- 1Epilepsy Centre, Clinical Neurosciences Department, King's College NHS Foundation Trust, London, United Kingdom
- 2Maurice Wohl Clinical Neuroscience Institute, Institute of Psychiatry, Psychology and Neuroscience, King's College London, London, United Kingdom
Introduction: Resistance to drug therapy is a major hurdle in new-onset refractory status epilepticus (NORSE) treatment and there is urgent need to develop new treatment approaches. Non-drug approaches such as neuromodulation offer significant benefits and should be investigated as new adjunct treatment modalities. An important unanswered question is whether desynchronizing networks by vagal nerve stimulation (VNS) may improve seizure control in NORSE patients.
Main text: We present a summary of published NORSE cases treated with VNS and our own data, discuss possible mechanisms of action, review VNS implantation timing, stimulation setting titration protocols and outcomes. Further, we propose avenues for future research.
Discussion: We advocate for consideration of VNS for NORSE both in early and late stages of the presentation and hypothesize a possible additional benefit from implantation in the acute phase of the disease. This should be pursued in the context of a clinical trial, harmonizing inclusion criteria, accuracy of documentation and treatment protocols. A study planned within our UK-wide NORSE-UK network will answer the question if VNS may confer benefits in aborting unremitting status epilepticus, modulate ictogenesis and reduce long-term chronic seizure burden.
1. Introduction
New-onset refractory status epilepticus (NORSE) and its subcategory febrile infection-related epilepsy syndrome (FIRES) are rare, devastating clinical presentations with <500 cases reported in the literature to date (1, 2). They are associated with high case fatality, long-term morbidity and their treatment is not supported by controlled studies (1, 2). Resistance to drug therapy is a major hurdle in managing this group of patients and there is urgent need to develop new treatment approaches. Functional disability is present in up to two thirds of NORSE survivors and subsequent chronic epilepsy is the norm (2–5), but individual reports and our own experience indicates that some people do have good cognitive and functional outcomes, even after prolonged status epilepticus (6). Whether desynchronizing networks by vagal nerve stimulation (VNS) may improve seizure control remains an unanswered question. Long-term studies of VNS in drug-refractory patients have demonstrated a >50% seizure reduction in up to 60% of patients (7, 8) but the evidence base for VNS having the potential to interrupt refractory status epilepticus acutely is low (Class IV) (9). Additionally, whether VNS modulates brain activity directly through electrical stimulation, or also indirectly, via modulation of the immune system, is not completely understood. We first evaluate the current evidence behind the use of VNS in adult and pediatric NORSE cases, then present our own center's experience and propose avenues for future research.
2. Key VNS anti-ictal and anti-epileptogenic mechanisms of action
The anti-ictal and anti-epileptogenic mechanisms of action of VNS have been studied extensively in both humans and animal models and comprise stimulation of serotonergic and noradrenergic centers in the brainstem (10), norepinephrine binding in the limbic system (11) and modulation of cortical γ-Aminobutyric acid (GABA) A receptor density (12). These studies however, have not explored the mechanism of action of VNS in models of status epilepticus, and it is unknown whether the same mechanisms are responsible for the acute or subacute interruption of status, as in the long-term reduction of chronic seizures. In this respect, it is interesting to note, that in a human study seizures that were acutely stimulated using VNS had a reduced ictal spread as well as reduced impact on cardiovascular function (13). It is also unknown, whether VNS stimulation early during status epilepticus may prevent the process of epileptigenesis to some extent. It is also conceivable, that the changes in receptor occupancy and density induced by VNS may act synergistically and over the longer-term with concomitantly administered antiseizure medicines (ASM), such as benzodiazepines. More recent developments have seen VNS being used as anti-inflammatory treatment: preclinical evidence suggests that VNS may regulate cytokine expression by upregulating High mobility group box protein 1 (HMGB1) through activation of the cholinergic anti-inflammatory pathway (CAP), a loop formed of ascending vagus afferents, autonomic brain stem, forebrain cortical structures and descending vagus efferents [reviewed in (14)]. Therefore, application of VNS in NORSE patients may provide an immediate and controllable way to modulate ictogenesis and further brain injury due to unremitting seizures and inflammation.
