
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol. , 29 June 2023
Sec. Pediatric Neurology
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1175370
 Lena-Luise Becker1,2,3
Lena-Luise Becker1,2,3 Alexander Gratopp4
Alexander Gratopp4 Christine Prager1,2
Christine Prager1,2 Christian E. Elger1,2,5
Christian E. Elger1,2,5 Angela M. Kaindl1,2,3*
Angela M. Kaindl1,2,3*Status epilepticus is one of the most common life-threatening neurological emergencies in childhood with the highest incidence in the first 5 years of life and high mortality and morbidity rates. Although it is known that a delayed treatment and a prolonged seizure can cause permanent brain damage, there is evidence that current treatments may be delayed and the medication doses administered are insufficient. Here, we summarize current knowledge on treatment of convulsive status epilepticus in childhood and propose a treatment algorithm. We performed a structured literature search via PubMed and
Status epilepticus (SE) refers to a prolonged clinical and/or electrographic seizure that does not cease within an expected time frame (1). SE is one of the most common life-threatening neurological emergencies in childhood, with about 17–23 episodes per 100,000 children annually and with the highest incidence in the first 5 years of life (1–9). SE can result in neurologic morbidity and has an overall mortality rate of up to 3% (1–9). Most common SE etiologies in children are prolonged febrile seizures, sudden discontinuation of anti-seizure medication (ASM) in children with treated epilepsy, acute central nervous system (CNS) insult, and chronic neurological conditions (10, 11).
The International League Against Epilepsy (ILAE) defined SE as “a condition resulting either from the failure of the mechanism responsible for seizure termination or from the initiation of mechanisms which lead to abnormally prolonged seizures (after time point t1). It is a condition that can have long-term consequences (after time point t2), including neuronal death, neuronal injury, and alteration of neuronal networks, depending on the type and duration of seizures” (12). The ILAE task force suggested treatment to be initiated at t1, and if t2 is reached, that treatment should be exacerbated to prevent long term damage (12). They further delineated t1, i.e., the time when a seizure is likely not to be self-limiting, to be 5 min in generalized tonic–clonic (GTC) SE, 10 min in focal SE with impaired awareness, and 10–15 min in absence SE, although there is no data on the latter (12, 13). Furthermore, they defined t2 to be 30 min for GTC SE and > 60 min for focal SE with impaired awareness (Table 1) (12). For other SE subtypes, including febrile seizure SE and focal SE with awareness, no duration has been proposed, leaving the older definition of >30 min (12). This earlier initiation and more aggressive therapeutic approach in the new ILAE definition is based on the knowledge that treatment becomes increasingly difficult the longer a SE lasts, in part due to receptor trafficking such as a reduction of GABAA receptor-mediated inhibition (1, 3, 14).
The correct choice of timing, dosage, and sequence of ASM is important, but the optimal pharmacologic treatment is largely unknown for the pediatric population and most guidelines focus on adult patients. Only a few randomized-controlled trials (RCT) exist for children that meet class I evidence (15, 16). Therefore, pharmaceutical management guidelines such as the ILAE pocket card1 often rely on expert opinions or experience with adult patients, giving rise to the need of controlled studies in pediatric patients and neonates.
Two studies identified that time until sufficient treatment is established is often prolonged and if treatment is started it is often not sufficiently high, partly due to out-of-hospital onset and intermittent SE (17–20). This calls for the establishment and distribution of guidelines, particularly for children, to aggressively address the treatment delay and to reduce putative permanent neuronal damage.
In the following, we summarize the current data on pharmacologic treatment of convulsive SE in childhood and propose a treatment algorithm for pediatric SE excluding neonates due to the absence of studies (Figure 1).
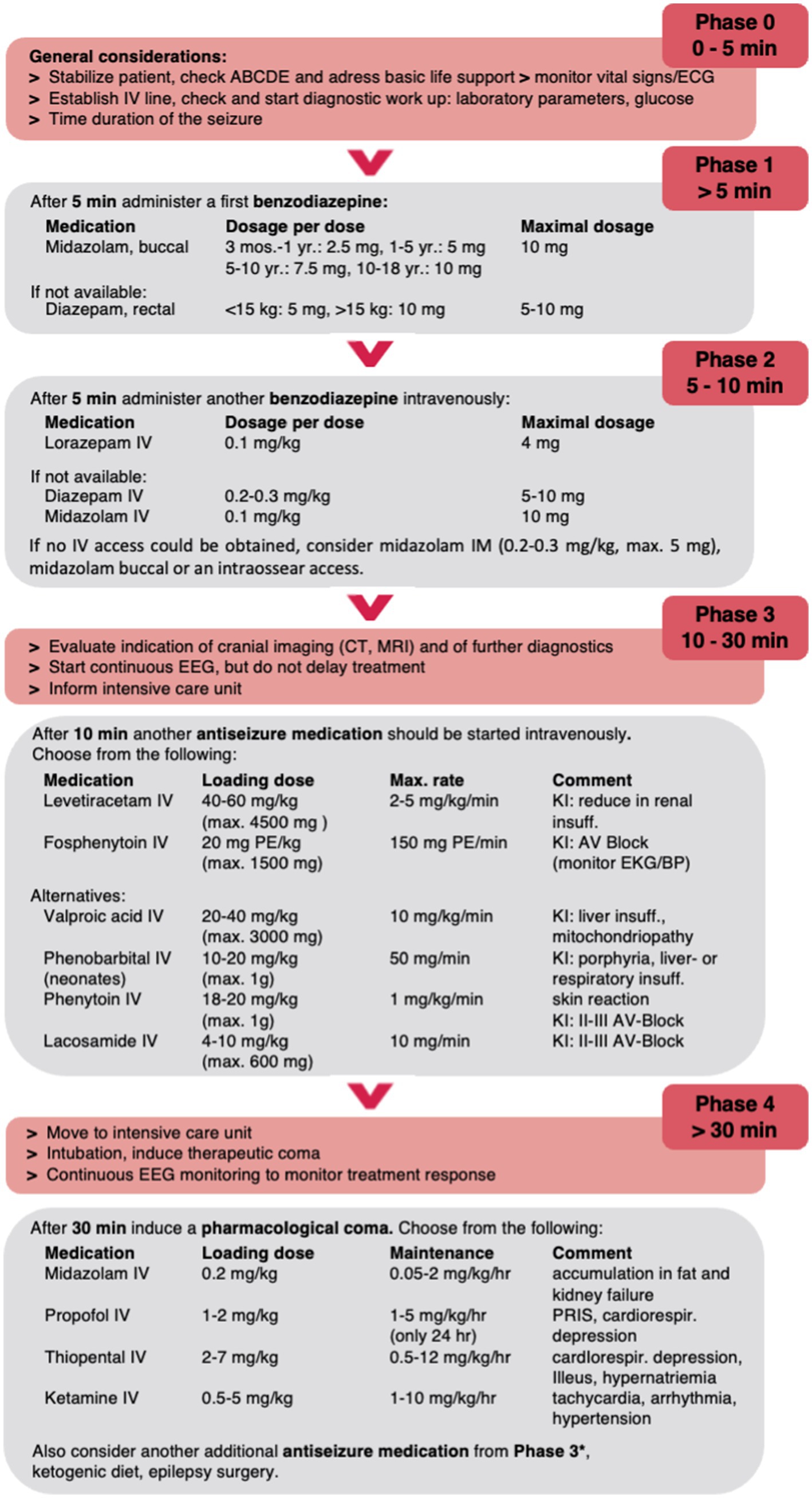
Figure 1. Proposed treatment algorithm for pediatric convulsive SE. Please note that this algorithm is designed to assist but not dismiss clinicians of their medical judgement of individual patient conditions and may need to be modified. The dose recommendations may vary between countries and guidelines; maximum doses are given in parentheses. *E.g., lacosamide IV or phenobarbital IV in high doses up to 140 mg/kg/d. PR, per rectum; B, buccal; IV, intravenous; IN, intranasal; IM, intramuscular; PE, phenytoin equivalent.
