- 1Department of Neurology, Weill Cornell Medicine-Qatar, Ar-Rayyan, Qatar
- 2Department of Neuroradiology, Hamad Medical Corporation, Doha, Qatar
- 3Department of Population Health Sciences, Weill Cornell Medicine-Qatar, Ar-Rayyan, Qatar
Objective: To describe the occurrence and features of Neurocystircercosis (NCC) in Qatar.
Background: Qatar has a mixed population of natives and expats. NCC is not endemic to the region, but clinical practice suggests its occurrence in large numbers.
Design/ methods: A database was created to summarize information retrospectively collected on patients with NCC seen through the national health system (HMC) between 2013 and 2018. We identified demographic and disease related variables (clinical manifestations, investigative findings, treatment and outcome) for all patients.
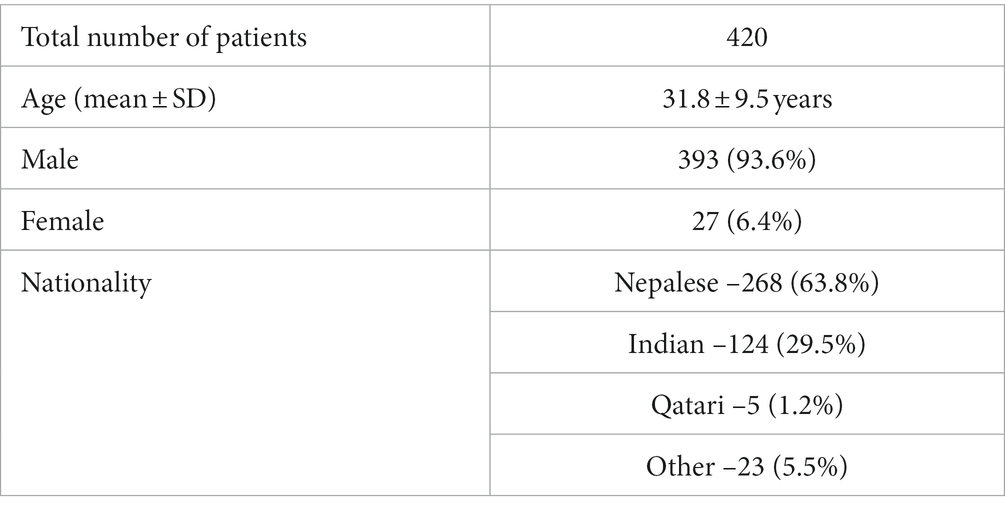
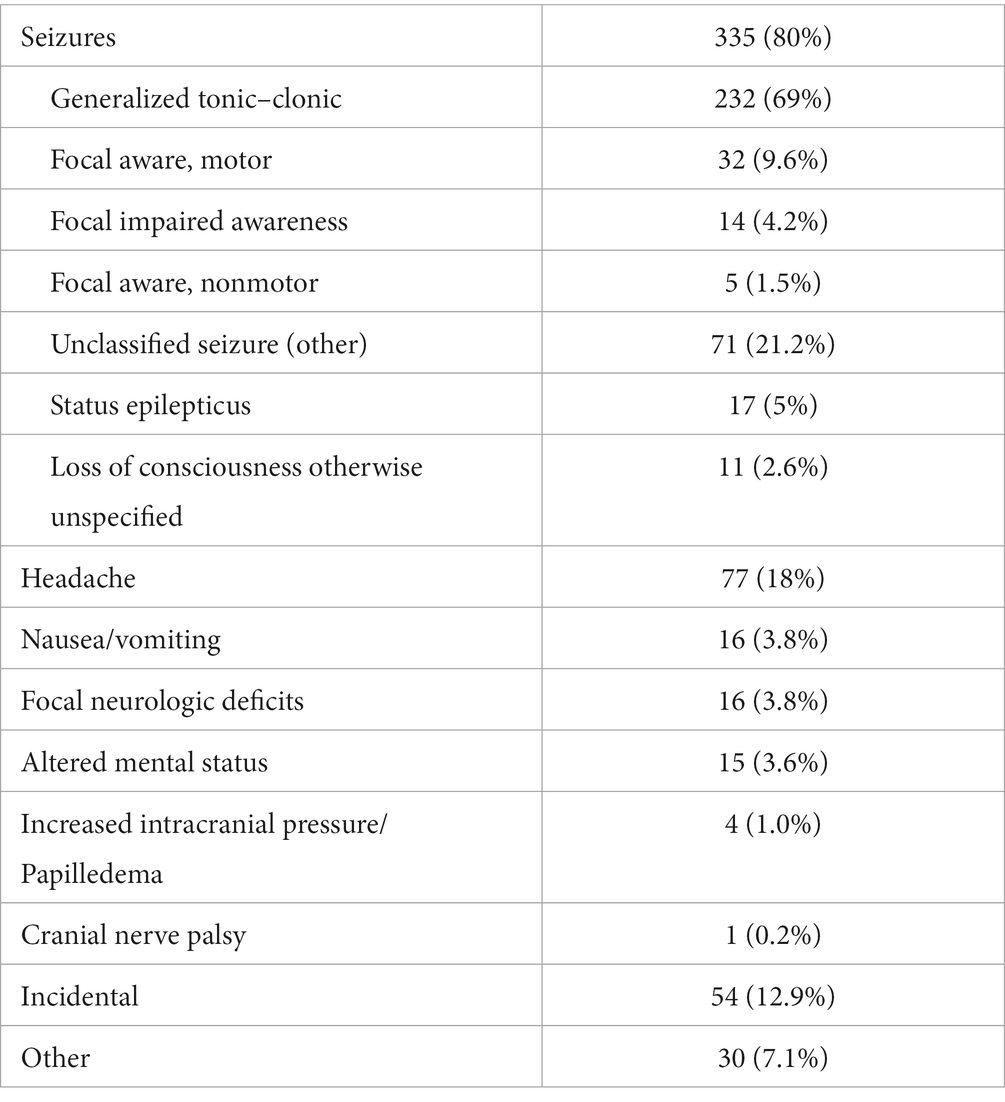
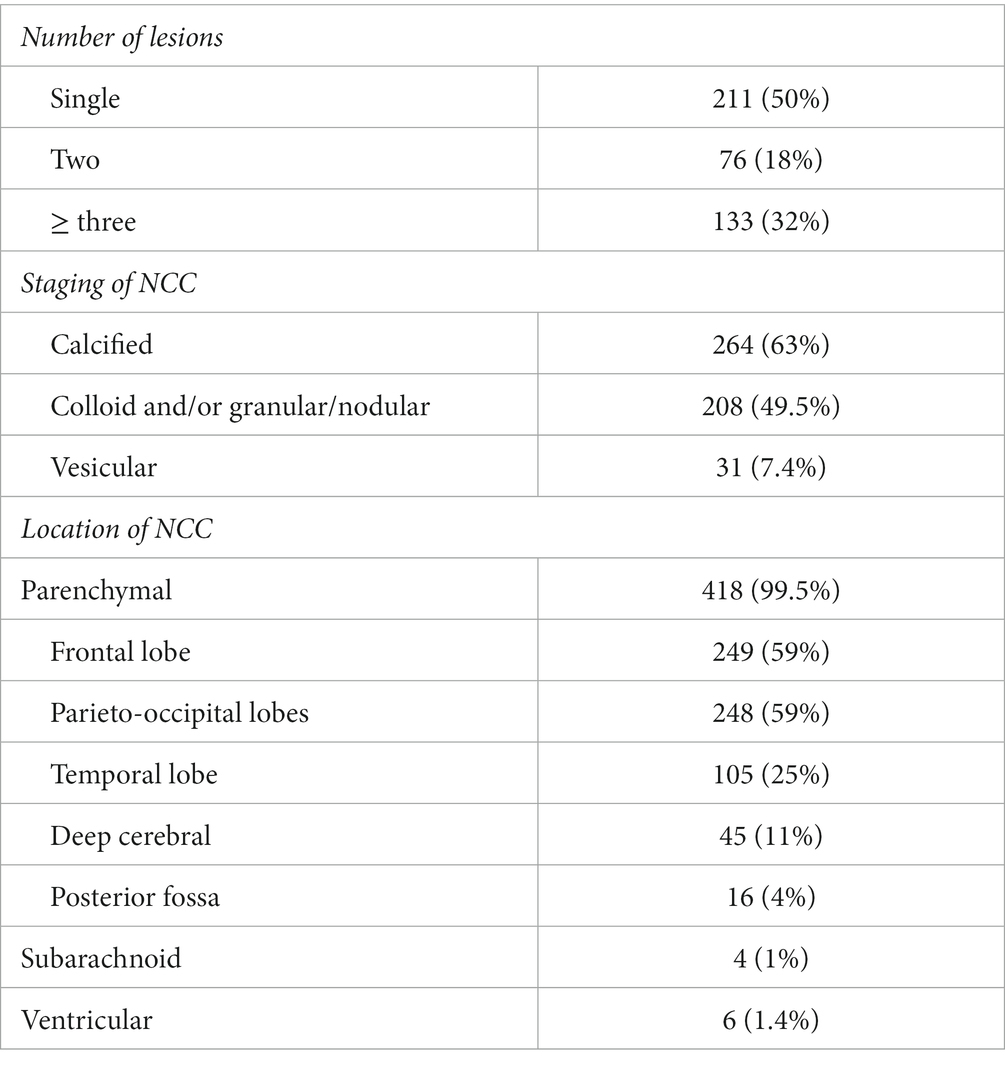
Results: Out of 420 identified NCC patients, 393 (93.6%) were men, and 98.3% were immigrants from NCC endemic countries such as Nepal (63.8%) and India (29.5%). Eighty percent of patients presented with seizures, with the majority (69%) experiencing generalized tonic–clonic seizures. Five percent presented with status epilepticus. Headaches, the second most common complaint, were reported in 18% of subjects. On imaging, 50% had a single lesion while 63% included pathology at the calcified stage. The lesions were parenchymal in 99.5% of