- 1Acupuncture and Moxibustion College, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
- 2Gastroenterology Department, Yongchuan Traditional Chinese Medicine Hospital Affiliated to Chongqing Medical University, Chongqing, China
- 3Acupuncture and Moxibustion College, Nanjing University of Traditional Chinese Medicine, Nanjing, Jiangsu, China
Background: Dysphagia is one of the common complications after stroke. It is closely related to lung infection and malnutrition. Neuromuscular electrical stimulation (NMES) is widely used in the treatment of post-stroke dysphagia, but the evidence-based medical evidence of NMES is limited. Therefore, this study aimed to evaluate the clinical efficacy of NMES in patients with post-stroke dysphagia by systematic review and meta-analysis.
Methods: We searched the CNKI, Wanfang, VIP, SinoMed, PubMed, Embase, Cochrane Library, and Web of Science databases for all randomized controlled trials (RCTs) of NMES in the treatment of post-stroke dysphagia from the establishment of the database to 9 June 2022. The risk of bias assessment tool recommended by Cochrane and the GRADE method was used to assess the risk of bias and the quality of evidence. RevMan 5.3 was used for statistical analysis. Sensitivity and subgroup analyses were performed to evaluate the intervention effect more specifically.
Results: A total of 46 RCTs and 3,346 patients with post-stroke dysphagia were included in this study. Our meta-analysis showed that NMES combined with routine swallowing therapy (ST) could effectively improve swallowing function in Penetration-Aspiration Scale (MD = −0.63, 95% CI [−1.15, −0.12], P = 0.01), Functional Oral Intake Scale (MD = 1.32, 95% CI [0.81, 1.83], P < 0.00001), Functional Dysphagia Scale (MD = − 8.81, 95% CI [−16.48, −1.15], P = 0.02), the Standardized Swallowing Assessment (MD = −6.39, 95% CI [−6.56, −6.22], P < 0.00001), the Videofluoroscopic Swallow Study (MD = 1.42, 95% CI [1.28, 1.57], P < 0.00001) and the Water swallow test (MD = −0.78, 95% CI [−0.84, −0.73], P < 0.00001). Furthermore, it could improve the quality of life (MD = 11.90, 95% CI [11.10, 12.70], P < 0.00001), increase the upward movement distance of hyoid bone (MD = 2.84, 95% CI [2.28, 3.40], P < 0.00001) and the forward movement distance of hyoid bone (MD = 4.28, 95% CI [3.93, 4.64], P < 0.00001), reduce the rate of complications (OR = 0.37, 95%CI [0.24, 0.57], P < 0.00001). Subgroup analyses showed that NMES+ST was more effective at 25 Hz, 7 mA or 0–15 mA, and at courses ( ≤ 4 weeks). Moreover, patients with an onset of fewer than 20 days and those older than 60 years appear to have more positive effects after treatment.
Conclusion: NMES combined with ST could effectively increase the forward and upward movement distance of the hyoid bone, improve the quality of life, reduce the rate of complications, and improve the swallowing function of patients with post-stroke dysphagia. However, its safety needs to be further confirmed.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO, identifier: CRD42022368416.
1. Introduction
Stroke has become the second leading cause of death and the first leading cause of disability worldwide due to its high morbidity, disability, and mortality (1, 2). Moreover, some studies have shown that dysphagia is one of the most common complications in stroke patients. Approximately 37%−78% of stroke patients have dysphagia (3). The clinical manifestations of dysphagia include swallowing disorder, drinking cough, salivation, and other symptoms. Dysphagia after stroke is closely related to malnutrition, dehydration, electrolyte disorder, pulmonary infection, anxiety, and depression (4, 5), and it also leads to prolonged hospitalization, decreased quality of life, and further increased risk of death (6). Currently, swallowing therapy (ST) is mainly used for post-stroke dysphagia, including swallowing muscle strength and coordination exercises, posture changes, and diet adjustments (7).
However, the single ST takes a long time and has poor patient compliance, with a limited effect on severe dysphagia. More than 10% of patients have residual swallowing problems after ST (8). Therefore, how to effectively improve the swallowing function of patients, achieve oral feeding, and reduce the rate of complications is of great significance (4).
Neuromuscular electrical stimulation (NMES) has been a promising treatment for dysphagia in recent years. It can improve swallowing function by stimulating peripheral nerves to trigger swallowing muscle contraction, promote motor cortex repair, and enhance motor relearning ability (9).
Although some reviews claimed that NMES contributed to the rehabilitation of patients with dysphagia after stroke (10, 11), the number of evaluation measures used in these reviews is small, the number of included trials is limited, and the frequency, current intensity, and duration of electrical stimulation are not explored. Therefore, our study conducted a meta-analysis of the clinical efficacy of NMES in the treatment of post-stroke dysphagia in recent years to further provide valuable guidance and evidence-based medical evidence for the clinical use of NMES in the treatment of post-stroke dysphagia.
2. Methods
2.1. Protocol and registration
This study followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (12), and the protocol has been registered with PROSPERO (Registration number: CRD42022368416).
2.2. Data sources and search strategy
We searched eight scientific databases, namely, CNKI, Wanfang, VIP, SinoMed, PubMed, Embase, Cochrane Library, and Web of Science. The retrieval time was from the establishment of the database to 9 June 2022. There were no restrictions concerning publication source or language. The searched MeSH terms are listed as follows: [“Transcutaneous Electric Stimulation”[MeSH] OR “Percutaneous Electric Nerve Stimulation” OR “Percutaneous Neuromodulation Therapy” OR “TENS” OR “PENS”] AND [“Stroke”[MeSH] OR “cerebral hemorrhage” OR “cerebral ischemia” OR “cerebrovascular disease”] AND [“Dysphagia”[MeSH] OR “Deglutition Disorder” OR “Swallowing Disorder”] AND [“randomized controlled trial”[MeSH] OR “RCT”]. In addition, a supplementary search was conducted for the references included in the literature. Specific information is given in the Supplementary material.
2.3. Inclusion criteria
The inclusion criteria for this meta-analysis were as follows: (1) patients with ischemic or hemorrhagic stroke with clear imaging evidence of relevant pathology on magnetic resonance imaging (MRI) or computed tomography (CT); (2) patients with dysphagia after stroke diagnosed by clinical examination; (3) the participant with no other neurological diseases or other dysphagia; and (4) the same ST intervention (acupuncture, transcranial electrical stimulation, and transcranial magnetic stimulation are not included) performed in the experimental and control groups except the experimental group that received NMES.
2.4. Exclusion criteria
The exclusion criteria for this meta-analysis were as follows: (1) non-RCT studies, such as cross-sectional studies, case–control studies, case reports, systematic reviews, and animal experiments; (2) studies in which the baseline consistency test was not given; (3) studies with incomplete data, or studies whose full text could not be obtained; and (4) repeatedly published articles.
2.5. Outcomes
The outcome indicators were as follows: (1) Functional Oral Intake Scale (FOIS); (2) Penetration-Aspiration Scale (PAS–Fluid); (3) Functional Dysphagia Scale (FDS); (4) the Swallowing Quality of Life questionnaire (SWAL-QOL); (5) the forward movement distance of the hyoid bone (FMHB); (6) the upward movement distance of the hyoid bone (UMHB); (7) the complication rate (CR); (8) the Standardized Swallowing Assessment (SSA); (9) the water swallow test (WST); and (10) the videofluoroscopic swallow study (VFSS).
2.6. Data extraction and management
The retrieved literature was imported into EndNote software for unified management. Two researchers (YW and LX) performed literature screening independently according to the proposed inclusion and exclusion criteria. First, we used EndNote to exclude duplicate literature and then conducted the preliminary screening. The two researchers independently read the title of the literature, keywords, and abstracts and initially excluded the documents that did not meet the inclusion criteria; after that, they downloaded and read the full text to determine whether the literature met the inclusion criteria. If necessary, we would contact the original author by mail or phone to obtain undetermined but important information for this study. The researchers independently extracted the data by a pre-designed data extraction form. The data extraction included (1) basic information about the study: research topic, first author, and publication year; (2) the number of cases, intervention, and course of treatment; (3) key elements of bias risk of assessment; and (4) outcome indicators and outcome statistics concerned. If there was any disagreement, it would be referred to a third researcher (LZ) to determine the final result.