A literature search was performed using PubMed (1978 until August 30th, 2022) with the MeSH Terms “status epilepticus” and “child” selecting only clinical studies, clinical trials, RCT, and observational clinical studies. Additionally, ClinicalTrails.org was searched by completed studies on “status epilepticus in children” (last August 30th, 2022). All prospective and retrospective studies including >2 children (age < 18 years) comparing two and more treatment options for status epilepticus were included. Studies were excluded if only one patient was reported, two drug treatment regimens were not compared, or the publication language was not English. Information on the number of patients, the study type (retrospective, single/multi center, randomized controlled study, etc.), and treatment regime including dosage and duration of treatment and outcome (cessation of status epilepticus) were extracted. The flow diagram in Supplementary Figure S1 shows the review process in line with the PRISMA guidelines. Additional literature was identified by evaluating available reviews and flowcharts on this topic. The literature was divided into the commonly used treatment algorithm phases and summarized (Supplementary Table S1). No statistical analysis or meta-analyses was performed and the review was not registered; no protocol was prepared.
Basic life support (airway, breathing, circulation, disability) needs to be addressed in every patient with a seizure. This includes monitoring of vital parameters, heart rate, and oxygen saturation. It is essential to start timing a seizure from the very beginning to intensify treatment appropriately (12, 16).
The seizure semiology details should be recorded, and seizure type(s) determined. A detailed history about preexisting conditions/ comorbidities including an epilepsy diagnosis or recurrent status epilepticus with previous or existing ASM dosages should be conducted. An extended neurological exam should be performed as soon as possible to identify focal neurologic deficits and specific clinical signs that indicate a seizure etiology.
The initial work-up can be initiated while establishing intravenous (IV) access and should include the analysis of blood gas, electrolytes, and glucose levels, a blood count, a toxicology screen, and ASM levels, if appropriate. If IV access cannot be obtained, intraosseous (or central) access should be pursued (16).
An electroencephalogram (EEG) should be sought as early as possible. An EEG can help identify a focal seizure onset, clarify non-motor seizures or paroxysmal events (under muscle relaxing drugs), and monitor sedation (12).
Cranial imaging studies using magnetic resonance imaging (MRI) or computer tomography (CT) need to be considered in every child with SE, particularly in children without previously history of epilepsy, brain surgery, an implanted shunt systems, or in those at risk of complications that might result in SE (16, 21, 22). Imaging can reveal tumors, inflammation, ischemia, cerebrospinal fluid (CSF) retention with herniation, and other possible patterns important for treatment initiation and ultimately outcome.
The first-line treatment for children with convulsive SE in the pre-hospital setting is a non-IV applied benzodiazepine (Figure 1). Available benzodiazepines are diazepam (rectal, IV), midazolam (buccal, intramuscular (IM), intranasal (IN), IV), and lorazepam (IV, IN, buccal) without evidence for the best agent and application in children (23).
Rectal diazepam is usually given at a dose of 0.2–0.5 mg/kg (proposed doses <15 kg 5 mg, >15 kg 10 mg, maximum single dose 10 mg, maximum two doses). Diazepam is lipophilic and rapidly penetrates the blood–brain barrier, leading to onset within a few minutes, a maximum effect after 10–20 min, and a long half-life of 20–100 h (24). Buccal midazolam is given at 0.2–0.5 mg/kg (proposed doses: 3 months - < 1 year 2.5 mg, 1– <5 years 5 mg, 5– <10 years 7.5 mg, >10 years 10 mg, maximum single dose 10 mg, maximum two doses) with a maximum effect expected after approximately 10 min and a shorter half-life than diazepam of 3–4 h (24). In a recent Cochrane review on drug management of children with GTCS including convulsive SE, no evidence for efficacy between the latter two drugs was identified (24). Another meta-analysis on midazolam, lorazepam, and diazepam for the treatment of SE in children found a superior effect for non-IV midazolam (buccal, IN, or IM) in comparison to non-IV diazepam (23). In conclusion, buccal midazolam has been reported to be at least as effective in cessation of SE, equally safe, and also more socially acceptable than rectal diazepam (10, 15, 16, 25–33). Buccal midazolam is also the most cost-effective drug in the United States (34).
There is insufficient evidence to support the use of alternatives such as IN (or IM) midazolam and IN or buccal lorazepam to buccal midazolam or rectal diazepam in the pre-hospital setting (15). Previous studies, however, have suggested that IN midazolam (0.2 mg/kg/dose) is more effective than rectal diazepam (0.5 mg/kg/dose) (35–37). McTague et al. highlighted that in general buccal and IN application of ASMs resulted in similar seizure cessation rates as IV-applied ASMs (15, 38–44). The anticonvulsant effect of sublingual lorazepam has been reported to start with a delay of about 20 min after drug application, and this treatment is therefore inappropriate for acute SE treatment (45). Paraldehyde (IM, rectal) is not recommended anymore due to less availability in clinics and also superiority of currently accepted treatments (15, 46).
Due the efficacy of buccal midazolam, the data on cost-effectiveness of midazolam, and the ease of administration through the buccal routes, we propose to use buccal midazolam as the best option for prolonged seizures >5 min in a pre-hospital setting (Figure 1).
In most guidelines the first drug treatment phase after stabilization of the patient in the emergency hospital setting is the administration of a benzodiazepine. The effect of benzodiazepines can decrease drastically as SE progresses due to GABAA receptor internalization (14). Therefore, early treatment and sufficient dosage are essential. If benzodiazepines such as buccal midazolam or rectal diazepam have already been administered in the pre-hospital setting, care should be given not to overdose, i.e., apply more than two doses including the pre-hospital doses and thereby exceeding the maximum dosage (Figure 1) (47). An excess benzodiazepine exposure increases the risk of respiratory depression (47).
There is still limited data regarding the possible superior effect of IV versus non-IV benzodiazepine administration, especially midazolam (15, 16, 23). In general, IV lorazepam, midazolam, and diazepam result in similar rates of seizure cessation and respiratory depression, but lead in some studies to more rapid seizure cessation than buccal midazolam or rectal diazepam (48, 49). Another possible administration is IM midazolam. In the multi-center, double-blind, RCT RAMPART trial (Rapid Anticonvulsant Medication Prior to Arrival Trial), IM midazolam (13–40 kg: 5 mg) was as effective as IV lorazepam (13–40 kg: 2 mg) in SE treatment in the pre-hospital setting in a mixed adult and pediatric cohort of 893 patients including 120 children (73% versus 63%) (50–52). This result was not statistically significant when studying only the 120 children, and the prolonged time to achieve an IV line for the application of lorazepam needs to be considered. This result is supported by two further meta-analyses including the Cochrane review with similar or better seizure cessation rate of IM midazolam than IV diazepam/lorazepam (15, 23, 53–55).
If an IV access has been obtained, IV lorazepam (0.1 mg/kg/dose, maximum single dose 4 mg, maximum two doses), IV diazepam (0.2–0.3 mg/kg/dose, maximum single dose 10 mg, maximum two doses), or IV midazolam (0.1 mg/kg/dose, maximum single dose 5 mg) should be considered (24). The effect of these drugs should be apparent rapidly, within 0.5–5 min. There is insufficient evidence to favor either of these IV drugs with respect to seizure control (15, 48). In addition, there is no clear significant difference between the SE cessation effect of IVs midazolam versus IV diazepam, IV midazolam versus IV lorazepam, or IV lorazepam versus combined IV diazepam/phenytoin (15, 27, 56–58). However, a second dose to control seizures had to be applied less often if lorazepam was given compared to diazepam; no significant difference was seen when comparing the effect of midazolam versus lorazepam or diazepam versus midazolam (48). IV lorazepam has been associated with fewer adverse events, such as respiratory depression, excessive somnolence, and sedation, than IV diazepam (15, 59). Favoring lorazepam as the first-line treatment over diazepam has been criticized recently due to limited data on a superior effect (58, 60).
If the seizure does not terminate 5 min following initial benzodiazepine administration, then a second benzodiazepine dose should be administered. An application of more than two consecutive doses of benzodiazepines (including any dose given in the pre-hospital setting) increases the risk of respiratory depression and is associated with sedation (47, 61).
In conclusion, we suggest applying IV lorazepam if IV access is available given that it is at least as effective as or more effective than IV diazepam/midazolam and has been suggested to have fewer side effects. If no IV access is available, buccal and especially IM midazolam are also acceptable first-line anticonvulsants for convulsive SE treatment in the hospital setting (Figure 1).