cases, predominantly in the frontal lobe (59%). Thirteen percent were diagnosed incidentally on imaging, mainly in the form of isolated calcified non enhancing lesions. Albendazole was received by 55% of patients, and phenytoin was the most prescribed anti-seizure drug (57%). When long term follow up was available, 70% of the patients presenting with seizures were completely seizure free.
Conclusion: NCC is prevalent in Qatar, mainly within the large Southeast Asian immigrant population. NCC is currently a significant contributor to the epilepsy burden in Qatar, often with a good outcome regarding seizure control. NCC with intraparenchymal single lesion shares a large proportion of our cohort.
Introduction
Neurocysticercosis (NCC) is the most common central nervous system (CNS) parasitic infection in humans and is an important cause of acquired epilepsy around the world (1–5). Areas in which NCC is endemic include Latin America, sub-Saharan Africa, the Indian Subcontinent and Southeast Asia. However, an increasing number of cases are surfacing in nonendemic regions most likely due to migration and travel (1–6). NCC is acquired via the fecal-oral route when humans ingest Taenia solium tapeworm eggs (1–5). Following ingestion, Taenia eggs develop into larvae referred to as cysticerci which are fluid-filled cysts that lodge in soft tissues such as the brain, and typically evolve over years (1–4). The most common clinical manifestation of NCC is seizures (1–5), (7–10). NCC can also cause increased intracranial pressure which results in a variety of symptoms including headache, nausea, and vomiting (1–5).
Qatar is a small country in the Arabian Peninsula, with a mixed population of natives and expats. NCC is not endemic to the region (4, 6), but clinical practice suggests its occurrence in increasing numbers. This report aims to describe the occurrence and features of NCC in Qatar.
Methods
Data was retrospectively collected on patients with definitive or probable NCC seen through the national health system at Hamad Medical Corporation (HMC) between 2013 and 2018. HMC hosts the main adult comprehensive neurology services in the country. Definitive NCC was mainly based on visualizing the scolex on imaging, or much less often on histology. Probable NCC had highly suggestive lesions on imaging with congruent clinical manifestations, typically with history of residence or travel in endemic regions (11, 12). After Institutional Review Board approval, demographic and disease-related variables (clinical manifestations, radiological/EEG findings, treatment, and outcomes) were identified for all patients. A database was created to summarize the gathered information. In the statistical analysis, the demographic and clinical characteristics of patients were described as mean and standard deviation for numerical variables and as frequency distribution for categorical variables. All analyses are carried out using IBM-SPSS (version 27, Armonk NY, United States).