2.7. Assessment of risk of bias
According to the bias of risk assessment tool recommended by Cochrane (13), the included literature studies were evaluated, including the random sequence production of the literature, the allocation concealment, the implementation of the blind method, whether the blind method was implemented for the result evaluation, the integrity of the result data, whether the results were selectively reported, and whether there were other biases. When the evaluators (YW and LX) had different opinions, they would discuss or ask for a third party (LZ). The risk of bias figure was drawn by RevMan5.3 software.
2.8. Data synthesis and statistical analysis
2.8.1. Measurement of therapeutic effects
In this study, odds ratio (OR) with a corresponding 95% confidence interval (CI) was used for binary variable data, and mean difference (MD) was used for continuous variable data.
2.8.2. Assessment of heterogeneity
After extracting and collating relevant data, this study used RevMan5.3 software for data analysis, and then used I2 statistics and Q-test (χ2) to assess the heterogeneity of results. The heterogeneity was considered low when P > 0.10 and I2 <50% (14, 15). The heterogeneity was considered high when P < 0.10 or I2 > 50%.
2.8.3. Data synthesis
If the heterogeneity of each group was small (P > 0.10, I2 <50%), the fixed effect model was used. When the heterogeneity was considerable (P < 0.10, I2 > 50%), the random effect model was used after excluding the influence of significant clinical heterogeneity.
2.8.4. Subgroup analysis and sensitivity analysis
When P < 0.10 or I2 > 50% in the χ2 test, the source of heterogeneity was identified by extracting eligible articles one by one to make the sensitivity analysis. If not, subgroup analyses would be performed to identify the sources of heterogeneity according to age, duration of disease, duration of treatment, intensity of electrical stimulation, and frequency of electrical stimulation.
2.8.5. Grading of quality of evidence
This study used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method (16) to assess the quality of evidence. GRADE divides the quality of evidence into four levels: High quality: further research is unlikely to change our confidence in effect estimates. Medium quality: further research may significantly impact our confidence in effect estimates and may change estimates. Low quality: further research is likely to have a meaningful impact on our confidence in effect estimates and may change estimates. Very low quality: any estimate of the effect is very uncertain (17). Two researchers (YW and LX) independently assessed the quality of the relevant evidence, and a third researcher (LZ) was notified of any disagreement for consultation.
3. Result
3.1. Literature search results
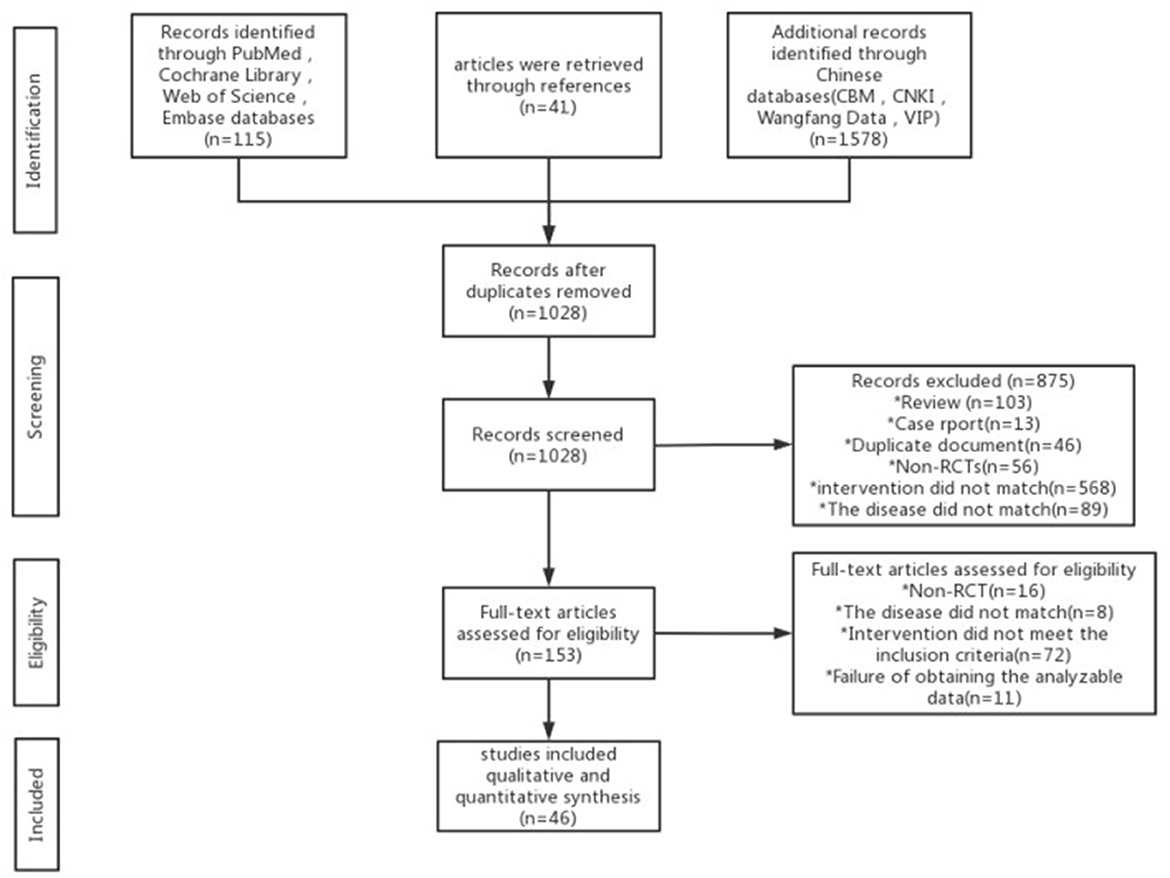
A total of 1,734 articles were retrieved, and 706 duplicate articles were excluded. After reading the title and abstract, we excluded 875 articles. After reading the complete text, 107 articles were excluded, and 46 articles were included finally. The research selection process is detailed in Figure 1.
3.2. Characteristics of the included studies
A total of 46 RCT studies were included, including 3,346 patients with post-stroke dysphagia. Among them, 1,679 patients received NMES + ST, and the other 1,667 received ST. The included studies were from China, the United States, Britain, Italy, Spain, and South Korea, and the treatment course ranged from 2 to 12 weeks, as shown in Table 1.