When benzodiazepines fail to terminate a convulsive SE, non-benzodiazepine ASM such as phenytoin, fosphenytoin, and phenobarbital are applied (Figure 1). There are no RCTs comparing the voltage-gated sodium channel inhibitor fosphenytoin to the positive allosteric GABAA receptor modulator phenobarbital in children, but fosphenytoin is usually preferred in pediatric SE treatment guidelines (except in neonates where phenobarbital is preferred), given its fewer cardiorespiratory depression side effects when compared to phenobarbital given after benzodiazepines (16, 62). Phenobarbital can cause respiratory depression, hypotension, and bradycardia and should thus be given only in an intensive care setting. When phenobarbital and fosphenytoin are used sequentially, fosphenytoin has been suggested to precede phenobarbital, especially when benzodiazepines have already been used, on account of its better safety profile and the lower likelihood of cardiorespiratory depression (62, 63). There is insufficient data about the comparative efficacy of phenytoin and fosphenytoin; however, fosphenytoin is better tolerated compared with phenytoin with respect to cardiac arrhythmias, blood pressure imbalance, and local skin reactions (16). Phenytoin and fosphenytoin are hepatic enzyme inducers and can subsequently lower other drug levels such as those of carbamazepine, oxcarbazepine, valproate, levetiracetam, lacosamide, lamotrigine, and topiramate (64, 65). Despite fosphenytoin (and phenytoin) being contraindicated in the daily treatment of Dravet syndrome, there is currently no proven contraindication in the acute setting though mostly other ASMs are applied (66).
There is increasing evidence to support the use of alternatives to IV fosphenytoin and IV phenobarbital for treatment of pediatric convulsive SE such as the off-label administration of levetiracetam, briveracetam, valproic acid, and lacosamide. These drugs are frequently applied when registered treatment options fail. There is some evidence that there is no difference in whether these second-line ASMs are already in the patients’ home medication or not (67).
For levetiracetam, several RCTs have shown its benefit in treatment of pediatric convulsive SE despite not being approved for this application. In a recent trial, a superior effect of levetiracetam (40 mg/kg/dose) over phenytoin (20 mg/kg/dose) (93% versus 83%) in cessation of benzodiazepine-refractory SE was demonstrated in a large cohort of 600 children (mean age 3–4 years) (68). This superior effect could not be confirmed in the ConSEPT trial (Convulsive Status Epilepticus Pediatric Trial) (69). In the latter, 233 children (3 months - 13 years) who presented to one of 13 emergency departments in Australia and New Zealand over a 2.5-year time period were treated with IV or intraosseous infusions of phenytoin (20 mg/kg/dose) or levetiracetam (40 mg/kg/dose). Clinical cessation of seizure activity 5 min after the end of the drug infusion occurred in 60% of patients in the phenytoin group and 50% of patients in the levetiracetam group. It needs to be noted, however, that the infusion times varied between the two groups (20 min for phenytoin, 5 min for levetiracetam). Given that SE duration comes with more difficulties in controlling SE this can be a bias. In the EcLiPSE trial (Emergency Treatment with Levetiracetam of Phenytoin in Status Epilepticus), 404 children (6 months −18 years) who presented with SE to one of 30 emergency departments in the UK over a 3-year time period were treated similarly to the ConSEPT with phenytoin (20 mg/kg/dose) or levetiracetam (40 mg/kg/dose) through IV or intraosseous infusions with differing infusion times (70). Deviating from the ConSEPT trial, the primary outcome was not seizure cessation 5 min after end of the drug infusion, but rather time from randomization to cessation of convulsive SE. Here, convulsive SE occurred in 64% of patients in the phenytoin group within a median time of 45 min and 70% of patients in the levetiracetam group within a median time of 35 min. The authors conclude that though no significant superiority of levetiracetam over phenytoin was identified, levetiracetam could be an appropriate alternative to phenytoin as the first-choice, second-line ASM in the treatment of pediatric convulsive SE given their data and the safety profiles of the drugs (70). In the most recent large double-blind RCT ESETT (Established Status Epilepticus Treatment Trial), the efficacy of fosphenytoin (20 mg/kg, max. 1,200 mg), valproate (40 mg/kg, max. 3,000 mg), and levetiracetam (60 mg/kg, max. 4,500 mg) in 225 children >2 years and 237 adults with benzodiazepine-refractory convulsive SE were included. There was no difference in efficacy or safety in children between the three groups with seizure ceasing within 1 h with levetirazetam in 52%, with fosphenytoin in 49%, and with valproate in 52% of patients (71–73). Other studies also underlined the similar effect of levetirazetam in comparison to valproate and fosphenytoin, and it being well tolerated in general (74–76).
In conclusion, levetiracetam is overall well tolerated with only low rates of increased aggressiveness, irritability, nausea, and vomiting. In our own hospital setting, off-label levetiracetam is an equally established drug to fosphenytoin in children given the low side-effect profile.
In adults, brivaracetam has been reported to be a safe alternative to levetiracetam to treat SE (77, 78), but results of an ongoing RCT for children are not available yet, except for one study that includes pediatric cases with absence SE (79). Given the faster transition of the brain–blood barrier of brivaracetam versus levetiracetam, reaching maximum concentration in the brain within minutes following IV application, brivaracetam treatment is a promising approach, however, it is currently not approved for SE treatment (80).
Sodium valproate, which modulates sodium and calcium channels and the metabolism of GABA, has been shown to be effective in treatment of pediatric convulsive SE and in adults to be superior in SE cessation (e.g., than phenytoin) (10, 16, 64, 81, 82). In a RCT on 100 children with diazepam-refractory SE, valproic acid (20 mg/kg/dose) and phenytoin (20 mg/kg/dose) were similarly efficient in ceasing seizure activity with no significant difference in side-effects (83). In a further RCT, IV valproic acid (20 mg/kg/dose) was more effective in SE cessation (90% versus 77% seizure cessation) and better tolerated (adverse effects 24% versus 74%) than IV phenobarbital (20 mg/kg/dose) in 60 children younger than 2 years-of-age (84). Sodium valproate can be administered rapidly IV through an infusion pump with rare adverse effects such as hypotension, blood count drop, platelet dysfunction, hypersensitivity, (acute hemorrhagic) pancreatitis, and hyperammonemia (85). A major concern is valproic acid hepatotoxicity, particularly in children who are under 2 years of age and who could have a metabolic/mitochondrial disorder. Particularly, mutations in the DNA polymerase gamma gene (POLG) cannot be ruled out in the acute setting unless in-depth genetic testing has already been performed and results are readily available (86). Valproate is a strong hepatic enzyme inhibitor, and it may raise other drug levels such as those of carbamazepine, lamotrigine, phenobarbital, and rufinamide (85, 87). Especially when combining with drugs favoring hyperammonemia, e.g., phenobarbital, clinicians should be concerned about valproate-induced hyperammonemic encephalopathy, a rare complication characterized by decreased consciousness, neurological deficits, and vomiting (88).
Only a few studies focused on the effect of lacosamide in the treatment of pediatric SE, most likely due to the fact that lacosamide is licensed for children older than 4 years and focal-onset seizures only (not for SE treatment) and the subsequent off-label use in younger children (89). A review on six retrospective studies including 36 pediatric patients with various SE subtypes showed an overall success rate of 45–78% (age range 1 month to 17 years) with doses between 4 to 10 mg/kg/dose (89). Of the 36 lacosamide-treated children, one had bradycardia and two had delayed oculogyric crisis and chorea. Otherwise, no serious side effects were reported. Since lacosamide can cause PR/QT prolongation, it should be administered with electrocardiogram (ECG) monitoring (89, 90). Lang et al. recently reported a 70% cessation rate of seizure series or SE in a heterogenous mixed pediatric and adult cohort of 119 patients who received additional lacosamide (median dose 300 mg) to the hospital’s ASM protocol with a good tolerability (91). In a study with 196 adult and pediatric patients, lacosamide and levetirazetam had a higher SE cessation rate than valproate and phenytoin as a first or second ASM in benzodiazepine-refractory SE (92). Overall, due to missing RCT studies comparing lacosamide in children to approved ASMs, no recommendation can be made at this point. In our hospital, lacosamide is applied as an ASM in phase 4.
In conclusion, we suggest applying IV levetiracetam or IV fosphenytoin; alternatively, valpoic acid IV or phenobarbital can be used (Figure 1).