Results
Demographics
This study included 420 patients with a mean age (years) of 31.8 ± 9.5. Three hundred ninety three (93.6%) were male, and 27 (6.4%) were female. With regards to nationality, the majority of patients were Nepalese (268; 63.8%), and Indian (124, 29.5%). Other nationalities included Bangladeshi, Sri Lankan, Pakistani, Thai, Filipino, Qatari, North Korean, Congolese, Djiboutians, Egyptian, South African, and Ethiopian. Over 98% of patients were immigrants from endemic countries, and only 1.2% were local Qataris (Table 1).
Seizure types and other clinical manifestations
Three hundred thirty-five (80%) patients presented with seizures. Among these patients, 69% experienced generalized tonic–clonic seizures by description. The other less common seizure types were focal aware motor seizures (9.6%), focal aware nonmotor seizures (1.5%), and focal impaired awareness seizures (4.2%). 22% of subjects reported more than one seizure type. Additionally, 5% of patients experienced status epilepticus.
Other clinical manifestations included headache (18%), nausea and vomiting (3.8%), focal neurologic deficits (3.8%), and altered mental status (3.6%). The less common presentations included cranial nerve (CN) palsy which was experienced by one patient, and papilledema and intracranial hypertension (IIH) which were experienced by four patients. Fifty-four patients (13%) were diagnosed incidentally on imaging, mainly in the form of isolated calcified non enhancing lesions (Table 2).
Diagnostic features
Radiological findings: Four hundred seven (97%) patients underwent computed tomography (CT) scans, and 196 (47%) underwent magnetic resonance imaging (MRI). Approximately 53% of patients had only CT scans, 3.1% had only MRIs, and 43.6% had both. All patients were classified in terms of the number of lesions, stages and location. A single lesion was seen in 50% of patients, two lesions in 18%, and three or more lesions in 32%. With regards to the staging of NCC lesions, 7.4% of cases had cysts at the vesicular stage, 49.5% had degenerating cysts (colloid and/or granular/nodular stages), and 63% had calcified lesions. Thus, almost 20% had lesions at different stages concomitantly. The majority of patients (418, 99.5%) had parenchymal lesions. Only six patients had ventricular cysts, and four had subarachnoid ones. We did not identify any cases with spinal cysts. Intraparenchymal lesions were found in the frontal lobe in 59% of cases, in the temporal lobe in 25%, in the parieto-occipital lobes in 59%, in deep cerebral structures in 11% and in the brainstem/cerebellum in 4% (Table 3).
EEG findings: Thirty-eight (9%) patients underwent EEGs. Among them, only nine patients had abnormal findings with only two cases showing epileptiform discharges (spikes) while the remaining seven had focal or generalized slowing.
Treatment and outcome
Albendazole was the antiparasitic agent of choice, received by 55% of patients, with 49% also receiving dexamethasone. Only three patients underwent neurosurgical procedures.
Phenytoin is the most used anti-seizure medication (ASM), given in 57% of cases, followed by levetiracetam in 14%, valproate and carbamazepine in 3% each. Of the patients with seizures, 90% were on a single ASM, 4% were on two or more ASMs, and 6% were not taking any ASMs on their last documented note.
With regards to outcome, 119 (28%) patients had a clinic follow up beyond 6 months from the initial diagnosis. Approximately half of them, 59 subjects, underwent head reimaging. Of the 103 patients who initially presented with seizures and had reliable follow up, 72 (70%) were completely seizure free as per the last documented clinic note.
The majority of reimaged patients (51%) underwent MRI, 39% underwent CT, and 10% underwent both MRI and CT. The NCC disappeared on reimaging in only 12% of subjects, with 80% showing either an unaltered appearance or just partial improvement without complete resolution.
Discussion
In the current study, we report on more than 400 patients with NCC in a country (Qatar) where the disease was historically nonexistent due to the absence of swine farming. This is by far the largest reported series from the Middle East and Arabian Peninsula (13–16). The infection is likely to have been acquired abroad as most patients are immigrants from endemic countries, notably Nepal and India. This trend is also reported in North American series, where the majority of patients are immigrants from Central and South America with the onset of symptoms occurring years after immigration (17–21). The predominance of young male patients in our database can be attributed to the disproportionately large southeast Asian male population living in Qatar (Planning and Statistics Authority), but other case series from the United States have also noted a male to female ratio of up to 1.5–2:1 (17, 18). Similar patterns of sex distribution for NCC are present in some reports from endemic countries as well; a retrospective analysis showed that 71.4% of NCC patients in Nepal were male (22).