3. Reported cases of VNS use in NORSE/FIRES
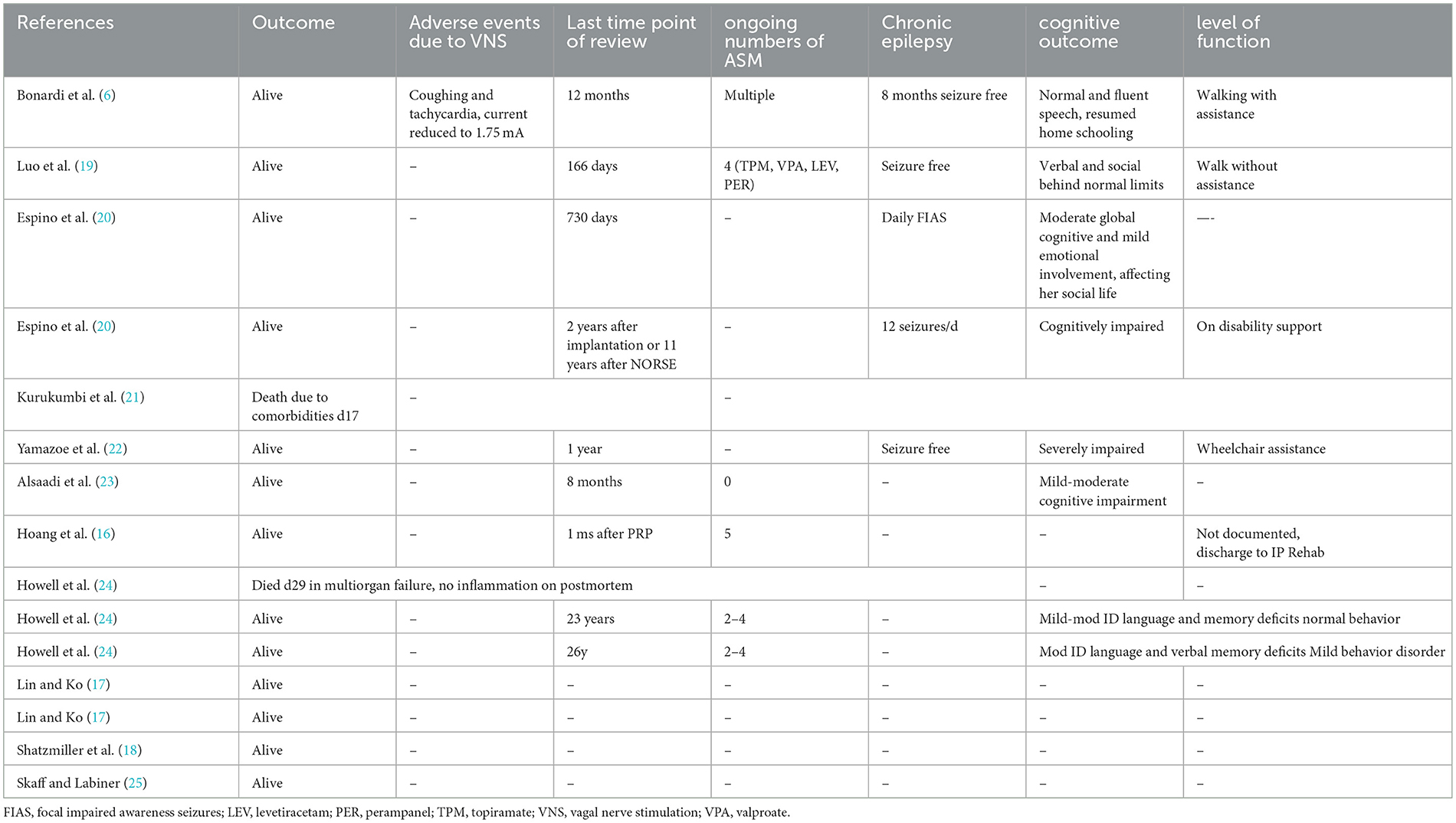
We searched ClinicalTrials.gov, and PubMed databases using the following search strategy (Supplementary Figure 1 Search criteria): (“VNS” OR “vagal nerve stimulation” OR “vagus nerve stimulation”) AND (“New-onset refractory status epilepticus” OR “NORSE” OR “FIRES” OR “Febrile infection-related epilepsy syndrome”), including cases summarized in previous systematic reviews (9, 15). We reviewed individual case descriptions and excluded patients that did not fulfill the current definitions of NORSE and FIRES (1). Reports included individual case reports and case series; four reports were published as abstracts only (16–18). The amount of detail included in the cases reviewed varied substantially and the description of VNS stimulation parameters and titration protocols was not uniform. Of the 15 cases of NORSE treated with VNS identified (Table 1), 10 were adult (age range 19–49 years) and five pediatric cases (age range 17 months−14 years), nine were male and six female. Eight cases fulfilled criteria for the subtype FIRES, including all five