In approximately 10–40% of children, the first- and second-line drugs fail to stop convulsive SE, and the SE is regarded as refractory (RSE). In 7% of children, the SE develops into super-refractory SE (SRSE), where the SE is ongoing after 24 h into induction of a pharmacological coma or proceeding after its withdrawal (11). Both events are highly associated with long-term neurological morbidity and mortality (RSE: 16–43.5%). Therefore, aggressive treatment of SE at this time point (t2) is essential to avoid permanent neuronal damage. There is no clear evidence to guide pharmaceutical treatment in this phase. Therefore, treatment is based on case series and expert opinions without controlled trials being available (11). Additionally, specific treatment for the underlining etiology should be started (see chapter: pharmacological considerations in specific pediatric SE subtypes).
Most patients are transferred at this point to the intensive care unit to receive further second-line drugs and/or general anesthetic drugs such as thiopental, pentobarbital, midazolam, or propofol (third-line agents) with continuous EEG (cEEG) monitoring (16, 93, 94) (Figure 1). There are limited data supporting which third-line ASM to choose and the recommended speed of titration (11). cEEG is essential to guide the pharmacological coma as well as exclusion of non-convulsive SE (NCSE) or electrographic seizures. It is unclear whether the treatment goal should merely be the termination of seizures or the induction of a burst-suppression pattern. In addition, it remains unclear how long a patient should be maintained in a pharmacologic coma. Expert opinion usually opts for 24–48 h of electrographic seizure control prior to a gradual withdrawal of continuous infusions (11, 95–97). If seizures continue despite treatment or recur during the weaning period of continuous infusion(s), a further trial for an additional 24–48 h is usually recommended (11).
In a midazolam-induced coma, dosing usually involves an initial loading dose of 0.2 mg/kg in a 2 mg/min infusion followed by an infusion at 0.05–2 mg/kg/h titrated as needed to achieve clinical and electrographic seizure suppression and/or EEG burst-suppression (11, 98). Pentobarbital is usually initially given at a loading dose of 5 mg/kg in a 50 mg/min infusion, and a further pulse of 5 mg/kg at similar 50 mg/min infusion speed can be subsequently administered, if needed (10, 11) This is followed by an infusion at 0.5–5 mg/kg/h that is titrated as needed to achieve clinical and electrographic seizure suppression and/or EEG burst-suppression (10, 11, 99, 100). If seizures persist with midazolam or pentobarbital, then escalating dosing through additional boluses is needed to rapidly increase levels or terminate seizures (98). Propofol (up to 5 mg/kg/h) is an additional safe and effective option, but recommended for use only for 24 h since it may cause propofol infusion syndrome (PRIS), which is associated with a high mortality rate (101, 102). Another possibility is the usage of inhalational anesthetics such as isoflurane to terminate an SRSE. While two pediatric clinical series reported seizure cessation in 94.4%, there is, unfortunately, a high relapse rate after discontinuing the treatment (103).
Though no RCT trials exist, very high-dose phenobarbital has been reported by several authors as an approach to control RSE. In our hospital, we increase the dose of phenobarbital to escalate plasma levels of 80 ug/ml. This is in line with previous reports where dosages ranged from 40 to 140 mg/kg/d with plasma levels of 30–340 ug/ml (104–107).
A promising future therapeutic approach in RSE is treatment with the noncompetitive NMDA receptor antagonist ketamine or (S)-ketamine (loading: 0.5–5 mg/kg, maintenance 1–10 mg/kg/h) (108). Only a small number of case series exist for such treatment in children (108). In adults, data on (S)-ketamine are available from open observational studies with response rates of up to 64% (108). A prospective study (KETASER01) comparing the effectiveness of ketamine to diazepam, thiopental, and propofol in children (1–18 years) in RSE is currently being conducted (109, 110).
Additional ASM application (e.g., phenytoin, valproate, levetiracetam, or topiramate) can be reasonable if these drugs have not been tried, if seizures become less frequent, or if they appear to be fragmenting (111). Further options including cannabidiol, bexanolone, ketogenic diet, and hypothermia cannot be classified as standard therapy but may become options when other measures fail to cease SE (93–95, 112–117). In any case of RSE due to structural epilepsy, epilepsy surgery needs to be considered urgently.
Specific pediatric SE subtypes require specific considerations with respect to pharmacologic treatment. In tonic SE, benzodiazepines should be avoided and therapy may be initiated with fosphenytoin or phenobarbital (or levetiracetam) treatment. Absence SE can often be stopped through treatment with clonazepam, phenobarbital, or valproate. In febrile prolonged seizures, the identification of the primary fever source is essential. A lumbar puncture can help diagnose meningoencephalitis and help in decision making with respect to early antiviral or antibacterial treatment (11).
Early genetic consultation and, if available, rapid whole exome/genome sequencing should be initiated if a genetic etiology is suspected to offer individualized treatment options (118).
In patients with unknown or autoimmune etiologies and non-infectious encephalitis, extensive diagnostic work-up should be performed. Treatment with IV immunoglobulins (1–2 g/kg) over 3–5 days should be considered after securing sufficient blood and CSF sample specimens for later analysis. As soon as infectious encephalitis has been ruled out, IV corticosteroids (methylprednisolone: 20–30 mg/kg/d for 3–5 days) and plasmapheresis or immunoabsorption need to be considered (11).
Seizures in febrile infection-related epilepsy syndrome (FIRES) are highly difficult to treat and usually remain refractory to standard therapy (116, 119). Treatment approaches include ASM, ketogenic diet, and pharmacologic coma induction. Given the putative causal role of inflammation in FIRES, immunomodulatory therapies have been initiated such as IV corticosteroids, IV immunoglobulins, plasma exchange or immunoabsorption, and/or further drug treatment with, e.g., tacrolimus, rituximab, cyclophosphamide, and anakinra (116, 119).
In SE the correct choice of timing, dosage, and sequence of ASM is important, but the optimal pharmacologic treatment is largely unknown for the pediatric and especially neonatal population as most guidelines focus on adult patients. Although it is known that prolonged seizures are more difficult to treat the longer they continue, as this can lead to permanent brain damage and death, sufficient treatment is often delayed and if treatment is started it if often not sufficiently high.
Limitations of this study are that the review does not include meta-analyses and statistics and includes the recommendation from pre-existing systematic reviews. The study is also limited by the number of identified studies.
This study and our proposed algorithm therefore highlight the immense importance of the establishment and distribution of guidelines, particularly for children, to aggressively address the treatment delay and to reduce putative permanent neuronal damage.