Not surprisingly, 80% of patients in our cohort presented with seizures. This is consistent with the current knowledge that seizures are the predominant manifestation of NCC (1–5), (7–10). Previous reports from Qatar alluded to the contribution of NCC to the overall seizure burden. In a study by Pathan et al. investigating the computed tomography abnormalities in patients presenting with first seizure to the emergency department in Qatar, the most common ones consisted of frank neurocysticercosis (9.2%) and calcified (6%) lesions (23). In another study mapping epilepsy in Qatar by Haddad et al., the authors found that NCC was the etiology for epilepsy in 10% of subjects of Southeast Asian background (24).
On brain imaging, 50% had a single lesion while 63% included lesions at the calcified stage. The lesions were parenchymal in 99.5% of cases, predominantly in the frontal lobe (59%). The abundance of single lesion cases in our cohort is reminiscent of described case series coming from Nepal and India, the countries of origin for most of our NCC cases. In Nepal, single lesions were seen in 62% of patients (22). A study from India also found that 64% of patients with probable NCC had single lesions (25). Many more reports suggest that single granulomas are more frequent in India compared to Latin America (5, 26). The paucity of extraparenchymal lesions in our cohort follows the same explanation. In Indian series, subarachnoid and ventricular localizations are less frequently identified than in Latin American series (5, 26, 27). Such heterogeneity within NCC is noted in non-endemic countries depending on the native geographical background of the patient population. North American series contain a higher rate of subarachnoid and ventricular NCC compared to ours (17–19, 21), again reflecting the Latin American background where the likely distant infestation occurred in many of their patients. We also found that 13% of our subjects were diagnosed incidentally on imaging, mainly in the form of isolated calcified non enhancing lesions. NCC can be asymptomatic (1, 5, 28). This should be recognized in non-endemic areas also; in a report of 48 patients diagnosed in Kuwait from 2014 to 2019, nine (18.7%) presented no apparent related symptoms (16).
Even though 80% of our NCC patients presented with seizures, EEGs were performed in less than 10% of subjects. The treating physicians were apparently comfortable with a diagnosis of seizures based on the clinical description and the correlated etiology on brain imaging. Only two out of 39 EEG studies showed epileptiform discharges. This experience, albeit limited, suggests the routine EEG to be clearly lacking in sensitivity in the context of NCC and seizures. In the existing literature, the rate of EEG interictal epileptiform discharges vary widely across the different publications, ranging from 6 to 75% of patients with NCC (29). Spikes are more likely to be detected in patients with enhancing lesions or viable cysts compared to patients with pure calcifications (29, 30).
As for treatment choices, Albendazole was received by 55% of patients, which roughly corresponds to the rate of NCC subjects with other than pure calcifications on imaging. According to the latest reviews and guidelines, antihelmintic therapy is recommended for potentially viable parenchymal NCC. Albendazole alone or in combination with Praziquantel seem to enhance the destruction of parasitic cysts and may lead to fewer seizure recurrences (31–34). Phenytoin was the most prescribed ASM (57%). This choice over newer ASMs is best explained by affordability and global availability, with most of our patients being immigrant laborers on limited income. The current literature does not offer any strong evidence for the choice of ASM in the context of NCC. We found a single open label study comparing carbamazepine and levetiracetam; although there was a trend for better seizure control with carbamazepine, patients treated with the later experienced a higher rate of drug related side effects (35). There is no reliable evidence regarding the duration of ASM treatment either, with the persistence of calcified lesions predicting a higher risk for seizure recurrence (1, 32, 36). We need larger multicenter, randomized, and controlled trials to address the questions of choice and duration of ASMs.
Outcome was only measurable in a fraction of our subjects. Most of our patients were immigrants on short term contracts, and thus returned to their home countries and were lost to follow up. The overall seizure outcome was favorable in the majority, with 70% noted to be seizure free on the last clinic visit. This is compatible with the reported NCC literature. In a study from a rural community in South India, 80% of NCC subjects achieved seizure freedom for more than 2 years. The remaining patients had rather infrequent seizures (37). Goyal et al. reported on 200 patients with epilepsy due to NCC, who were followed for a minimum of 1 year. Only 17 patients (8.5%) had recurrence of their seizures, including 13 (6.5%) who fulfilled the ILAE criteria for drug resistant epilepsy (38, 39). Seizure freedom was often achieved with antiseizure monotherapy (37, 38). Furthermore, amongst 512 patients with intractable epilepsy in Brazil where NCC is endemic, isolated NCC was found in only eight patients (1.56%) (40). Thus, despite the high contribution of NCC to the burden of epilepsy especially in endemic countries, NCC only rarely leads to intractable epilepsy.