3.3. Bias risk evaluation of the included studies
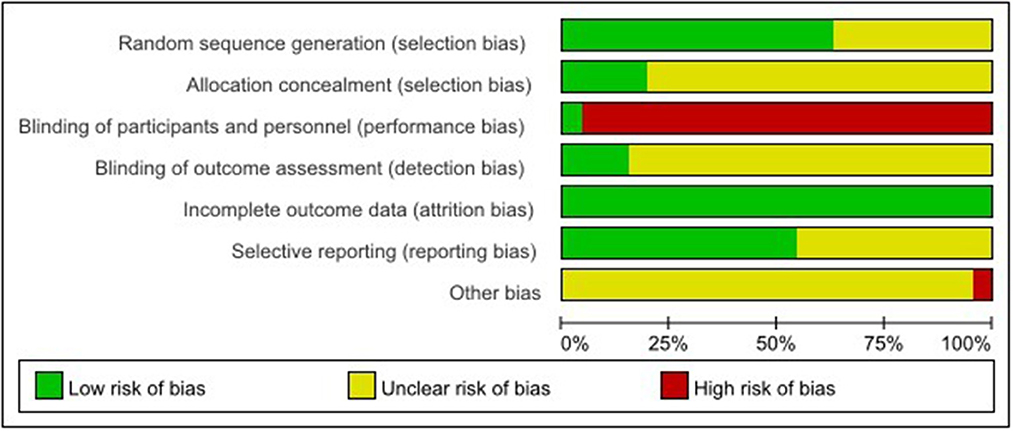
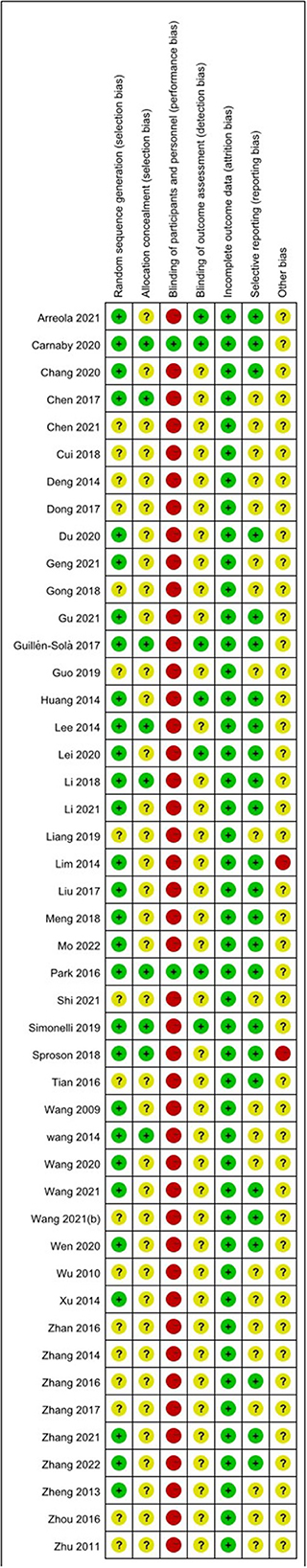
The risk of bias assessment is shown in Figures 2, 3.
3.3.1. Generation of random sequence
Among the 46 studies included, 29 studies (18, 20, 22, 23, 25–27, 29, 32–34, 36, 37, 39, 42, 46, 48, 51–62) selected and reported appropriate randomization methods, such as random number table, so they were assessed as low risk of bias, the other 17 studies (19, 21, 24, 28, 30, 31, 35, 38, 40, 41, 43–45, 47, 49, 50, 63) only mentioned randomization allocation, so they were assessed as having the unclear risk of bias.
3.3.2. Allocation concealment
Nine studies (20, 32, 51–53, 55, 57, 59, 62) followed the appropriate protocol to hide treatment allocation, so they were considered to have a low risk of bias. A total of 37 studies did not mention whether they followed the allocation hiding principle, so they were assessed as having an unclear risk of bias.
3.3.3. Blinding of participants and personnel
Two studies (55, 57) explicitly proposed that the control group used sham NMES to blind participants and personnel, so they were assessed as having a low risk of bias because studies did not show that the control group received sham NMES. Instead, they only mentioned that the control group received ST and the experimental group received NMES + ST. We considered that participants were not blinded and rated studies as high risk of bias.
3.3.4. Blinding of outcome assessment
Seven studies (26, 53, 55–58, 62) reported the blinding of outcome assessment, identifying it as a low risk of bias. The other 39 studies did not report whether to adopt the blinding of outcome assessment, identified as the unclear risk of bias.
3.3.5. Incomplete outcome information
All studies thoroughly reported the test results data, so they were identified as having a low risk of bias.
3.3.6. Selective reporting of study results
A total of 25 studies (18, 22, 25–27, 29, 31, 33, 35, 37, 39, 46, 51–63) reported the registration and ethical review of clinical RCTs, so the risk of bias was low. The remaining 21 studies were identified as having an unclear risk of bias.
3.3.7. Other bias sources
Because there were high drop-out rates in two (52, 60) studies, we rated them as high risk. In the remaining 44 studies, we did not observe any potential study bias, so they were identified as the unclear risk of bias.
3.4. Results of the meta-analysis
3.4.1. Standardized swallowing assessment
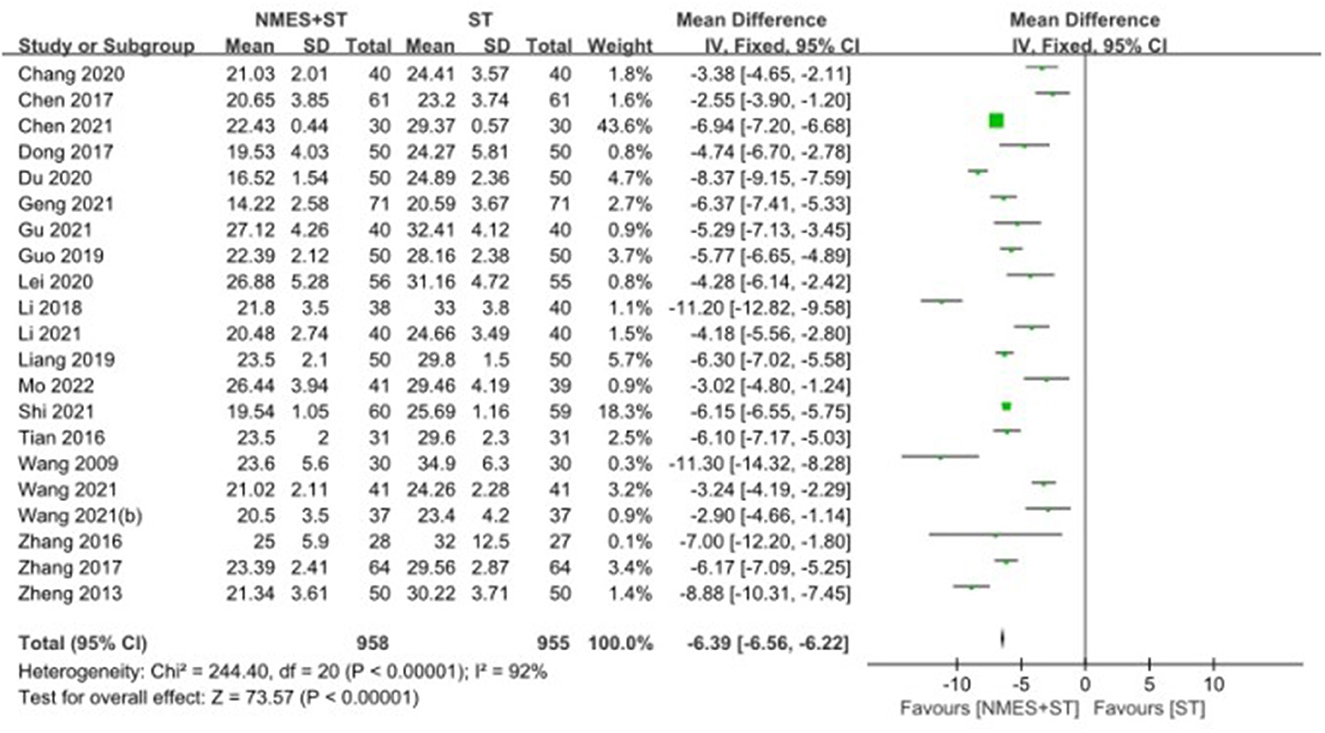
A total of 21 studies reported changes in SSA in patients with post-stroke dysphagia after NMES + ST. The effect of NMES + ST on the improvement of swallowing function in patients with post-stroke dysphagia was better than single ST, and the difference was statistically significant [MD = −6.39, 95% CI (−6.56, −6.22), P < 0.00001, I2 = 92%], but there was heterogeneity as shown in Figure 4.