AK and LLB drafted the initial manuscript and performed the systematic literature search, which was reviewed and revised by AG, CP, and CE. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Our research is supported by the German Research Foundation (DFG; SFB1315, FOR3004) and the Günter Endres Fond through the Einstein Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1175370/full#supplementary-material
1. Sánchez Fernández, I, Vendrame, M, Kapur, K, Klehm, J, Uysal, S, Gedik, M, et al. Comparison of pediatric patients with status epilepticus lasting 5-29 min versus >/=30 min. Epilepsy Behav. (2014) 37:1–6. doi: 10.1016/j.yebeh.2014.05.018
2. Chin, RF, Neville, BG, Peckham, C, Bedford, H, Wade, A, Scott, RC, et al. Incidence, cause, and short-term outcome of convulsive status epilepticus in childhood: prospective population-based study. Lancet. (2006) 368:222–9. doi: 10.1016/S0140-6736(06)69043-0
3. Dham, BS, Hunter, K, and Rincon, F. The epidemiology of status epilepticus in the United States. Neurocrit Care. (2014) 20:476–83. doi: 10.1007/s12028-013-9935-x
4. Maytal, J, Shinnar, S, Moshe, SL, and Alvarez, LA. Low morbidity and mortality of status epilepticus in children. Pediatrics. (1989) 83:323–31. doi: 10.1542/peds.83.3.323
5. Raspall-Chaure, M, Chin, RF, Neville, BG, and Scott, RC. Outcome of paediatric convulsive status epilepticus: a systematic review. Lancet Neurol. (2006) 5:769–79. doi: 10.1016/S1474-4422(06)70546-4
6. Schubert-Bast, S, Zöllner, JP, Ansorge, S, Hapfelmeier, J, Bonthapally, V, Eldar-Lissai, A, et al. Burden and epidemiology of status epilepticus in infants, children, and adolescents: a population-based study on German health insurance data. Epilepsia. (2019) 60:911–20. doi: 10.1111/epi.14729
7. Wu, YW, Shek, DW, Garcia, PA, Zhao, S, and Johnston, SC. Incidence and mortality of generalized convulsive status epilepticus in California. Neurology. (2002) 58:1070–6. doi: 10.1212/WNL.58.7.1070
8. Singh, RK, Stephens, S, Berl, MM, Chang, T, Brown, K, Vezina, LG, et al. Prospective study of new-onset seizures presenting as status epilepticus in childhood. Neurology. (2010) 74:636–42. doi: 10.1212/WNL.0b013e3181d0cca2
9. DeLorenzo, RJ, Hauser, WA, Towne, AR, Boggs, JG, Pellock, JM, Penberthy, L, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. (1996) 46:1029–35. doi: 10.1212/WNL.46.4.1029
10. Capovilla, G, Beccaria, F, Beghi, E, Minicucci, F, Sartori, S, and Vecchi, M. Treatment of convulsive status epilepticus in childhood: recommendations of the Italian league against epilepsy. Epilepsia. (2013) 54:23–34. doi: 10.1111/epi.12307
11. Vasquez, A, Farias-Moeller, R, and Tatum, W. Pediatric refractory and super-refractory status epilepticus. Seizure. (2019) 68:62–71. doi: 10.1016/j.seizure.2018.05.012
12. Trinka, E, Cock, H, Hesdorffer, D, Rossetti, AO, Scheffer, IE, Shinnar, S, et al. A definition and classification of status epilepticus--report of the ILAE task force on classification of status epilepticus. Epilepsia. (2015) 56:1515–23. doi: 10.1111/epi.13121
13. Shinnar, S, Berg, AT, Moshe, SL, and Shinnar, R. How long do new-onset seizures in children last? Ann Neurol. (2001) 49:659–64. doi: 10.1002/ana.1018
14. Sanchez Fernandez, I, Goodkin, HP, and Scott, RC. Pathophysiology of convulsive status epilepticus. Seizure. (2019) 68:16–21. doi: 10.1016/j.seizure.2018.08.002
15. McTague, A, Martland, T, and Appleton, R, Cochrane Epilepsy Group. Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children. Cochrane Database Syst Rev. (2018) 2018:CD001905. doi: 10.1002/14651858.CD001905.pub3
16. Glauser, T, Shinnar, S, Gloss, D, Alldredge, B, Arya, R, Bainbridge, J, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the guideline Committee of the American Epilepsy Society. Epilepsy Curr. (2016) 16:48–61. doi: 10.5698/1535-7597-16.1.48
17. Sánchez Fernández, I, Gaínza-Lein, M, Abend, NS, Anderson, AE, Arya, R, Brenton, JN, et al. Factors associated with treatment delays in pediatric refractory convulsive status epilepticus. Neurology. (2018) 90:e1692–701. doi: 10.1212/WNL.0000000000005488
18. Sánchez Fernández, I, Jackson, MC, Abend, NS, Arya, R, Brenton, JN, Carpenter, JL, et al. Refractory status epilepticus in children with and without prior epilepsy or status epilepticus. Neurology. (2017) 88:386–94. doi: 10.1212/WNL.0000000000003550
19. Sheehan, T, Amengual-Gual, M, Vasquez, A, Abend, NS, Anderson, A, Appavu, B, et al. Benzodiazepine administration patterns before escalation to second-line medications in pediatric refractory convulsive status epilepticus. Epilepsia. (2021) 62:2766–77. doi: 10.1111/epi.17043
20. Sathe, AG, Underwood, E, Coles, LD, Elm, JJ, Silbergleit, R, Chamberlain, JM, et al. Patterns of benzodiazepine underdosing in the established status epilepticus treatment trial. Epilepsia. (2021) 62:795–806. doi: 10.1111/epi.16825
21. Mendes, A, and Sampaio, L. Brain magnetic resonance in status epilepticus: a focused review. Seizure. (2016) 38:63–7. doi: 10.1016/j.seizure.2016.04.007
22. Meletti, S, Monti, G, Mirandola, L, Vaudano, AE, and Giovannini, G. Neuroimaging of status epilepticus. Epilepsia. (2018) 59:113–9. doi: 10.1111/epi.14499
23. Zhao, ZY, Wang, HY, Wen, B, Yang, ZB, Feng, K, and Fan, JC. A comparison of midazolam, lorazepam, and diazepam for the treatment of status epilepticus in children: a network meta-analysis. J Child Neurol. (2016) 31:1093–107. doi: 10.1177/0883073816638757
24. Singh, A, Stredny, CM, and Loddenkemper, T. Pharmacotherapy for pediatric convulsive status epilepticus. CNS Drugs. (2020) 34:47–63. doi: 10.1007/s40263-019-00690-8
25. Scott, LJ, Lyseng-Williamson, KA, and Garnock-Jones, KP. Oromucosal midazolam: a guide to its use in paediatric patients with prolonged acute convulsive seizures. CNS Drugs. (2012) 26:893–7. doi: 10.2165/11209350-000000000-00000
26. Mpimbaza, A, Ndeezi, G, Staedke, S, Rosenthal, PJ, and Byarugaba, J. Comparison of buccal midazolam with rectal diazepam in the treatment of prolonged seizures in Ugandan children: a randomized clinical trial. Pediatrics. (2008) 121:e58–64. doi: 10.1542/peds.2007-0930
27. Brigo, F, Nardone, R, Tezzon, F, and Trinka, E. Nonintravenous midazolam versus intravenous or rectal diazepam for the treatment of early status epilepticus: a systematic review with meta-analysis. Epilepsy Behav. (2015) 49:325–36. doi: 10.1016/j.yebeh.2015.02.030
28. McMullan, J, Sasson, C, Pancioli, A, and Silbergleit, R. Midazolam versus diazepam for the treatment of status epilepticus in children and young adults: a meta-analysis. Acad Emerg Med. (2010) 17:575–82. doi: 10.1111/j.1553-2712.2010.00751.x
29. McIntyre, J, Robertson, S, Norris, E, Appleton, R, Whitehouse, WP, Phillips, B, et al. Safety and efficacy of buccal midazolam versus rectal diazepam for emergency treatment of seizures in children: a randomised controlled trial. Lancet. (2005) 366:205–10. doi: 10.1016/S0140-6736(05)66909-7
30. Yoshinaga, H, Benitez, A, Takeda, S, Fournier, M, and Kugler, AR. A phase 3 open-label study of the efficacy, safety and pharmacokinetics of buccally administered midazolam hydrochloride for the treatment of status epilepticus in pediatric Japanese subjects. Epilepsy Res. (2021) 174:106651. doi: 10.1016/j.eplepsyres.2021.106651
31. Momen, AA, Azizi Malamiri, R, Nikkhah, A, Jafari, M, Fayezi, A, Riahi, K, et al. Efficacy and safety of intramuscular midazolam versus rectal diazepam in controlling status epilepticus in children. Eur J Paediatr Neurol. (2015) 19:149–54. doi: 10.1016/j.ejpn.2014.11.007
32. Scott, RC, Besag, FM, and Neville, BG. Buccal midazolam and rectal diazepam for treatment of prolonged seizures in childhood and adolescence: a randomised trial. Lancet. (1999) 353:623–6. doi: 10.1016/S0140-6736(98)06425-3
33. Ashrafi, MR, Khosroshahi, N, Karimi, P, Malamiri, RA, Bavarian, B, Zarch, AV, et al. Efficacy and usability of buccal midazolam in controlling acute prolonged convulsive seizures in children. Eur J Paediatr Neurol. (2010) 14:434–8. doi: 10.1016/j.ejpn.2010.05.009
34. Sanchez Fernandez, I, Gainza-Lein, M, and Loddenkemper, T. Nonintravenous rescue medications for pediatric status epilepticus: a cost-effectiveness analysis. Epilepsia. (2017) 58:1349–59. doi: 10.1111/epi.13812
35. Fişgin, T, Gurer, Y, Tezic, T, Senbil, N, Zorlu, P, Okuyaz, C, et al. Effects of intranasal midazolam and rectal diazepam on acute convulsions in children: prospective randomized study. J Child Neurol. (2002) 17:123–6. doi: 10.1177/088307380201700206
36. Holsti, M, Sill, BL, Firth, SD, Filloux, FM, Joyce, SM, and Furnival, RA. Prehospital intranasal midazolam for the treatment of pediatric seizures. Pediatr Emerg Care. (2007) 23:148–53. doi: 10.1097/PEC.0b013e3180328c92
37. Baysun, S, Aydin, ÖF, Atmaca, E, and Gürer, YKY. A comparison of buccal midazolam and rectal diazepam for the acute treatment of seizures. Clin Pediatr (Phila). (2005) 44:771–6. doi: 10.1177/000992280504400904
38. Rudra, N, Ghosh, T, and Roy, UK. A comparative study on intranasal versus intravenous lorazepam in the Management of Acute Seizure in children. Folia Med. (2021) 63:958–64. doi: 10.3897/folmed.63.e60938
39. von Blomberg, A, Kay, L, Knake, S, Fuest, S, Zöllner, JP, Reif, PS, et al. Efficacy, tolerability, and safety of concentrated intranasal midazolam spray as emergency medication in epilepsy patients during video-EEG monitoring. CNS Drugs. (2020) 34:545–53. doi: 10.1007/s40263-020-00720-w
40. Mahmoudian, T, and Mohammadi Zadeh, M. Comparison of intranasal midazolam with intravenous diazepam for treating acute seizures in children. Epilepsy Behav. (2004) 5:253–5. doi: 10.1016/j.yebeh.2004.01.003
41. Arya, R, Gulati, S, Kabra, M, Sahu, JK, and Kalra, V. Intranasal versus intravenous lorazepam for control of acute seizures in children: a randomized open-label study. Epilepsia. (2011) 52:788–93. doi: 10.1111/j.1528-1167.2010.02949.x
42. Javadzadeh, M, Sheibani, K, Hashemieh, M, and Saneifard, H. Intranasal midazolam compared with intravenous diazepam in patients suffering from acute seizure: a randomized clinical trial. Iran J Pediatr. (2012) 22:1–8.