pediatric cases. An etiology was identified in six adult cases [four NMDAR encephalitis (17, 18, 20, 23), one Human Parvovirus B19 infection (25), one AntiGluR encephalitis (22)], eight cases described negative investigation results and could be classified as cryptogenic (cNORSE) (16, 18–21, 24, 25). When documented, patients had tried multiple antiseizure medications (seven cases, average >5) and anesthetic agents (10 cases, average >3) with propofol, ketamine and midazolam the most commonly used anesthetic agents in order of frequency of use. Five patients were started on the ketogenic diet (16, 17, 19), without aborting status epilepticus, although adequacy of ketosis was never documented. In nine cases, a trial of immunosuppression was described, with most patients undergoing a combination regime of pulsed steroids [six cases (6, 16, 18–20, 22)], followed by ivIg [six cases (6, 16, 18–20, 22)], Plasma exchange [four cases (6, 16, 22, 25)] or Rituximab [two cases (19, 25)].
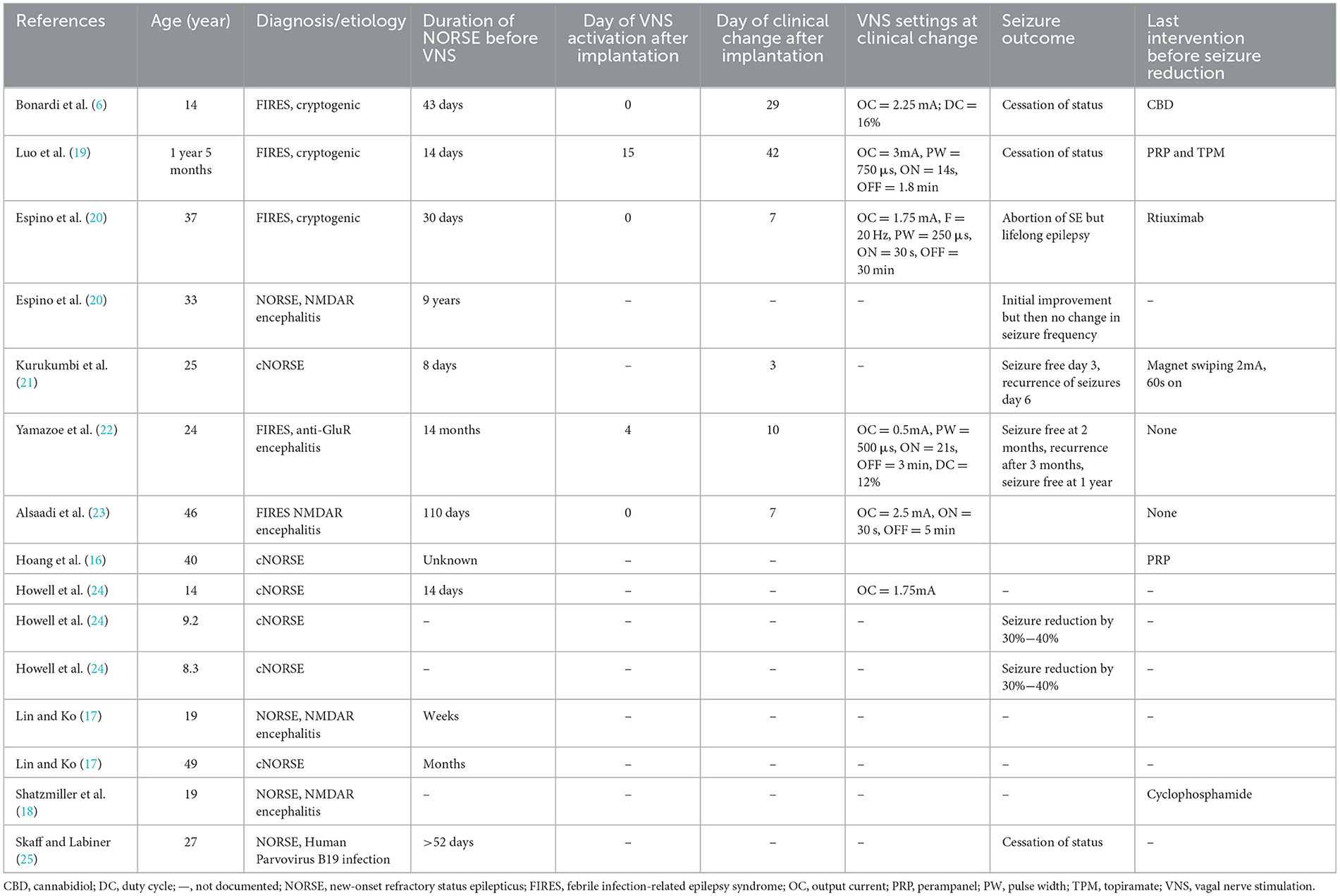
Table 1. Patient characteristics, details of VNS implantation and stimulation parameters in published NORSE cases.
