.1161/01.CIR.0000163561.90680.1C
20. CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet. (1997) 349:1641–9. doi: 10.1016/S0140-6736(97)04010-5
21. The International Stroke Trial (IST): a randomised trial of aspirin subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet. (1997) 349:1569–81. doi: 10.1016/S0140-6736(97)04011-7
22. Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. (2018) 379:215–25. doi: 10.1056/NEJMoa1800410
23. Chimowitz MI, Kokkinos J, Strong J, Brown MB, Levine SR, Silliman S, et al. The Warfarin-Aspirin symptomatic intracranial disease study. Neurology. (1995) 45:1488–93. doi: 10.1212/WNL.45.8.1488
24. Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. (2013) 369:11–9. doi: 10.1056/NEJMoa1215340
25. Wong KSL, Chen C, Fu J, Chang HM, Suwanwela NC, Huang YN, et al. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study). Lancet Neurol. (2010) 9:489–97. doi: 10.1016/S1474-4422(10)70060-0
26. Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. New Engl J Med. (2011) 365:993–1003. doi: 10.1056/NEJMoa1105335
27. The EC/IC Bypass Study Group. Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. N Engl J Med. (1985) 313:1191–200. doi: 10.1056/NEJM198511073131904
28. Grubb RL Jr, Powers WJ, Clarke WR, Videen TO, Adams HP Jr., Derdyne CP. Carotid occlusion study investigator: surgical results of Carotid occlusion surgery study. J Neurosurgery. (2013) 118:25–33. doi: 10.3171/2012.9.JNS12551
29. Low SW, Teo K, Lwin S, Yeo LL, Paliwal PR, Ahmad A, et al. Improvement in cerebral hemodynamic parameters and outcome after superficial temporal artery- middle cerebral artery bypass in patients with severe steno-occlusive disease of intracranial carotid and middle cerebral artery. J Neurosurg. (2015) 123:662–9. doi: 10.3171/2014.11.JNS141553
30. Teo KAC, Chou N, Sein L, Yeo TT, Sharma VK. Long-term outcome in extracranial-intracranial bypass surgery for severe steno-occlusive disease of intracranial internal carotid or middle cerebral artery. Clin Neurol Neurosurg. (2018) 169:149–53. doi: 10.1016/j.clineuro.2018.04.003
31. Bose A, Hartmann M, Henkes H, Liu HM, Teng MM, Szikora I, et al. A novel, self-expanding, nitinol stent in medically refractory intracranial atherosclerotic stenoses: the Wingspan study. Stroke. (2007) 38:1531–137. doi: 10.1161/STROKEAHA.106.477711
32. Zaidat OO, Klucznik R, Alexander MJ, Chaloupka J, Lutsep H, Barnwell S, et al. The NIH registry on use of the Wingspan stent for symptomatic 70–99% intracranial arterial stenosis. Neurology. (2008) 70:1518–24. doi: 10.1212/01.wnl.0000306308.08229.a3
33. Gupta R, Zaidat O, Majid A. US multicenter experience with the wingspan stent system for the treatment of intracranial atheromatous disease: periprocedural results. Stroke. (2007) 38:881–7. doi: 10.1161/01.STR.0000257963.65728.e8
34. Zaidat OO, Fitzsimmons B-F, Woodward BK, Wang Z, Killer-Oberpfalzer M, Wakhloo A, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA. (2015) 313:1240–8. doi: 10.1001/jama.2015.1693
35. Gao P, Wang D, Zhao Z, Cai Y, Li T, Shi H, et al. Multicenter prospective trial of stent placement in patients with symptomatic high-grade intracranial stenosis. Am J Neuroradiol. (2016) 37:1275–80. doi: 10.3174/ajnr.A4698
36. Alexander MJ, Zauner A, Chaloupka JC, Baxter B, Callison RC, Gupta R, et al. WEAVE trial sites and interventionalists. Final results in 152 on-label patients. Stroke. (2019) 50:889–94. doi: 10.1161/STROKEAHA.118.023996
37. Kim J, Ban SP, Kim YD, Kwon OK. Long-term outcomes of drug-eluting stent implantation in patients with symptomatic extra- and intracranial atherosclerotic stenoses. J Cerebrovasc Endovasc Neurosurg. (2020) 14:216–24. doi: 10.7461/jcen.2020.E2020.09.001
38. Bonetti PO, Holmes DR, Lerman A, Barsness GW. Enhanced external counterpulsation for ischemic heart disease: what's behind the curtain? J Am Coll Cardiol. (2003) 41:1918–25. doi: 10.1016/S0735-1097(03)00428-5
39. Lawson WE. Current use of enhanced external counterpulsation and patient selection. Clin Cardiol. (2002) 25:1116–21. doi: 10.1002/clc.4960251406
40. Linnemeier G. Enhanced external counterpulsation – a therapeutic option for patients with chronic cardiovascular problems. J Cardiovasc Manag. (2002) 13:20–5.