43. Lahat, E, Goldman, M, Barr, J, Bistritzer, T, and Berkovitch, M. Comparison of intranasal midazolam with intravenous diazepam for treating febrile seizures in children: prospective randomised study. BMJ. (2000) 321:83–6. doi: 10.1136/bmj.321.7253.83
44. Talukdar, B, and Chakrabarty, B. Efficacy of buccal midazolam compared to intravenous diazepam in controlling convulsions in children: a randomized controlled trial. Brain and Development. (2009) 31:744–9. doi: 10.1016/j.braindev.2008.11.006
45. Kadel, J, Bauer, S, Hermsen, AM, Immisch, I, Kay, L, Klein, KM, et al. Use of emergency medication in adult patients with epilepsy: a multicentre cohort study from Germany. CNS Drugs. (2018) 32:771–81. doi: 10.1007/s40263-018-0544-2
46. Ahmad, S, Ellis, JC, Kamwendo, H, and Molyneux, E. Efficacy and safety of intranasal lorazepam versus intramuscular paraldehyde for protracted convulsions in children: an open randomised trial. Lancet. (2006) 367:1591–7. doi: 10.1016/S0140-6736(06)68696-0
47. Stewart, WA, Harrison, R, and Dooley, JM. Respiratory depression in the acute management of seizures. Arch Dis Child. (2002) 87:225–6. doi: 10.1136/adc.87.3.225
48. Gathwala, G, Goel, M, Singh, J, and Mittal, K. Intravenous diazepam, midazolam and lorazepam in acute seizure control. Indian J Pediatr. (2012) 79:327–32. doi: 10.1007/s12098-011-0505-y
49. Yoshikawa, H, Yamazaki, S, Abe, T, and Oda, Y. Midazolam as a first-line agent for status epilepticus in children. Brain and Development. (2000) 22:239–42. doi: 10.1016/S0387-7604(00)00116-9
50. Meurer, WJ, Silbergleit, R, Nicholas, KS, Burke, JF, and Durkalski, V. Accounting for repeat enrollments during an emergency clinical trial: the rapid anticonvulsant medications prior to arrival trial (RAMPART). Acad Emerg Med. (2015) 22:373–7. doi: 10.1111/acem.12596
51. Silbergleit, R, Lowenstein, D, Durkalski, V, and Conwit, R, on behalf of the NETT Investigators. Lessons from the RAMPART study--and which is the best route of administration of benzodiazepines in status epilepticus. Epilepsia. (2013) 54:74–7. doi: 10.1111/epi.12284
52. Silbergleit, R, Durkalski, V, Lowenstein, D, Conwit, R, Pancioli, A, Palesch, Y, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. (2012) 366:591–600. doi: 10.1056/NEJMoa1107494
53. Shah, I, and Deshmukh, CT. Intramuscular midazolam vs intravenous diazepam for acute seizures. Indian J Pediatr. (2005) 72:667–70. doi: 10.1007/BF02724074
54. Chamberlain, JM, Altieri, MA, Futterman, C, Young, GM, Ochsenschlager, DW, and Waisman, Y. A prospective, randomized study comparing intramuscular midazolam with intravenous diazepam for the treatment of seizures in children. Pediatr Emerg Care. (1997) 13:92–4. doi: 10.1097/00006565-199704000-00002
55. Welch, RD, Nicholas, K, Durkalski-Mauldin, VL, Lowenstein, DH, Conwit, R, Mahajan, PV, et al. Intramuscular midazolam versus intravenous lorazepam for the prehospital treatment of status epilepticus in the pediatric population. Epilepsia. (2015) 56:254–62. doi: 10.1111/epi.12905
56. Giang, DW, and McBride, MC. Lorazepam versus diazepam for the treatment of status epilepticus. Pediatr Neurol. (1988) 4:358–61. doi: 10.1016/0887-8994(88)90083-5
57. Sreenath, TG, Gupta, P, Sharma, KK, and Krishnamurthy, S. Lorazepam versus diazepam-phenytoin combination in the treatment of convulsive status epilepticus in children: a randomized controlled trial. Eur J Paediatr Neurol. (2010) 14:162–8. doi: 10.1016/j.ejpn.2009.02.004
58. Chamberlain, JM, Okada, P, Holsti, M, Mahajan, P, Brown, KM, Vance, C, et al. Lorazepam vs diazepam for pediatric status epilepticus: a randomized clinical trial. JAMA. (2014) 311:1652–60. doi: 10.1001/jama.2014.2625
59. Appleton, R, Sweeney, A, Choonara, I, Robson, J, and Molyneux, E. Lorazepam versus diazepam in the acute treatment of epileptic seizures and status epilepticus. Dev Med Child Neurol. (1995) 37:682–8. doi: 10.1111/j.1469-8749.1995.tb15014.x
60. Brigo, F, Bragazzi, NL, Bacigaluppi, S, Nardone, R, and Trinka, E. Is intravenous lorazepam really more effective and safe than intravenous diazepam as first-line treatment for convulsive status epilepticus? A systematic review with meta-analysis of randomized controlled trials. Epilepsy Behav. (2016) 64:29–36. doi: 10.1016/j.yebeh.2016.09.020
61. Chin, RF, Neville, BG, Peckham, C, Wade, A, Bedford, H, and Scott, RC. Treatment of community-onset, childhood convulsive status epilepticus: a prospective, population-based study. Lancet Neurol. (2008) 7:696–703. doi: 10.1016/S1474-4422(08)70141-8
62. Appleton, R, Choonara, I, Martland, T, Phillips, B, Scott, R, and Whitehouse, W. The treatment of convulsive status epilepticus in children. The status epilepticus working party, members of the status epilepticus working party. Arch Dis Child. (2000) 83:415–9.