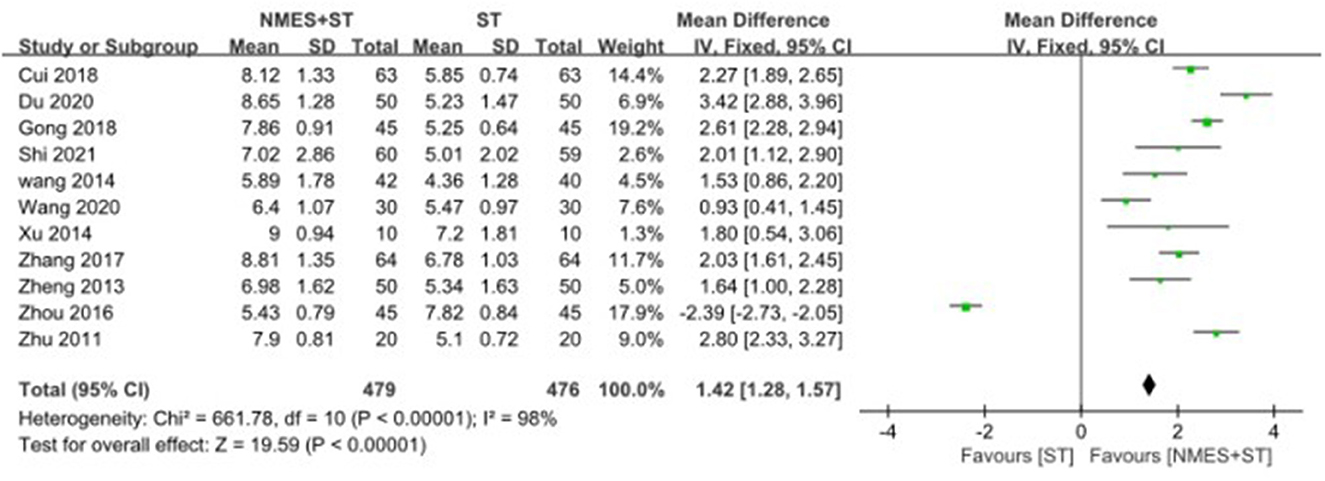
3.4.2. Videofluoroscopic swallow study
A total of 11 studies reported changes in VFSS after treatment, and NMES + ST was more effective [MD = 1.42, 95% CI (1.28, 1.57), P < 0.00001, I2 = 98%], but there was a high heterogeneity as shown in Figure 5.
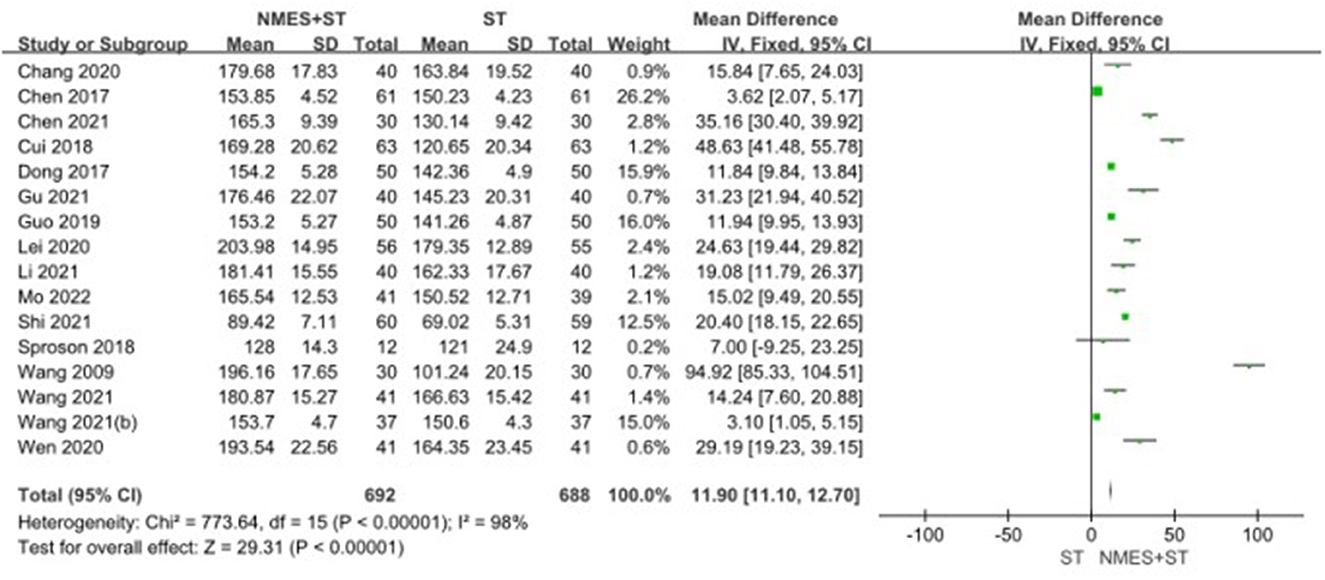
3.4.3. The swallowing quality of life questionnaire
A total of 16 studies reported changes in SWAL-QOL scores after treatment. Compared with ST, NMES + ST improved the quality of life of patients with dysphagia more significantly [MD = 11.90, 95% CI (11.10, 12.70), P < 0.00001, I2 = 98%]. Nevertheless, there was heterogeneity as shown in Figure 6.
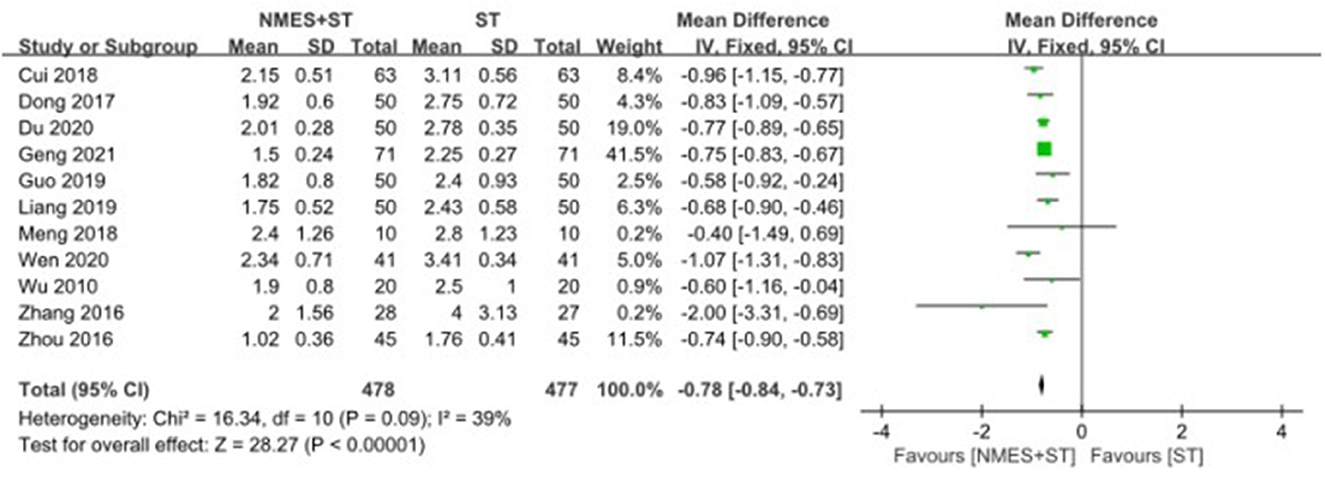
3.4.4. Water swallow test
A total of 11 studies used the water swallow test to evaluate the swallowing function of patients. The efficacy of NMES + ST was better than that of single ST, with the statistical difference [MD = −0.78, 95% CI (−0.84, −0.73), P < 0.00001, I2 = 39%], as shown in Figure 7.