Our study has some limitations. It is a retrospective chart review, and more than half of our subjects lacked long term follow up. We do not have serological antibody testing at our disposition to support the NCC diagnosis. However, the enzyme linked immunotransfer blot (EITB), despite being the serological test of choice, has a poor sensitivity in patients with single parenchymal or with only calcified NCC (32). Such patients make up a large proportion of our series and thus would have not benefited much from such testing.
This is by far the largest NCC series from our region, the Middle East and the Arabian Peninsula. NCC is clearly prevalent in Qatar and is predominantly noted in the Southeast Asian immigrant population. NCC is a significant contributor to the epilepsy burden in Qatar, often with a good outcome regarding seizure control. NCC with intraparenchymal single lesion seems to be a common radiological presentation in our cohort. Physicians, especially neurologists and emergency practitioners, should be fully aware of its presence and complications.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Hamad Medical Corporation -Medical Research Center. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
NH, GM, AE, BM, HE, and ZM contributed to the conception and design of the study. YS, MA, FA-M, and MS organized the database. NH, YS, and ZM performed the statistical analysis. NH, YS, AN, FW, BM, and ZM wrote the first draft of the manuscript. All authors contributed and approved the final manuscript version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Garcia, HH, Nash, TE, and Del Brutto, OH. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol. (2014) 13:1202–15. doi: 10.1016/S1474-4422(14)70094-8
2. Del Brutto, OH. Neurocysticercosis. Handb Clin Neurol. (2014) 121:1445–59. doi: 10.1016/B978-0-7020-4088-7.00097-3
4. Burneo, JG, and Cavazos, JE. Neurocysticercosis and epilepsy. Epilepsy Curr. (2014) 14:23–8. doi: 10.5698/1535-7511-14.s2.23
5. Carpio, A, Fleury, A, and Hauser, WA. Neurocysticercosis: five new things. Neurol Clin Pract. (2013) 3:118–25. doi: 10.1212/CPJ.0b013e31828d9f17
6. Donadeu, M, Lightowlers, MW, Fahrion, AS, Kessels, J, and Abela-Ridder, B. Taenia solium: WHO endemicity map update. Wkly Epidemiol Rec. (2016) 91:595–9.
7. Singh, G, Burneo, JG, and Sander, JW. From seizures to epilepsy and its substrates: neurocysticercosis. Epilepsia. (2013) 54:783–92. doi: 10.1111/epi.12159
8. Nash, TE, Mahanty, S, Loeb, JA, Theodore, WH, Friedman, A, Sander, JW, et al. Neurocysticercosis: a natural human model of epileptogenesis. Epilepsia. (2015) 56:177–83. doi: 10.1111/epi.12849
9. Del Brutto, OH, Arroyo, G, Del Brutto, VJ, Zambrano, M, and García, HH. On the relationship between calcified neurocysticercosis and epilepsy in an endemic village: a large-scale, computed tomography-based population study in rural Ecuador. Epilepsia. (2017) 58:1955–61. doi: 10.1111/epi.13892
10. Bustos, J, Gonzales, I, Saavedra, H, Handali, S, and Garcia, HH, Cysticercosis Working Group in Peru. Neurocysticercosis. Neurocysticercosis. A frequent cause of seizures, epilepsy, and other neurological morbidity in most of the world. J Neurol Sci. (2021) 427:117527. doi: 10.1016/j.jns.2021.117527
11. del Brutto, OH, Nash, TE, White, AC Jr, Rajshekhar, V, Wilkins, PP, Singh, G, et al. Revised diagnostic criteria for neurocysticercosis. J Neurol Sci. (2017) 372:202–10. doi: 10.1016/j.jns.2016.11.045
12. Del Brutto, OH. Twenty-five years of evolution of standard diagnostic criteria for neurocysticercosis. How have they impacted diagnosis and patient outcomes? Expert Rev Neurother. (2020) 20:147–55. doi: 10.1080/14737175.2020.1707667
13. Al-Khuwaitir, TS, Al-Moghairi, AM, El Zain, FN, and Al-Zayer, WS. Neurocysticercosis in central Saudi Arabia. Neurosciences (Riyadh). (2005) 10:226–9.