63. Srivastava, K, Bhartiya, S, Gavli, V, Patil, R, and Rajadhyaksha, S. Efficacy, tolerability and serum phenytoin levels after intravenous Fosphenytoin loading dose in children with status epilepticus. Indian Pediatr. (2020) 57:218–21. doi: 10.1007/s13312-020-1754-5
64. Misra, UK, Kalita, J, and Patel, R. Sodium valproate vs phenytoin in status epilepticus: a pilot study. Neurology. (2006) 67:340–2. doi: 10.1212/01.wnl.0000224880.35053.26
65. Gilad, R, Izkovitz, N, Dabby, R, Rapoport, A, Sadeh, M, Weller, B, et al. Treatment of status epilepticus and acute repetitive seizures with i.v. valproic acid vs phenytoin. Acta Neurol Scand. (2008) 118:296–300. doi: 10.1111/j.1600-0404.2008.01097.x
66. Wirrell, EC, Hood, V, Knupp, KG, Meskis, MA, Nabbout, R, Scheffer, IE, et al. International consensus on diagnosis and management of Dravet syndrome. Epilepsia. (2022) 63:1761–77. doi: 10.1111/epi.17274
67. Wabl, R, Terman, SW, Kwok, M, Elm, J, Chamberlain, J, Silbergleit, R, et al. Efficacy of home anticonvulsant Administration for Second-Line Status Epilepticus Treatment. Neurology. (2021) 97:e720–7. doi: 10.1212/WNL.0000000000012414
68. Noureen, N, Khan, S, Khursheed, A, Iqbal, I, Maryam, M, Sharib, SM, et al. Clinical efficacy and safety of injectable levetiracetam versus phenytoin as second-line therapy in the Management of Generalized Convulsive Status Epilepticus in children: An open-label randomized controlled trial. J Clin Neurol. (2019) 15:468–72. doi: 10.3988/jcn.2019.15.4.468
69. Dalziel, SR, Borland, ML, Furyk, J, Bonisch, M, Neutze, J, Donath, S, et al. Levetiracetam versus phenytoin for second-line treatment of convulsive status epilepticus in children (ConSEPT): an open-label, multicentre, randomised controlled trial. Lancet. (2019) 393:2135–45. doi: 10.1016/S0140-6736(19)30722-6
70. Lyttle, MD, Rainford, NEA, Gamble, C, Messahel, S, Humphreys, A, Hickey, H, et al. Levetiracetam versus phenytoin for second-line treatment of paediatric convulsive status epilepticus (EcLiPSE): a multicentre, open-label, randomised trial. Lancet. (2019) 393:2125–34. doi: 10.1016/S0140-6736(19)30724-X
71. Chamberlain, JM, Kapur, J, Shinnar, S, Elm, J, Holsti, M, Babcock, L, et al. Efficacy of levetiracetam, fosphenytoin, and valproate for established status epilepticus by age group (ESETT): a double-blind, responsive-adaptive, randomised controlled trial. Lancet. (2020) 395:1217–24. doi: 10.1016/S0140-6736(20)30611-5
72. Kapur, J, Elm, J, Chamberlain, JM, Barsan, W, Cloyd, J, Lowenstein, D, et al. Randomized trial of three anticonvulsant medications for status epilepticus. N Engl J Med. (2019) 381:2103–13. doi: 10.1056/NEJMoa1905795
73. Sathe, AG, Mishra, U, Ivaturi, V, Brundage, RC, Cloyd, JC, Elm, JJ, et al. Early exposure of Fosphenytoin, levetiracetam, and Valproic Acid after high-dose intravenous Administration in Young Children with Benzodiazepine-Refractory Status Epilepticus. J Clin Pharmacol. (2021) 61:763–8. doi: 10.1002/jcph.1801
74. Vignesh, V, Rameshkumar, R, and Mahadevan, S. Comparison of phenytoin, valproate and levetiracetam in pediatric convulsive status epilepticus: a randomized double-blind controlled clinical trial. Indian Pediatr. (2020) 57:222–7. doi: 10.1007/s13312-020-1755-4
75. Nalisetty, S, Kandasamy, S, Sridharan, B, Vijayakumar, V, Sangaralingam, T, and Krishnamoorthi, N. Clinical effectiveness of levetiracetam compared to Fosphenytoin in the treatment of benzodiazepine refractory convulsive status epilepticus. Indian J Pediatr. (2020) 87:512–9. doi: 10.1007/s12098-020-03221-2
76. Lang, N, Esser, W, Evers, S, Kellinghaus, C, Nguento, A, Schlegel, U, et al. Intravenous levetiracetam in clinical practice--results from an independent registry. Seizure. (2015) 29:109–13. doi: 10.1016/j.seizure.2015.03.018
77. Brigo, F, Lattanzi, S, Nardone, R, and Trinka, E. Intravenous brivaracetam in the treatment of status epilepticus: a systematic review. CNS Drugs. (2019) 33:771–81. doi: 10.1007/s40263-019-00652-0
78. Santamarina, E, Parejo Carbonell, B, Sala, J, Gutiérrez-Viedma, Á, Miró, J, Asensio, M, et al. Use of intravenous brivaracetam in status epilepticus: a multicenter registry. Epilepsia. (2019) 60:1593–601. doi: 10.1111/epi.16094
79. Strzelczyk, A, Kay, L, Bauer, S, Immisch, I, Klein, KM, Knake, S, et al. Use of brivaracetam in genetic generalized epilepsies and for acute, intravenous treatment of absence status epilepticus. Epilepsia. (2018) 59:1549–56. doi: 10.1111/epi.14476
80. Nicolas, JM, Hannestad, J, Holden, D, Kervyn, S, Nabulsi, N, Tytgat, D, et al. Brivaracetam, a selective high-affinity synaptic vesicle protein 2A (SV2A) ligand with preclinical evidence of high brain permeability and fast onset of action. Epilepsia. (2016) 57:201–9. doi: 10.1111/epi.13267
81. Appleton, RE, Rainford, NE, Gamble, C, Messahel, S, Humphreys, A, Hickey, H, et al. Levetiracetam as an alternative to phenytoin for second-line emergency treatment of children with convulsive status epilepticus: the EcLiPSE RCT. Health Technol Assess. (2020) 24:1–96. doi: 10.3310/hta24580
82. Abbaskhanian, A, Sheidaee, K, and Charati, JY. Comparison of the effect of continuous intravenous infusion of sodium valproate and midazolam on management of status epilepticus in children. Arch Pediatr. (2021) 28:696–701. doi: 10.1016/j.arcped.2021.09.011
83. Agarwal, P, Kumar, N, Chandra, R, Gupta, G, Antony, AR, and Garg, N. Randomized study of intravenous valproate and phenytoin in status epilepticus. Seizure. (2007) 16:527–32. doi: 10.1016/j.seizure.2007.04.012
84. Malamiri, RA, Ghaempanah, M, Khosroshahi, N, Nikkhah, A, Bavarian, B, and Ashrafi, MR. Efficacy and safety of intravenous sodium valproate versus phenobarbital in controlling convulsive status epilepticus and acute prolonged convulsive seizures in children: a randomised trial. Eur J Paediatr Neurol. (2012) 16:536–41. doi: 10.1016/j.ejpn.2012.01.012