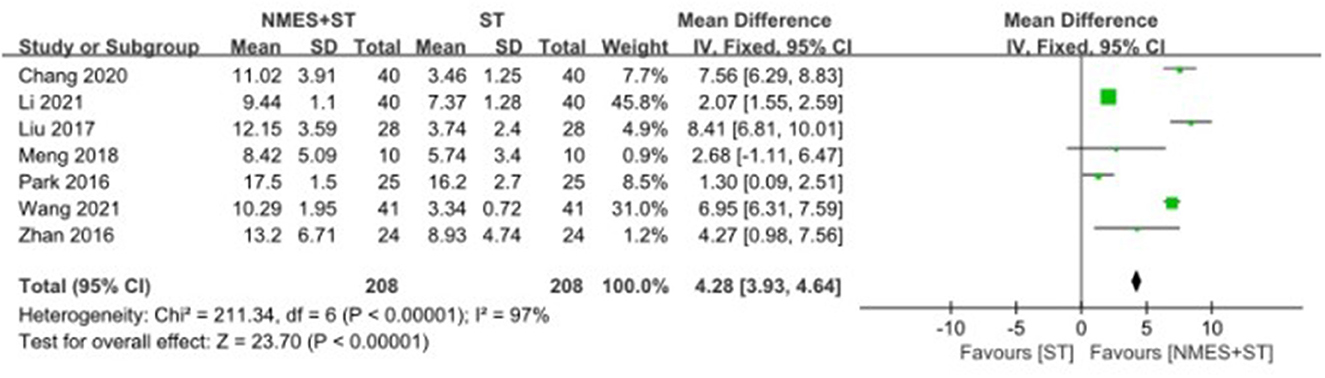
3.4.5. Forward movement distance of the hyoid bone
Seven studies reported changes in the forward movement distance of the hyoid bone after treatment. NMES + ST increased the forward movement distance of hyoid bone compared with single ST [MD = 4.28, 95% CI (3.93, 4.64), P < 0.00001, I2 = 97%], but there was heterogeneity, as shown in Figure 8.
3.4.6. Upward movement distance of the hyoid bone
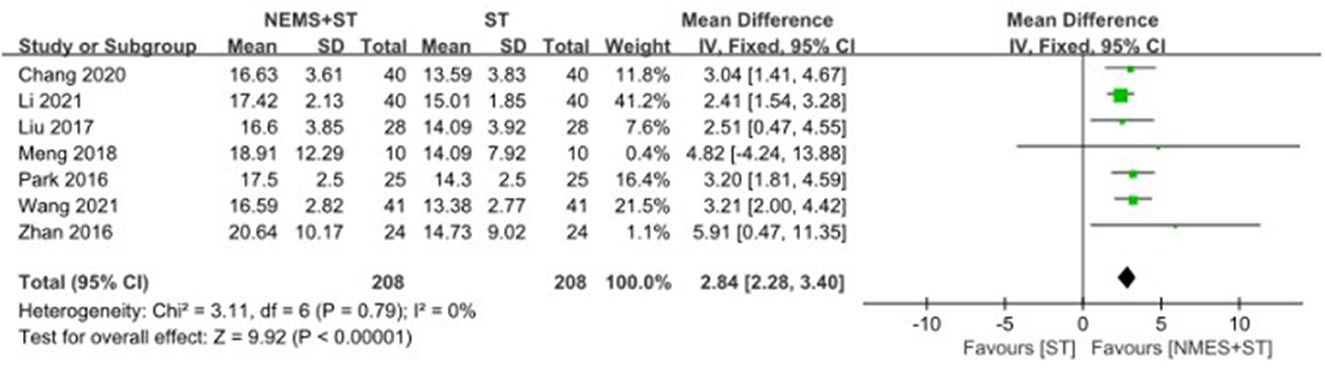
Seven studies reported changes in the upward movement distance of the hyoid bone after treatment. NMES + ST significantly increased the upward movement distance of the hyoid bone compared with ST [MD = 2.84, 95% CI (2.28, 3.40), P < 0.00001, I2 = 0%], as shown in Figure 9.
3.4.7. Complication rate
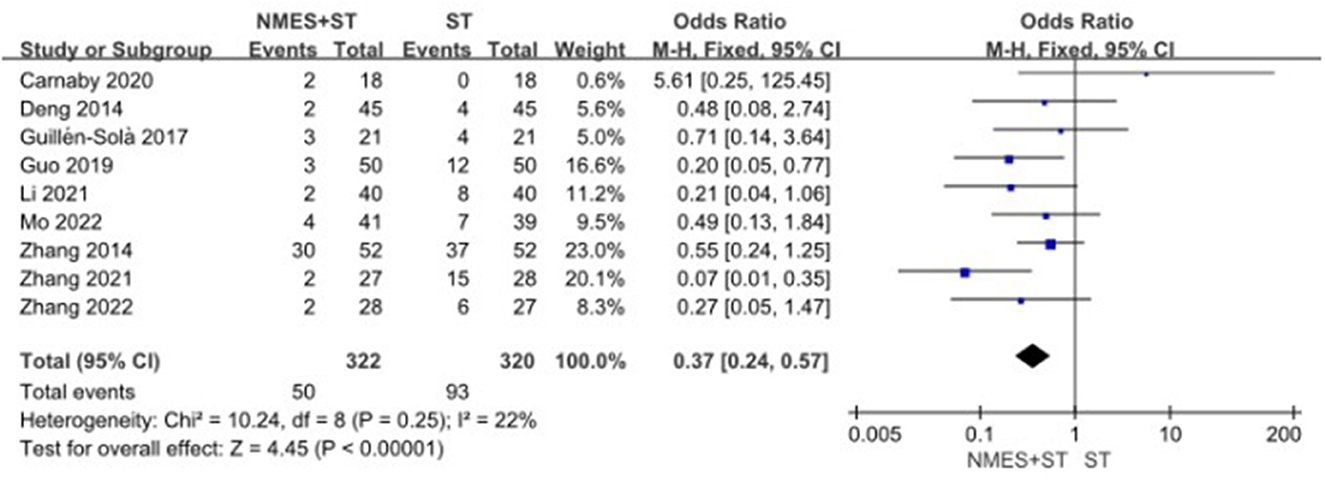
Nine studies evaluated and recorded the rate of complications in patients with dysphagia. The results showed that NMES + ST could significantly reduce the rate of complications such as pneumonia and malnutrition compared with single ST (OR = 0.37 95% CI [0.24, 0.57], P < 0.00001, I2 = 22%), as shown in Figure 10.
3.4.8. Penetration-aspiration scale
Six studies reported changes in PAS after treatment, with NMES + ST achieving better clinical efficacy than ST [MD = −0.63, 95% CI (−1.15, −0.12), P = 0.01, I2 = 14%; as shown in the Supplementary material].
3.4.9. Functional oral intake scale
Five studies reported changes in FOIS after treatment, and NMES + ST was better than ST [MD = 1.32, 95% CI (0.81, 1.83), P < 0.00001, I2 = 18%; as shown in the Supplementary material].
3.4.10. Functional dysphagia scale
Three studies evaluated swallowing function according to FDS, and the clinical effect of NMES + ST may be more positive [MD = −8.81, 95% CI (−16.48, −1.15), P = 0.02, I2 = 57%; as shown in the Supplementary material].
3.5. Subgroup analysis
3.5.1. Subgroup analysis of SSA
A subgroup analysis showed that 25 Hz electrical stimulation [MD = −7.00, 95% CI (−12.20, −1.80), P = 0.008] had a more positive clinical effect on dysphagia after stroke than 10–50 Hz electrical stimulation [MD = −6.17, 95% CI (−7.09, −5.25), P < 0.00001], 30–80 Hz electrical stimulation [MD = −5.62, 95% CI (−8.18, −3.06), P < 0.0001], 40–80 Hz electrical stimulation [MD = −3.02, 95% CI (−4.80, −1.24), P = 0.0009], 80 Hz electrical stimulation [MD = −5.13, 95% CI (−7.68, −2.59), P < 0.0001]. In the study, 7 mA electrical stimulation [MD = −11.20, 95% CI (−12.82, −9.58), P < 0.00001] was better than 0–25 mA [MD = −5.83, 95% CI (−7.48, −4.19), P < 0.00001], 0–15 mA [MD = −8.08, 95% CI (−11.80, −4.37), P < 0.0001], 5–11 mA [MD = −3.85, 95% CI (−6.53, −1.16), P = 0.005], 5–25 mA [MD = −6.37, 95% CI (−6.99, −5.75), P < 0.00001], 0–30 mA [MD = −4.28, 95% CI (−6.14, −2.42), P < 0.00001]. A 4-week treatment course [MD = −6.29, 95% CI (−7.16, −5.42), P < 0.00001] might have better clinical efficacy, and older patients (age > 60 years old; MD = −6.33, 95% CI [−7.10, −5.56], P < 0.00001) may have a more significant positive effect on post-stroke dysphagia than younger patients (age <60 years old; MD = −4.58, 95% CI [−6.03, −3.14], P < 0.00001). However, in the duration of each treatment and course of the disease, there is no statistical difference between subgroups (as shown in the Supplementary material).