14. Khan, FY, Imam, YZ, Kamel, H, and Shafaee, M. Neurocysticercosis in Qatari patients: case reports. Travel Med Infect Dis. (2011) 9:298–302. doi: 10.1016/j.tmaid.2011.07.002
15. Del Brutto, OH. Neurocysticercosis on the Arabian peninsula, 2003-2011. Emerg Infect Dis. (2013) 19:172–4. doi: 10.3201/eid1901.120432
16. Iqbal, J, Ahmad, S, al-Awadhi, M, Masud, A, Mohsin, Z, Abdulrasoul, AY, et al. A large case series of Neurocysticercosis in Kuwait, a nonendemic Arabian gulf country in the Middle East region. Microorganisms. (2021) 9:1221. doi: 10.3390/microorganisms9061221
17. Wallin, MT, and Kurtzke, JF. Neurocysticercosis in the United States: review of an important emerging infection. Neurology. (2004) 63:1559–64. doi: 10.1212/01.wnl.0000142979.98182.ff
18. Serpa, JA, Graviss, EA, Kass, JS, and White, AC Jr. Neurocysticercosis in Houston, Texas: an update. Medicine (Baltimore). (2011) 90:81–6. doi: 10.1097/MD.0b013e318206d13e
19. Serpa, JA, and White, ACJr. Neurocysticercosis in the United States. Pathog Glob Health. (2012) 106:256–260. doi: 10.1179/2047773212Y.0000000028
20. Del Brutto, OH. A review of cases of human cysticercosis in Canada. Can J Neurol Sci. (2012) 39:319–22. doi: 10.1017/s0317167100013445
21. Spallone, A, Woroch, L, Sweeney, K, Seidman, R, and Marcos, LA. The burden of Neurocysticercosis at a single New York hospital. J Pathog. (2020) 2020:8174240–9. doi: 10.1155/2020/8174240
22. Ojha, R, Shah, DB, Shrestha, A, Koirala, S, Dahal, A, Adhikari, K, et al. Neurocysticercosis in Nepal: a retrospective clinical analysis. Neuroimmunol Neuroinflamm. (2015) 2:167–70. doi: 10.4103/2347-8659.160865
23. Pathan, SA, Abosalah, S, Nadeem, S, Ali, A, Hameed, AA, Marathe, M, et al. Computed tomography abnormalities and epidemiology of adult patients presenting with first seizure to the emergency department in Qatar. Acad Emerg Med. (2014) 21:1264–8. doi: 10.1111/acem.12508
24. Haddad, N, Melikyan, G, al Hail, H, al Jurdi, A, Aqeel, F, Elzafarany, A, et al. Epilepsy in Qatar: causes, treatment, and outcome. Epilepsy Behav. (2016) 63:98–102. doi: 10.1016/j.yebeh.2016.07.043
25. Sahu, PS, Patro, S, Jena, PK, Swain, SK, and Das, BK. Imaging and serological-evidence of Neurocysticercosis among patients with seizures in Odisha, an unexplored eastern Coastal Province in India. J Clin Diagn Res. (2015) 9:DC06–10. doi: 10.7860/JCDR/2015/12609.5967
26. Singh, G. Neurocysticercosos in South-Central America and the Indian subcontinent A comparative evaluation. Arq Neuropsiquiatr. (1997) 55:349–56. doi: 10.1590/s0004-282x1997000300001
27. Fleury, A, Escobar, A, Fragoso, G, Sciutto, E, and Larralde, C. Clinical heterogeneity of human neurocysticercosis results from complex interactions among parasite, host and environmental factors. Trans R Soc Trop Med Hyg. (2010) 104:243–50. doi: 10.1016/j.trstmh.2010.01.005
28. Moyano, LM, O'Neal, SE, Ayvar, V, Gonzalvez, G, Gamboa, R, Vilchez, P, et al. Cysticercosis working Group in Peru. High prevalence of asymptomatic Neurocysticercosis in an endemic rural community in Peru. PLoS Negl Trop Dis. (2016) 10:e0005130. doi: 10.1371/journal.pntd.0005130
29. Duque, KR, and Burneo, JG. Clinical presentation of neurocysticercosis-related epilepsy. Epilepsy Behav. (2017) 76:151–7. doi: 10.1016/j.yebeh.2017.08.008