85. Rahman, M, and Valproic Acid, NH. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing (2022).
86. Rahman, S, and Copeland, WC. POLG-related disorders and their neurological manifestations. Nat Rev Neurol. (2019) 15:40–52. doi: 10.1038/s41582-018-0101-0
87. Brodie, MJ, Mintzer, S, Pack, AM, Gidal, BE, Vecht, CJ, and Schmidt, D. Enzyme induction with antiepileptic drugs: cause for concern? Epilepsia. (2013) 54:11–27. doi: 10.1111/j.1528-1167.2012.03671.x
88. Segura-Bruna, N, Rodriguez-Campello, A, Puente, V, and Roquer, J. Valproate-induced hyperammonemic encephalopathy. Acta Neurol Scand. (2006) 114:1–7. doi: 10.1111/j.1600-0404.2006.00655.x
89. Strzelczyk, A, Zöllner, JP, Willems, LM, Jost, J, Paule, E, Schubert-Bast, S, et al. Lacosamide in status epilepticus: systematic review of current evidence. Epilepsia. (2017) 58:933–50. doi: 10.1111/epi.13716
90. Ngampoopun, M, Suwanpakdee, P, Jaisupa, N, and Nabangchang, C. Effectiveness and adverse effect of intravenous Lacosamide in nonconvulsive status epilepticus and acute repetitive seizures in children. Neurol Res Int. (2018) 2018:1–5. doi: 10.1155/2018/8432859
91. Lang, N, Lange, M, Schmitt, FC, Bös, M, Weber, Y, Evers, S, et al. Intravenous lacosamide in clinical practice-results from an independent registry. Seizure. (2016) 39:5–9. doi: 10.1016/j.seizure.2016.01.008
92. Misra, UK, and Kalita, J. A comparison of four antiepileptic drugs in status epilepticus: experience from India. Int J Neurosci. (2016) 126:1013–9. doi: 10.3109/00207454.2015.1095743
93. Abend, NS, and Dlugos, DJ. Treatment of refractory status epilepticus: literature review and a proposed protocol. Pediatr Neurol. (2008) 38:377–90. doi: 10.1016/j.pediatrneurol.2008.01.001
94. Abend, NS, Bearden, D, Helbig, I, McGuire, J, Narula, S, Panzer, JA, et al. Status epilepticus and refractory status epilepticus management. Semin Pediatr Neurol. (2014) 21:263–74. doi: 10.1016/j.spen.2014.12.006
95. Smith, DM, McGinnis, EL, Walleigh, DJ, and Abend, NS. Management of Status Epilepticus in children. J Clin Med. (2016) 5:47. doi: 10.3390/jcm5040047
96. Neurocritical Care Society Status Epilepticus Guideline Writing CommitteeBrophy, GM, Bell, R, Claassen, J, Alldredge, B, Bleck, TP, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. (2012) 17:3–23. doi: 10.1007/s12028-012-9695-z
97. The Neurocritical Care Society Status Epilepticus Guideline Writing CommitteeRiviello, JJ Jr, Claassen, J, LaRoche, SM, Sperling, MR, Alldredge, B, et al. Treatment of status epilepticus: an international survey of experts. Neurocrit Care. (2013) 18:193–200. doi: 10.1007/s12028-012-9790-1
98. Tasker, RC, Goodkin, HP, Sánchez Fernández, I, Chapman, KE, Abend, NS, Arya, R, et al. Refractory status epilepticus in children: intention to treat with continuous infusions of midazolam and pentobarbital. Pediatr Crit Care Med. (2016) 17:968–75. doi: 10.1097/PCC.0000000000000900
99. Ozdemir, D, Gulez, P, Uran, N, Yendur, G, Kavakli, T, and Aydin, A. Efficacy of continuous midazolam infusion and mortality in childhood refractory generalized convulsive status epilepticus. Seizure. (2005) 14:129–32. doi: 10.1016/j.seizure.2004.12.005
100. Koul, R, Chacko, A, Javed, H, and Al, RK. Eight-year study of childhood status epilepticus: midazolam infusion in management and outcome. J Child Neurol. (2002) 17:908–10. doi: 10.1177/08830738020170123002
101. Hemphill, S, McMenamin, L, Bellamy, MC, and Hopkins, PM. Propofol infusion syndrome: a structured literature review and analysis of published case reports. Br J Anaesth. (2019) 122:448–59. doi: 10.1016/j.bja.2018.12.025
102. van Gestel, JP, Blussé van Oud-Alblas, HJ, Malingré, M, Ververs, FF, Braun, KP, and van Nieuwenhuizen, O. Propofol and thiopental for refractory status epilepticus in children. Neurology. (2005) 65:591–2. doi: 10.1212/01.wnl.0000173066.89001.f9
103. Zeiler, FA, Zeiler, KJ, Teitelbaum, J, Gillman, LM, and West, M. Modern inhalational anesthetics for refractory status epilepticus. Can J Neurol Sci. (2015) 42:106–15. doi: 10.1017/cjn.2014.121
104. Lee, WK, Liu, KT, and Young, BW. Very-high-dose phenobarbital for childhood refractory status epilepticus. Pediatr Neurol. (2006) 34:63–5. doi: 10.1016/j.pediatrneurol.2005.06.014
105. Crawford TOMitchell, WG, Fishman, LS, and Snodgrass, SR. Very-high-dose phenobarbital for refractory status epilepticus in children. Neurology. (1988) 38:1035–40. doi: 10.1212/WNL.38.7.1035
106. Tiamkao, S, Mayurasakorn, N, Suko, P, Jitpimolmard, S, Arunpongpaisal, S, Phuttharak, W, et al. Very high dose phenobarbital for refractory status epilepticus. J Med Assoc Thail. (2007) 90:2597–600.
107. Watanabe, S, Okumura, Y, and Aiba, H. A case of acute encephalitis with refractory repetitive partial seizures successfully controlled by very-high-dose phenobarbital therapy found in a boy. No To Hattatsu. (2014) 46:443–6.
108. Höfler, J, Rohracher, A, Kalss, G, Zimmermann, G, Dobesberger, J, Pilz, G, et al. (S)-ketamine in refractory and super-refractory status epilepticus: a retrospective study. CNS Drugs. (2016) 30:869–76. doi: 10.1007/s40263-016-0371-2
109. Rosati, A, Ilvento, L, L'Erario, M, de Masi, S, Biggeri, A, Fabbro, G, et al. Efficacy of ketamine in refractory convulsive status epilepticus in children: a protocol for a sequential design, multicentre, randomised, controlled, open-label, non-profit trial (KETASER01). BMJ Open. (2016) 6:e011565. doi: 10.1136/bmjopen-2016-011565
110. Rosati, A, L'Erario, M, Ilvento, L, Cecchi, C, Pisano, T, Mirabile, L, et al. Efficacy and safety of ketamine in refractory status epilepticus in children. Neurology. (2012) 79:2355–8. doi: 10.1212/WNL.0b013e318278b685
111. Mehta, V, Singhi, P, and Singhi, S. Intravenous sodium valproate versus diazepam infusion for the control of refractory status epilepticus in children: a randomized controlled trial. J Child Neurol. (2007) 22:1191–7. doi: 10.1177/0883073807306248
112. Wilkes, R, and Tasker, RC. Pediatric intensive care treatment of uncontrolled status epilepticus. Crit Care Clin. (2013) 29:239–57. doi: 10.1016/j.ccc.2012.11.007
113. Tasker, RC, and Vitali, SH. Continuous infusion, general anesthesia and other intensive care treatment for uncontrolled status epilepticus. Curr Opin Pediatr. (2014) 26:682–9. doi: 10.1097/MOP.0000000000000149
114. Koo, CM, and Kang, HC. Could cannabidiol be a treatment option for intractable childhood and adolescent epilepsy? J Epilepsy Res. (2017) 7:16–20. doi: 10.14581/jer.17003
115. Elia, M, Klepper, J, Leiendecker, B, and Hartmann, H. Ketogenic diets in the treatment of epilepsy. Curr Pharm Des. (2017) 23:5691–701. doi: 10.2174/1381612823666170809101517
116. Gaspard, N, Hirsch, LJ, Sculier, C, Loddenkemper, T, van Baalen, A, Lancrenon, J, et al. New-onset refractory status epilepticus (NORSE) and febrile infection-related epilepsy syndrome (FIRES): state of the art and perspectives. Epilepsia. (2018) 59:745–52. doi: 10.1111/epi.14022
117. Rosenthal, ES, Claassen, J, Wainwright, MS, Husain, AM, Vaitkevicius, H, Raines, S, et al. Brexanolone as adjunctive therapy in super-refractory status epilepticus. Ann Neurol. (2017) 82:342–52. doi: 10.1002/ana.25008
118. Śmigiel, R, Biela, M, Szmyd, K, Błoch, M, Szmida, E, Skiba, P, et al. Rapid whole-exome sequencing as a diagnostic tool in a neonatal/pediatric intensive care unit. J Clin Med. (2020) 9:2220. doi: 10.3390/jcm9072220
119. Hirsch, LJ, Gaspard, N, van Baalen, A, Nabbout, R, Demeret, S, Loddenkemper, T, et al. Proposed consensus definitions for new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related conditions. Epilepsia. (2018) 59:739–44. doi: 10.1111/epi.14016
Keywords: status epilepticus, pediatric, treatment, epilepsy, benzodiazepine
Citation: Becker L-L, Gratopp A, Prager C, Elger CE and Kaindl AM (2023) Treatment of pediatric convulsive status epilepticus. Front. Neurol. 14:1175370. doi: 10.3389/fneur.2023.1175370
Received: 27 February 2023; Accepted: 12 June 2023;
Published: 29 June 2023.
Edited by:
Carlotta Spagnoli, Santa Maria Nuova Hospital, ItalyReviewed by:
Lucio Parmeggiani, Servizio di Neurologia e Neuroriabilitazione dell'Età Evolutiva - Ospedale di Bolzano, ItalyCopyright © 2023 Becker, Gratopp, Prager, Elger and Kaindl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angela M. Kaindl, YW5nZWxhLmthaW5kbEBjaGFyaXRlLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.