3.5.2. Subgroup analysis of VFSS
The subgroup analysis showed that the treatment group within 4 weeks [MD = 2.24, 95% CI (1.62, 2.86), P < 0.00001] was better than the 4-week treatment group [MD = 2.09, 95% CI (1.46, 2.71), P < 0.00001] and the treatment group over 4 weeks [MD = −2.39, 95% CI (−2.73, −2.05), P < 0.00001]. There was no significant difference in clinical efficacy between subgroups in age, course of the disease, duration of each treatment, intensity of electrical stimulation, and frequency of electrical stimulation (as shown in the Supplementary material).
3.5.3. Subgroup analysis of SWAL-QOL
Subgroup analysis showed that 0–15 mA electrical stimulation [MD = 94.92, 95% CI (85.33, 104.51), P < 0.00001] was compared with 0–25mA [MD = 21.00, 95% CI (10.04, 31.96), P = 0.0002], 5–11 mA [MD = 27.68, 95% CI (−3.73, 59.09), P = 0.08], 0–30 mA [MD = 24.63, 95% CI (19.44, 29.82), P < 0.00001], 14–20 mA [MD = 29.19, 95% CI (19.23, 39.15), P < 0.00001] might produce better influence. Patients [the day from onset <20 days; MD = 30.32, 95% CI (12.27, 48.37), P = 0.001] may have better clinical efficacy, and older patients (age > 60 years old; MD = 27.50, 95% CI [18.58, 36.42], P < 0.00001) was better than younger patients (age <60 years old; MD = 13.25, 95% CI [5.67, 20.84], P = 0.0006). However, there were no statistical differences between subgroups in electrical stimulation frequency, course of treatment, and duration of each treatment (as shown in the Supplementary material).
3.6. Publication bias
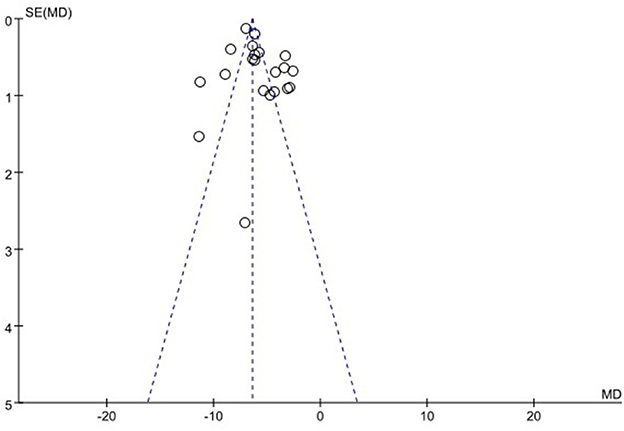
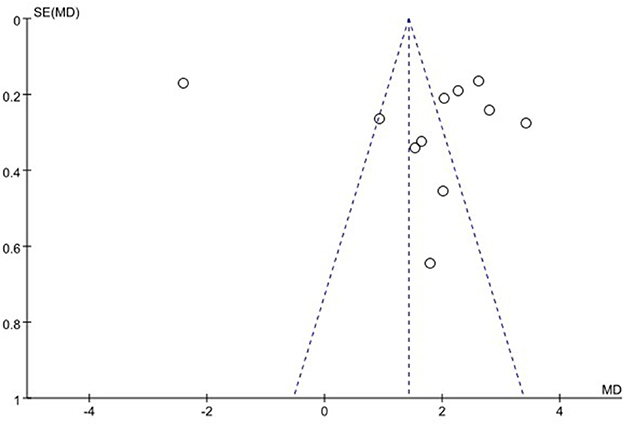
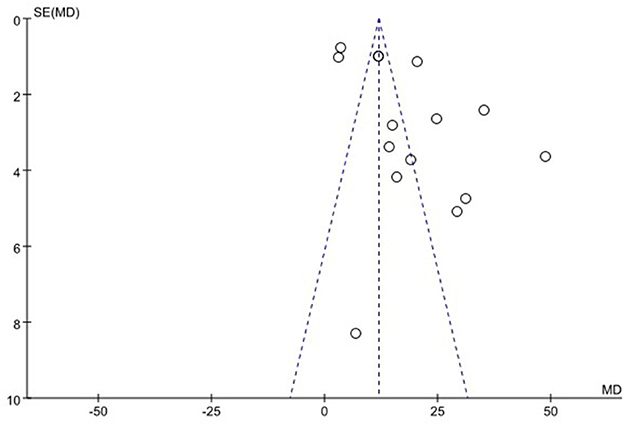
We used funnel plots to evaluate the publication bias of SSA, VFSS, SWAL-QOL, and WST, respectively. The results showed that the funnel plots of SSA and WST were relatively symmetric, and they might not have publication bias, while the funnel plots of VFSS and SWAL-QOL were asymmetric. It might have publication bias, as shown in Figures 11–13, Supplementary material.
3.7. Sensitivity analysis
Sensitivity analysis was performed because I2 of SSA (92%), VFSS (98%), SWAL-QOL (98%), FMHB (97%), and FDS (57%) were >50%. After sensitivity analysis of FDS, we excluded one study that could have led to high heterogeneity. FDS analysis showed the disappearance of high heterogeneity (I2 = 0; Supplementary material). However, after sensitivity analysis of other relevant literature data, the responsible articles leading to high heterogeneity were not determined. During the analysis, we found that some subgroups still had high heterogeneity. Subsequently, sensitivity analysis was conducted again for each subgroup, but the source of heterogeneity was still not identified. Therefore, meta-regression analysis was used to explain the high heterogeneity. However, it was not performed due to the small number of studies included in FMHB (as shown in the Supplementary material).
3.7.1. Heterogeneity of SSA
According to the results of the meta-regression analysis, the high heterogeneity of SSA may be related to age (P = 0.032), but there was not significantly associated with the course of treatment (P = 0.160), course of disease (P = 0.091), duration of each treatment (P = 0.096), the intensity of electrical stimulation (P = 0.320), and frequency of electrical stimulation (P = 0.802).
3.7.2. Heterogeneity of SWAL-QOL
The heterogeneity of SWAL-QOL results might be due to the age (P = 0.024), course of treatment (P = 0.003), course of disease (P = 0.002), and duration of each treatment (P = 0.003), but there was no significant correlation with frequency of electrical stimulation (P = 0.782) and intensity of electrical stimulation (P = 0.287).
3.7.3. Heterogeneity of VFSS
The heterogeneity of VFSS results might be due to the course of treatment (P = 0.022), but it might not be related to age (P = 0.147), course of disease (P = 0.345), treatment time (P = 0.124), the intensity of electrical stimulation (P = 0.459), and frequency of electrical stimulation (P = 0.542).