30. Chayasirisobhon, S, Menoni, R, Chayasirisobhon, W, and Locke, GE. Correlation of electroencephalography and the active and inactive forms of neurocysticercosis. Clin Electroencephalogr. (1999) 30:9–11. doi: 10.1177/155005949903000106
31. Baird, RA, Wiebe, S, Zunt, JR, Halperin, JJ, Gronseth, G, and Roos, KL. Evidence-based guideline: treatment of parenchymal neurocysticercosis: report of the guideline development Subcommittee of the American Academy of neurology. Neurology. (2013) 80:1424–9. doi: 10.1212/WNL.0b013e31828c2f3e
32. White, AC Jr, Coyle, CM, Rajshekhar, V, Singh, G, Hauser, WA, Mohanty, A, et al. Diagnosis and treatment of Neurocysticercosis: 2017 clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. (2018) 66:e49–75. doi: 10.1093/cid/cix1084
33. Monk, EJM, Abba, K, and Ranganathan, LN. Anthelmintics for people with neurocysticercosis. Cochrane Database Syst Rev. (2021) 2021:CD000215. doi: 10.1002/14651858.CD000215.pub5
34. Garcia, HH. Parasitic infections of the nervous system. Continuum (Minneap Minn). (2021) 27:943–62. doi: 10.1212/CON.0000000000000986
35. Santhosh, AP, Kumar Goyal, M, Modi, M, Kharbanda, PS, Ahuja, CK, Tandyala, N, et al. Carbamazepine versus levetiracetam in epilepsy due to neurocysticercosis. Acta Neurol Scand. (2021) 143:242–7. doi: 10.1111/ane.13355
36. Cochrane Epilepsy GroupWalton, D, Castell, H, Collie, C, Wood, GK, Sharma, M, et al. Antiepileptic drugs for seizure control in people with neurocysticercosis. Cochrane Database Syst Rev. (2021) 2021:CD009027. doi: 10.1002/14651858.CD009027.pub4
37. Murthy, JMK, and Seshadri, V. Prevalence, clinical characteristics, and seizure outcomes of epilepsy due to calcific clinical stage of neurocysticercosis: study in a rural community in South India. Epilepsy Behav. (2019) 98:168–72. doi: 10.1016/j.yebeh.2019.07.024
38. Goyal, M, Chand, P, Modi, M, Khandelwal, N, Kharbanda, PS, Lal, V, et al. Neurocysticercosis: an uncommon cause of drug-refractory epilepsy in north Indian population. Epilepsia. (2015) 56:1747–52. doi: 10.1111/epi.13130
39. Kwan, P, Arzimanoglou, A, Berg, AT, Brodie, MJ, Allen Hauser, W, Mathern, G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia. (2010) 51:1069–77. doi: 10.1111/j.1528-1167.2009.02397.x
Keywords: neurocysticercosis, Qatar, seizures, epilepsy, calcified granuloma, antiseizure drugs, antihelminthic treatment
Citation: Haddad N, Shaheen Y, Abunaib M, Melikyan G, El Sotouhy A, Wahbeh F, Nauman A, Al-Maadid F, Soliman M, Mesraoua B, Elkhider H and Mahfoud Z (2023) Neurocysticercosis in non-endemic regions: The experience of Qatar. Front. Neurol. 14:1173909. doi: 10.3389/fneur.2023.1173909
Edited by:
Hector H. Garcia, National Institute of Neurological Sciences (INCN), PeruReviewed by:
Gagandeep Singh, Icahn School of Medicine at Mount Sinai, United StatesOscar H. Del Brutto, Espiritu Santo University, Ecuador
Copyright © 2023 Haddad, Shaheen, Abunaib, Melikyan, El Sotouhy, Wahbeh, Nauman, Al-Maadid, Soliman, Mesraoua, Elkhider and Mahfoud. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Naim Haddad, bmFoMjAwM0BxYXRhci1tZWQuY29ybmVsbC5lZHU=
 Naim Haddad
Naim Haddad Yanal Shaheen1
Yanal Shaheen1 Ahmed El Sotouhy
Ahmed El Sotouhy Farah Wahbeh
Farah Wahbeh Ziyad Mahfoud
Ziyad Mahfoud