41. Werner D, Schneider M, Weise M, Nonnast-Daniel B, Daniel WG. Pneumatic external counterpulsation: a new noninvasive method to improve organ perfusion. Am J Cardiol. (1999) 84:950–952, A7-8. doi: 10.1016/S0002-9149(99)00477-4
42. Fitzgerald CP, Lawson WE, Hui JC, Kennard ED. IEPR Investigators. Enhanced external counterpulsation as initial revascularization treatment for angina refractory to medical therapy. Cardiology. (2003) 100:129–35. doi: 10.1159/000073930
43. Taguchi I, Ogawa K, Oida A, Abe S, Kaneko N, Sakio H. Comparison of hemodynamic effects of enhanced external counterpulsation and intra-aortic balloon pumping in patients with acute myocardial infarction. Am J Cardiol. (2000) 86:1139–41. doi: 10.1016/S0002-9149(00)01175-9
44. Yi YX, Zhu XP, Yang Y. Therapeutic hemodynamic effects of external counterpulsation on elderly patients with brain infarction during convalescence. Hunan Yi Ke Da XueXueBao. (2000) 25:45–7.
45. Lin W, Han J, Chen X, Xiong L, Wan Leung H, Leung TW, et al. Predictors of good functional outcome in counterpulsation-treated recent ischaemic stroke patients. BMJ Open. (2013) 3:e002932. doi: 10.1136/bmjopen-2013-002932
46. Alexandrov AW, Ribo M, Wong KS, Sugg RM, Garami Z, Jesurum JT, et al. Perfusion augmentation in acute stroke using mechanical counter-pulsation–phase iia, effect of external counterpulsation on middle cerebral artery mean flow velocity in five healthy subjects. Stroke. (2008) 39:2760–4. doi: 10.1161/STROKEAHA.107.512418
47. Sharma VK, Wong KS. Intracranial stenosis. In:Alexandrov V, , editors. Cerebrovascular Ultrasound in Stroke Prevention and Treatment. 2nd ed. New York, NY: Wiley-Blackwell Publishing (2011). p. 228–39.
48. Rathakrishnan R, Berne YI, Quek KK, Hong CS, Ong BK, Chan BP, et al. Validation of transcranial Doppler with CT angiography in cerebral ischaemia: a preliminary pilot study in Singapore. Ann Acad Med Singapore. (2008) 37:402–5. doi: 10.47102/annals-acadmedsg.V37N5p402
49. Sharma VK, Tsivgoulis G, Lao AY, Malkoff MD, Alexandrov AV. Noninvasive detection of diffuse intracranial disease. Stroke. (2007) 38:3175–81. doi: 10.1161/STROKEAHA.107.490755
50. Harper AM, Glass HI. Effect of alteration in the arterial carbon dioxide tension on the blood flow through the cerebral cortex at normal and low arterial blood pressures. JNNP. (1965) 28:449–52. doi: 10.1136/jnnp.28.5.449
51. Markus HS, Harrison MJ. Estimation of cerebrovascular reactivity using transcranial Doppler, including the use of breath-holding as the vasodilatory stimulus. Stroke. (1992) 23:668–73. doi: 10.1161/01.STR.23.5.668
52. Silverstrini M, Vernieri F, Pasqualetti P, Matteis M, Passarelli F, Troisi E, et al. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid stenosis. JAMA. (2000) 283:2122–227. doi: 10.1001/jama.283.16.2122
53. Alexandrov AV, Sharma VK, Lao AY, Tsivgoulis G, Malkoff MD, Alexandrov AW. Reversed Robin Hood syndrome in acute ischemic stroke patients. Stroke. (2007) 38:3045–8. doi: 10.1161/STROKEAHA.107.482810
54. Sharma VK, Tsivgoulis G, Ning C, Teoh HL, Bairaktaris C, Chong VF, et al. Role of multimodal evaluation of cerebral hemodynamics in selecting patients with symptomatic carotid or middle cerebral artery steno-occlusive disease for revascularization. J Vasc Interv Neurol. (2008) 1:96–101.