3.1. Timing of VNS implantation and titration protocols
VNS was implanted in the acute phase of NORSE in five cases (range 14–30 days from onset), in the chronic phase of treatment-resistant epilepsy (TRE) in seven cases (range 43 days−9 years from onset, Table 2). For the purposes of this article, we defined the acute phase of NORSE, as occurring within the first 30 days of NORSE onset, hypothesizing this to be the phase of acute inflammation and epileptogenesis, based on our experience and the available literature on clinical, electrographic and imaging evolution in NORSE (2–4). Details of VNS parameters and titration paradigms are summarized in Table 2. When documented, VNS was activated either on the same day of implantation or within the first 2 weeks after implantation: output current was rapidly increased (range 0.25–0.75 mA/24 h) to peak amplitudes of 0.5–3 mA achieved over 7–21 days. The most commonly used initial stimulation frequencies were 20–30 Hz, pulse widths of 250–500 μs, the latter later widened to 750 μs. Duty cycle settings started in the “conventional” range (30 s on/3 min off) with increases every 2–7 days. Whilst most cases remained in the conventional cycling range, the fastest cycling documented was 7s on/14s off (24). VNS resulted in a significant clinical change in 10 cases, an average of 16.3 days after implantation when documented (range 3–42 days). Eight reports documented the last drug modification or intervention before status cessation, albeit this was performed long before the 24 h suggested by Redecker et al. (26) as the most appropriate measure for the evaluation of efficacy of an ASM in the treatment of SE: in one case Perampanel was added (16), one case had Perampanel and Topiramate introduced (19), one completed a course of Rituximab on the same day as seizures were aborted and hence may have drawn additional benefit from previous Rituximab treatments (20), one four pulses of cyclophosphamide (18) and one commenced on CBD oil (21), whilst in two cases VNS was the documented last intervention.
3.2. Outcomes
Cessation of super-refractory status was ascribed to VNS in two cases implanted in the acute (19, 20) and two in the chronic phase (21, 23). Status epilepticus is defined as refractory when it does not respond to first-line benzodiazepines and second-line antiseizure medicines, requiring general anesthesia: if refractory status persists or recurs 24 h or more after general anesthesia or recurs on withdrawing anesthetic medication it is defined as super-refractory (27, 28). Improvement in seizures but then recurrence was documented in two cases (20, 21), no effect in one (24), whilst sustained seizure reduction was documented in three cases: by 30%−40% in two (24), in one enabling weaning of anesthetic agents and leaving the ICU (23). Long-term outcomes were available for 12 cases (summarized in Table 2) and were documented between 1 month−26 years after implantation: two cases implanted in the acute phase died due to multiorgan failure or comorbidities (21, 24). Three patients were documented as seizure-free survivors, seven have ongoing chronic epilepsy (16, 20, 21, 24, 25). Bradycardia as side effect of Vagal Nerve Stimulation was the only adverse event due to VNS documented in one case (24). Functional outcomes were documented in eight survivors: the best cognitive outcome was documented in a 14-year old female (21) who resumed home schooling with normal and fluent speech. In both patients implanted in the acute and chronic phase, cognitive outcomes ranged from at least mild to severe cognitive impairment, whilst the only case described as walking without assistance was implanted in the chronic phase.