3.8. Quality of evidence
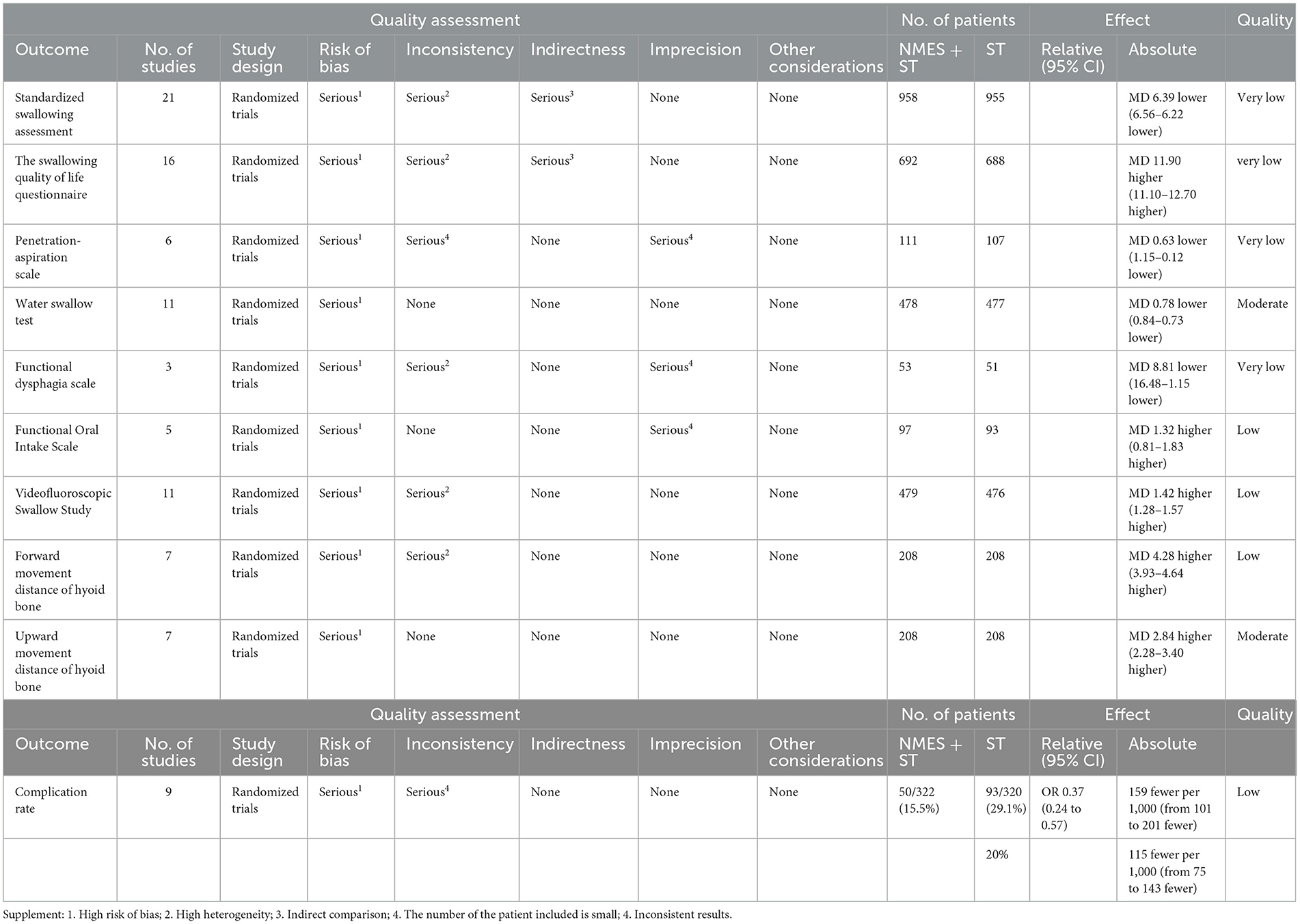
After the GRADE evaluation, the quality of evidence: WST and UMHB were rated as moderate quality. VFSS, CR, FMHB, and FOIS were rated as low quality. PAS, FDS, SSA, and SWAL-QOL were rated as very low quality. The low quality included the high risk of bias in the included studies, insufficient sample size, high heterogeneity, indirect comparison of trial results, and inconsistency as shown in Table 2.
3.9. Adverse events
Nine studies reported adverse events. Five studies (50–53, 62) reported that during the study period, patients in the experimental group did not report any adverse events, while Wang et al. (33) reported that one patient had a skin allergy to the electrode patch; one patient had a peculiar smell in the mouth, and the adverse reactions disappeared after stopping treatment. Arreola (56) and Lim (60) show that two patients reported skin tingling, which disappeared after electrical stimulation was stopped. Zhang et al. (63) reported that seven patients had localized skin redness or allergic reactions in the area of electrode placement, and the adverse reactions disappeared after the treatment was stopped, and no one withdrew because of skin reactions.
4. Discussion
This meta-analysis showed that NMES + ST could effectively increase the forward and upward movement distance of the hyoid bone, improve the quality of life of patients with dysphagia, reduce the rate of complications, and improve the swallowing function of patients.
In addition, subgroup analysis based on the course of the disease found that NMES + ST may have a more significant clinical influence on patients (the day from onset <20 days). This subgroup results emphasize the importance of early treatment of dysphagia after stroke, which may be related to the fact that NMES can enhance pharyngeal sensory feedback pathways, promote cortical reorganization, and increase pharyngeal motor performance in the contralateral motor cortex (64–66). Early treatment may be more effective in promoting cortical reorganization.
The subgroup analysis based on the course of treatment showed that 4 weeks or less might achieve more satisfactory clinical efficacy than the course of more than 4 weeks. The study (67) found that electrical stimulation may have a cumulative effect on brain activity, which is associated with the recovery of behavioral function. Therefore, we wondered if there was an upper threshold for the cumulative effect of electrical stimulation on the plasticity of the cerebral cortex, resulting in the limited recovery of swallowing function from excessive electrical stimulation.
In the subgroup analysis based on the frequency and intensity of electrical stimulation, the results of SSA as the outcome index showed that 25 Hz electrical stimulation and 7 mA electrical stimulation seemed to have a more positive effect. In contrast, the results of SWAL-QOL showed that the clinical efficacy of 0–15 mA electrical stimulation was more prominent. However, there was no statistical difference between the subgroups of electrical stimulation at different frequencies (P > 0.05). Studies (63, 68) confirmed that there were specific differences in clinical efficacy in different intensities and frequencies of electrical stimulation, which might be related to the different degrees of motor-evoked potentials (MEPs) induced by different frequencies and intensities of stimulation on the pharyngeal muscle. Pharyngeal muscle MEPs were closely related to the excitability of the swallowing cortex-medulla oblongata (69, 70) and could have a long-term effect on the reorganization of the cerebral cortex through nerve conduction, thereby promoting the recovery of swallowing function.
In age-based subgroup analysis, it appeared to be more clinically positive in patients (age > 60 years old) than in patients (age <60 years old). As people grow older, the human physiological function will also decrease (71), which may lead to the fact that routine ST does not provide the same recovery effect for older patients as for younger patients. In the control group, patients (age > 60 years old) achieved worse clinical efficacy in conventional ST than patients (age <60 years old). However, patients in the experimental group received NMES + ST. The addition of electrical stimulation can enhance the contraction of swallowing muscles, promote the repair of damaged nerves and the remodeling of the cerebral cortex, which may make it possible that patients (age > 60 years old) in the experimental group can obtain the same benefits as other patients (age <60 years old). For this reason, the relative benefit is more significant in patients over 60 than in patients under 60.
Swallowing is a complex neuromuscular reflex activity involving the cortical center, brainstem swallowing center, peripheral nerve, and other aspects. Stroke can cause damage to the cortical swallowing center, corticobulbar tract, brainstem swallowing center, cranial nerves (V, IX, X, XI, and XII), and spinal nerves (C1, C2, and C3), which leads to symptoms of swallowing disorder such as drinking cough, eating difficulties, dysarthria. Research (72, 73) has proved that specific intensity of electrical stimulation on the glossopharyngeal muscle group can enhance muscle contraction ability, increase the degree of activation, and prevent disuse muscle atrophy. Swallowing-related muscles are mainly composed of type I muscle fibers and type II muscle fibers. Type II muscle fibers are smaller than type I and are not easily polarized (73). When NMES stimulates muscle, type II muscle fibers that constitute swallowing muscles are preferentially activated. Traditional rehabilitation training activates type I muscle fibers (72). When simultaneous treatment is performed, type I and type II muscle fibers are simultaneously activated, and the glossopharyngeal muscle group can produce a stronger contraction. In addition, electrical stimulation can produce vasoactive peptides to cause local vasodilation, which can improve the blood circulation of the injured part (74), accelerate the regeneration and repair of the nerve to correctly project the regeneration track of the target organ, promote the regeneration of the axon and the maturation of the myelin sheath (75), and further promote the functional recovery and reorganization of the cerebral cortex and related neural connections and pathways (76, 77). The sufficient movement of the hyoid-laryngeal complex is the key to ensuring the effective and safe completion of swallowing activities (78, 79). At present, the range of motion of the hyoid bone is often used to measure the movement of the hyoid-laryngeal complex of patients with dysphagia. Furthermore, the upward and forward movement distance of the hyoid bone in patients with dysphagia is significantly lower than that of ordinary people (80–82). This study also shows that while the swallowing function of patients is improved, the distance of upward and forward movement of the hyoid bone is significantly increased, which is consistent with previous studies.