55. Jezzard P, Chappell MA, Okell TW. Arterial spin labeling for the measurement of cerebral perfusion and angiography. J Cereb Blood Flow Metab. (2018) 38:603–26. doi: 10.1177/0271678X17743240
56. Bokkers RP, van Osch MJ, van der Worp HB, de Borst GJ, Mali WP, Hendrikse J. Symptomatic carotid artery stenosis: impairment of cerebral autoregulation measured at the brain tissue level with arterial spin-labeling MR imaging. Radiology. (2010) 256:201–8. doi: 10.1148/radiol.10091262
57. Richter V, Helle M, van Osch MJP, Lindner T, Gersing AS, Tsantilas P, et al. MR Imaging of individual perfusion reorganization using superselective pseudocontinuous arterial spin-labeling in patients with complex extracranial steno-occlusive disease. AJNR Am J Neuroradiol. (2017) 38:703–11. doi: 10.3174/ajnr.A5090
58. Mithoefer JC, Mayer PW, Stocks JF. Effect of carbonic anhydrase inhibition on the cerebral circulation of anesthetized dog. Fed Proc. (1957) 16:88–9.
59. Ozgur HT, Kent Walsh T, Masaryk A, Seeger JF, Williams W, Krupinski E, et al. Correlation of cerebrovascular reserve asmeasured by acetazolamide-challenged SPECT with angiographic flow patterns and intra- or extracranial arterialstenosis. Am J Neuroradiol. (2001) 22:928–36.
60. Vorstrup S. Tomographic cerebral blood flow measurements in patients with ischemic cerebrovascular disease and evaluation of the vasodilatory capacity by the acetazolamide test. Acta Neurol Scand. (1988) 114:1–48.
61. Hirano T, Minematsu K, Hasegawa Y, Tanaka Y, Hayashida K, Yamaguchi T. Acetazolamide reactivity on 123I-IMP single photon emission computed tomography in patients with major cerebral artery occlusive disease: correlation with positron emission tomography parameters. J Cereb Blood Flow Metab. (1994) 14:763–70. doi: 10.1038/jcbfm.1994.97
62. Makin SD, Turpin S, Dennis MS, Wardlaw JM. Cognitive impairment after lacunar stroke: systematic review and meta-analysis of incidence, prevalence and comparison with other stroke subtypes. J Neurol Neurosurg Psychiatry. (2013) 84:893–900. doi: 10.1136/jnnp-2012-303645
63. Dong Y, Sharma VK, Chan BP-L, Venketasubramanian N, Teoh HL, Seet RCS, et al. The Montreal Cognitive Assessment (MoCA) is superior to the Mini-Mental State Examination (MMSE) for the detection of vascular cognitive impairment after acute stroke. J Neurol Sci. (2010) 299:15–8. doi: 10.1016/j.jns.2010.08.051
64. Dong Y, Teoh HL, Chan BPL, Ning C, Yeo TT, Sinha AK, et al. Changes in cerebral hemodynamic and cognitive parameters after external carotid-internal carotid bypass surgery in patients with severe steno-occlusive disease: a pilot study. J Neurol Sci. (2012) 322:112–6. doi: 10.1016/j.jns.2012.07.034
65. Yeo D, Gabriel C, Chen C, Lee S, Loenneker T, Wong M. Pilot validation of a customized neuropsychological battery in elderly Singaporeans. Neurol J South East Asia. (1997) 2:123.
66. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2014) 45:2160–236. doi: 10.1161/STR.0000000000000024
Keywords: ischemic stroke, intracranial stenosis, transcranial Doppler, enhanced external counter pulsation (EECP), cerebral vasodilatory reserve
Citation: Sharma VK, Gopinathan A, Tan BYQ, Loh PH, Hung J, Tang D, Chua C, Chan ACY, Ong JJY, Chin A, Jing M, Goh Y, Sunny S, Keat CH, Ka Z, Pandya S, Wong LYH, Chen JT, Yeo LLL, Chan BPL, Teoh HL and Sinha AK (2023) Enhanced external counter pulsation therapy in patients with symptomatic and severe intracranial steno-occlusive disease: a randomized clinical trial protocol. Front. Neurol. 14:1177500. doi: 10.3389/fneur.2023.1177500
Received: 01 March 2023; Accepted: 02 May 2023;
Published: 30 May 2023.
Edited by:
Mahesh P. Kate, University of Alberta Hospital, CanadaReviewed by:
Alberto Maud, Texas Tech University Health Sciences Center El Paso, United StatesCopyright © 2023 Sharma, Gopinathan, Tan, Loh, Hung, Tang, Chua, Chan, Ong, Chin, Jing, Goh, Sunny, Keat, Ka, Pandya, Wong, Chen, Yeo, Chan, Teoh and Sinha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vijay K. Sharma, bWRjdmtzQG51cy5lZHUuc2c=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.