4. King's college hospital experience
Two adult cases of NORSE were implanted at our center:
Case 1—Late implantation of VNS in TRE phase of NORSE
A 54-year old female was implanted in the chronic phase of NORSE (day 67 from onset) and had failed multiple standard antiseizure medications, anesthetic agents and trials of immunosuppression (steroids, ivIg, Plasma Exchange). Our patient had also undergone an unsuccessful trial of electroconvulsive therapy and repetitive transcranial magnetic stimulation. VNS was switched on immediately after insertion, initial stimulation started with 0.5 mA output current, and gradually increased to 2 mA, 30 Hz, 500 μS, with a duty cycle of 35% (30 s ON and 1.1 min OFF). Case Ictal activity on EEG resolved on day 2 after implantation, allowing gradual tapering of clonazepam and anesthetic agents, and leaving the ICU. She died 46 days after VNS implantation due to an obstructed tracheostomy and cardiac arrest.
Case 2—Early implantation of VNS in acute phase of NORSEin pregnancy
A 30-year old pregnant female in the first Trimester of pregnancy was implanted with VNS in the acute phase (day 26 from onset) of NORSE possibly linked with drug overdose. She had also failed multiple standard antiseizure medications, anesthetic agents and trials of immunosuppression including Anakinra. VNS was switched on the day of implantation with initial output current of 0.25 mA. Output current was further uptitrated to 1 mA in the following 72 h, and increased to 1.25 mA on day 7 post-op. Our patient experienced improvement of myoclonic jerks from day 7 post-implantation and became seizure-free from day 20 post implantation. She regained functional independence during inpatient rehabilitaton, delivered a premature but healthy baby at 33 weeks and has remained seizure free to date (last reviewed 8 months from onset).
5. Discussion and perspectives for future research
Studying rare and complex diseases such as NORSE in the real clinical world is challenging, as patients may be subject to multiple and concomitant interventions and the presence of publication bias toward cases with good outcomes is very likely. Due to the paucity of cases and the variable amount of information available within each report, the level of evidence supporting the use of VNS in NORSE is low. Nevertheless, we feel that from the cases summarized in the previous sections and our experience, some general conclusions can be drawn: overall, VNS was a well-tolerated intervention without significant adverse effects in the short or long term, both in cases implanted acutely or in the TRE phase, supporting its safety even in pregnancy. Whilst it is not possible to determine a stimulation threshold effect leading to seizure cessation, most patients had VNS switched on either immediately or within the first few weeks of implantation at conventional—not high frequency—cycling rates and the output current increased over a short period of time (days to weeks). In the three cases, including ours (22, 23), where VNS was the last intervention before seizure cessation, clinical changes occurred within 7–10 days of implantation and benefit was sustained long term, in keeping with a recent meta-analysis of the effect of VNS in refractory status epilepticus (9). Beneficial effects reported include not only cessation of status but also the ability to wean anesthesia and assess patients's level of consciousness and neurological status. These positive effects were reported when VNS is implanted both in the acute and TRE phase. Since most if not all NORSE survivors go on to develop chronic epilepsy (2, 4, 5), we suggest that implanting VNS in NORSE should always be considered for its chronic neuromodulatory effect to reduce seizure burden in the long term and may also aid reducing ASM burden. Whether earlier implantation allows earlier control of status by acutely desynchronizing ictal rhythms and limiting seizure spread, and whether it would lead to better functional outcomes is unknown and should be put to the test in future trials. At King's College Hospital, a Charles Sykes Memorial Grant is supporting the set up of a multi-center “N-of-1 trial” series to study the efficacy and mechanism of action of VNS in the treatment of NORSE. N-of-1 trials are considered to be among the most relevant and rigorous study designs for assessing individual patent's treatment efficacy in rare diseases, such as NORSE, where a conventional randomized trial design would not be feasible. We will embed this study into a UK-wide NORSE network (NORSE-UK), including all major tertiary neuroscience centers in the UK, and would welcome international collaborators. Our research will develop electrophysiological and serological biomarkers to predict and monitor response to VNS in NORSE, and may become relevant for the treatment of other drug resistant forms of SE.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LMR and RS designed the work. LMR wrote the manuscript. Both authors revised the manuscript, read and approved the final version.
Funding
LMR's epilepsy research is supported by the following grants: Epilepsy Research UK Pilot Grant (PGE 2003 Mantoan Ritter), Clinical Virology Network UK and Charles Sykes Memorial Fund (through the King's College Hospital Charity, D2169/72022/Mantoan/714).
Acknowledgments
We would like to acknowledge the contributions of all clinical colleagues in managing these clinically and emotionally challenging cases, the Kings' College Hospital Charity and the Association of British Neurologists that aided the efforts of the authors and for their support of NORSE-UK, as well as patient families for allowing us to share our learning and experiences to improve NORSE patient care.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1172898/full#supplementary-material
References
1. Hirsch LJ, Gaspard N, van Baalen A, Nabbout R, Demeret S, Loddenkemper T, et al. Proposed consensus definitions for new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related conditions. Epilepsia. (2018) 59:739–44. doi: 10.1111/epi.14016
2. Mantoan Ritter L, Nashef L. New-onset refractory status epilepticus (NORSE). Pract Neurol. (2021) 21:119–27. doi: 10.1136/practneurol-2020-002534
3. Sculier C, Gaspard N. New onset refractory status epilepticus (NORSE). Seizure. (2019) 68:72–8. doi: 10.1016/j.seizure.2018.09.018
4. Gaspard N, Foreman BP, Alvarez V. New-onset refractory status epilepticus: etiology, clinical features, and outcome. Neurology. (2015) 85:1604-13. doi: 10.1212/WNL.0000000000001940
5. Cabezudo-García P, Mena-Vázquez N, Ciano-Petersen NL, Oliver-Martos B, Serrano-Castro PJ. Functional outcomes of patients with NORSE and FIRES treated with immunotherapy: a systematic review. Neurologia. (2022). doi: 10.1016/j.nrleng.2022.03.004
6. Bonardi CM, Furlanis GM, Toldo I, Guarrera B, Luisi C, Pettenazzo A, et al. Myoclonic super-refractory status epilepticus with favourable evolution in a teenager with FIRES: is the association of vagus nerve stimulation and cannabidiol effective? Brain Dev. (2023) 45:293–9. doi: 10.1016/j.braindev.2023.01.004
7. Elliott RE, Morsi A, Kalhorn SP, Marcus J, Sellin J, Kang M, et al. Vagus nerve stimulation in 436 consecutive patients with treatment-resistant epilepsy: long-term outcomes and predictors of response. Epilepsy Behav. (2011) 20:57–63. doi: 10.1016/j.yebeh.2010.10.017
8. Englot DJ, Hassnain KH, Rolston JD, Harward SC, Sinha SR, Haglund MM. Quality-of-life metrics with vagus nerve stimulation for epilepsy from provider survey data. Epilepsy Behav. (2017) 66:4–9. doi: 10.1016/j.yebeh.2016.10.005
9. Dibué-Adjei M, Brigo F, Yamamoto T, Vonck K, Trinka E. Vagus nerve stimulation in refractory and super-refractory status epilepticus - a systematic review. Brain Stimul. (2019) 12:1101–10. doi: 10.1016/j.brs.2019.05.011
10. Cunningham JT, Mifflin SW, Gould GG, Frazer A. Induction of c-Fos and DeltaFosB immunoreactivity in rat brain by vagal nerve stimulation. Neuropsychopharmacology. (2008) 33:1884–95. doi: 10.1038/sj.npp.1301570
11. Raedt R, Clinckers R, Mollet L, Vonck K, El Tahry R, Wyckhuys T, et al. Increased hippocampal noradrenaline is a biomarker for efficacy of vagus nerve stimulation in a limbic seizure model. J Neurochem. (2011) 117:461–9. doi: 10.1111/j.1471-4159.2011.07214.x
12. Marrosu F, Serra A, Maleci A, Puligheddu M, Biggio G, Piga M. Correlation between GABA(A) receptor density and vagus nerve stimulation in individuals with drug-resistant partial epilepsy. Epilepsy Res. (2003) 55:59–70. doi: 10.1016/S0920-1211(03)00107-4
13. Ravan M, Sabesan S, D'Cruz O. On quantitative biomarkers of VNS therapy using EEG and ECG signals. IEEE Trans Biomed Eng. (2017) 64:419–28. doi: 10.1109/TBME.2016.2554559
14. Johnson RL, Wilson CG. A review of vagus nerve stimulation as a therapeutic intervention. J Inflamm Res. (2018) 11:203–13. doi: 10.2147/JIR.S163248
15. Zeiler FA, Zeiler KJ, Teitelbaum J, Gillman LM, West M, VNS. for refractory status epilepticus. Epilepsy Res. (2015) 112:100–13. doi: 10.1016/j.eplepsyres.2015.02.014
16. Hoang Q, Wohlt P, Rosenberg N. Treatment of super-refractory status epi-lepticus with perampanel in an intensive care unit. Crit Care Med. (2014) 42:A1652. doi: 10.1097/01.ccm.0000458717.41927.59
17. Lin K, Ko D. The use of ketogenic diet and vagus nerve stimulation in the setting of refractory status epilepticus in adults. Epilepsy Curr. (2012) 12:234. Available online at: http://epilepsycurrents.org/doi/pdf/10.5698/1535-7511-12.s1.1
18. Shatzmiller RA, Apelian RG, Cho J, Ko D, Millett DE. Asian woman presenting with new onset refractory status epilepticus: cyclophosphamide-responsive NMDA receptor encephalitis without tumor. Epilepsy Curr. (2011) 11(Suppl. 1).
19. Luo T, Wang Y, Lu G, Zhou Y, Wang Y. Vagus nerve stimulation for super-refractory status epilepticus in febrile infection-related epilepsy syndrome: a pediatric case report and literature review. Childs Nerv Syst. (2022) 38:1401–4. doi: 10.1007/s00381-021-05410-6
20. Espino PH, Burneo JG, Moscol G, Gofton T, MacDougall K, Suller Marti A. Long-term outcomes after NORSE: treatment with vagus nerve stimulation. Epilepsia Open. (2022) 7:822–8. doi: 10.1002/epi4.12654
21. Kurukumbi M, Leiphart J, Asif A, Wang J. Vagus nerve stimulation (VNS) in super refractory new onset refractory status epilepticus (NORSE). Case Rep Neurol Med. (2019) 2019:7852017. doi: 10.1155/2019/7852017
22. Yamazoe T, Okanishi T, Yamamoto A, Yamada T, Nishimura M, Fujimoto A, et al. New-onset refractory status epilepticus treated with vagus nerve stimulation: a case report. Seizure. (2017) 47:1e4. doi: 10.1016/j.seizure.2017.02.011
23. Alsaadi T, Shakra M, Turkawi L, Hamid J. VNS terminating refractory non-convulsive SE secondary to anti-NMDA encephalitis: a case report. Epilepsy Behav Case Rep. (2015) 3:39e42. doi: 10.1016/j.ebcr.2015.02.003
24. Howell KB, Katanyuwong K, Mackay MT, Bailey CA, Scheffer IE, Freeman JL, et al. Long-term follow-up of febrile infection-related epilepsy syndrome. Epilepsia. (2012) 53:101e10. doi: 10.1111/j.1528-1167.2011.03350.x
25. Skaff PT, Labiner DM. Status epilepticus due to human parvovirus B19 encephalitis in an immunocompetent adult. Neurology. (2001) 57:1336e7. doi: 10.1212/WNL.57.7.1336
26. Redecker J, Wittstock M, Rosche J. The efficacy of different kinds of intravenously applied antiepileptic drugs in the treatment of status epilepticus. How can it be determined? Epilepsy Behav. (2017) 71(Pt A):35e8. doi: 10.1016/j.yebeh.2017.03.018
27. Shorvon S, Ferlisi M. The treatment of super-refractory status epilepticus: a critical review of available therapies and a clinical treatment protocol. Brain. (2011) 134: 02–2818 doi: 10.1093/brain/awr215
Keywords: febrile infection-related epilepsy syndrome (FIRES), new onset refractory status epilepticus (NORSE), vagal nerve stimulation (VNS), neuromodulation, refractory status epilepticus (RSE)
Citation: Mantoan Ritter L and Selway R (2023) Perspective: Vagal nerve stimulation in the treatment of new-onset refractory status epilepticus. Front. Neurol. 14:1172898. doi: 10.3389/fneur.2023.1172898
Received: 24 February 2023; Accepted: 30 March 2023;
Published: 20 April 2023.
Edited by:
Julia Jacobs, University of Freiburg Medical Center, GermanyReviewed by:
Jay Gavvala, University of Texas Health Science Center at Houston, United StatesCopyright © 2023 Mantoan Ritter and Selway. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Mantoan Ritter, bGF1cmEubWFudG9hbkBrY2wuYWMudWs=
 Laura Mantoan Ritter
Laura Mantoan Ritter Richard Selway1
Richard Selway1