However, in the subgroup analysis of the frequency and intensity of electrical stimulation, there was high heterogeneity within subgroups possibly due to the different placement of NMES in different studies. Studies had found that when electrodes were placed on the suprahyoid muscle (83), thyrohyoid muscle (53), orbicularis oculi muscle (84), and masseter muscle (85), the swallowing functions were improved. Furthermore, a meta-analysis study showed that horizontal electrodes placed in the suprahyoid muscle or suprahyoid muscle and thyrohyoid muscle seem to have the best effect (11), so the electrode placement site is related to clinical efficacy. The heterogeneity of the treatment course subgroup may be related to the differences in the intensity and frequency of electrical stimulation, pulse duration, and swallowing rehabilitation. One study (86) proposed that the shorter the pulse duration, the greater the stimulation intensity needed to obtain a muscle response. The pulse duration is inversely proportional to the specificity of the stimulus applied, which may be responsible for the high heterogeneity in subgroups. In addition, the high heterogeneity of age and disease course subgroups may be related to the inconsistency of stroke type, disease severity, frequency of electrical stimulation, and electrical stimulation intensity among patients included in different studies, which still needs further exploration.
4.1. Strengths and weaknesses
This study included more clinical randomized controlled trials (46 RCTs) and case numbers (3,346 patients) than previous meta-analysis studies on NMES in treating post-stroke dysphagia. It evaluated the clinical efficacy of NMES + ST from 10 different outcome indicators, such as SSA, SWAL-QOL, and VFSS. In addition, subgroup analysis found that the clinical efficacy of NMES + ST in patients with a disease course of fewer than 20 days appeared to be more significant than that in patients with a disease course of more than 20 days, which provided evidence support for early intervention treatment. A treatment course of 4 weeks or less appears to be better than a course of more than 4 weeks, which will help reduce the cost of treatment and improve the potential cost-effectiveness of the intervention; Electrical stimulation with a frequency of 25 Hz and intensity of 7 mA or 0–15 mA appears to work better. This finding could help develop optimal stimulation parameters. The effect of electrical stimulation in patients over 60 years old is noticeable, promoting further attention to the treatment of swallowing disorders in the elderly. This study also has some limitations: (1) The majority of the 46 RCTs included in this study are from China, which may lead to regional bias. (2) Most of the included clinical trials do not report the blinding method used, which reduced the quality of the methodological study. There may be some placebo effect and observer bias, which may reduce the credibility of the clinical trial results. (3) Among 46 RCTs, only six studies followed-up visited with the patients, so the long-term clinical efficacy of NMES + ST on post-stroke dysphagia still needs to be further explored. (4) Adverse events were reported in only some studies, which resulted in insufficient evidence to support the safety of NMES + ST treatment. Future studies still need to strengthen the recording and reporting of adverse events. (5) Due to the high heterogeneity, some results' reliability in the study has been somewhat affected. (6) The use of multiple types of ST in different studies led to the fact that this study did not perform a subgroup analysis based on the type of ST received by the control group. We look forward to further exploration in subsequent studies.
5. Conclusion
Our study showed that NMES + ST could effectively increase the forward and upward movement distance of the hyoid bone, improve the quality of life of patients with post-stroke dysphagia, reduce the rate of complications, and promote the recovery of swallowing function. NMES with a frequency of 25 Hz, an intensity of 0–15 mA, and a treatment course of 4 weeks or less may have better results. Patients with an onset of fewer than 20 days and over 60 years old appear more effective with NMES + ST. However, there is insufficient evidence on the safety of NMES + ST for post-stroke dysphagia. Moreover, due to the small number of included literature and the low quality of evidence, more large-sample, high-quality, multi-center RCT studies are needed to prove the clinical efficacy of NMES + ST in the treatment of post-stroke dysphagia.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YW conceived the study and drafted the protocol. YW, LX, and LZ participated in literature screening, data extraction, and bias risk assessment. YW, LW, MJ, and LX are responsible for statistical analysis and manuscript writing. LZ was responsible for the planning and guidance of this article. All authors participated in the study and approved the published version of the manuscript.
Funding
The study was funded by the Sichuan Provincial Department of Education Central Guided Local Science and Technology Development Fund Project (2021ZYD0103).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1163045/full#supplementary-material
References
1. Naghavi M, Abajobir AA, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2017) 390:1151–210. doi: 10.1016/S0140-6736(17)32152-9
2. Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
3. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. (2005) 36:2756–63. doi: 10.1161/01.STR.0000190056.76543.eb
4. Foley NC, Martin RE, Salter KL, Teasell RW. A review of the relationship between dysphagia and malnutrition following stroke. J Rehabil Med. (2009) 41:707–13. doi: 10.2340/16501977-0415
5. Eslick GD, Talley NJ. Dysphagia: epidemiology, risk factors and impact on quality of life–a population-based study. Aliment Pharmacol Ther. (2008) 27:971–9. doi: 10.1111/j.1365-2036.2008.03664.x
6. Doeltgen SH, Bradnam LV, Young JA, Fong E. Transcranial non-invasive brain stimulation in swallowing rehabilitation following stroke—a review of the literature. Physiol Behav. (2015) 143:1–9. doi: 10.1016/j.physbeh.2015.02.025
7. Jongprasitkul H, Kitisomprayoonkul W. Effectiveness of conventional swallowing therapy in acute stroke patients with dysphagia. Rehabil Res Pract. (2020) 2020:1–5. doi: 10.1155/2020/2907293
8. Smithard DG, O'Neill PA, Martin DF, England R. Aspiration following stroke: is it related to the side of the stroke? Clin. Rehabil. (1997) 11:73–6. doi: 10.1177/026921559701100111
9. Steele CM. Electrical stimulation of the pharyngeal swallow: does the evidence support application in clinical practice? J Speech Lang Pathol Audiol. (2004) 28:77–83.
10. Chen YW, Chang KH, Chen HC, Liang WM, Wang YH, Lin YN. The effects of surface neuromuscular electrical stimulation on post-stroke dysphagia: a systemic review and meta-analysis. Clin Rehabil. (2016) 30:24–35. doi: 10.1177/0269215515571681
11. Doan TN, Ho WC, Wang LH, Chang FC, Tran TTQ, Chou LW. Therapeutic effect and optimal electrode placement of transcutaneous neuromuscular electrical stimulation in patients with post-stroke dysphagia: a systematic review and meta-analysis of randomized controlled trials. Life. (2022) 12:875. doi: 10.3390/life12060875
12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
13. Cumpston M, Li T, Page MJ, Chandler J, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cohrane Database Syst Rev. (2019) 10. doi: 10.1002/14651858.ED000142
14. Tan SW, Wu A, Cheng LJ, Wong SH, Lau Y, Lau ST. The effectiveness of transcranial stimulation in improving swallowing outcomes in adults with poststroke dysphagia: a systematic review and meta-analysis. Dysphagia. (2022) 37:1796–813. doi: 10.1007/s00455-022-10424-6
15. Li SH, Hu WS, Wu QF, Sun JG. The efficacy of bloodletting therapy in patients with acute gouty arthritis: a systematic review and meta-analysis. Complement Ther Clin Pract. (2022) 46:101503. doi: 10.1016/j.ctcp.2021.101503
16. Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Interpreting results and drawing conclusions. In:Chandler J, Thomas J, Higgins JPT, Page MJ, Cumpston M, Li T, et al., , editors. Cochrane Handbook for Systematic Reviews of Interventions. New York, NY: Wiley (2019), p. 403–31. doi: 10.1002/9781119536604.ch15
17. Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin. Epidemiol. (2013) 66:719–25. doi: 10.1016/j.jclinepi.2012.03.013
18. Chang E. Effect of neuromuscular electrical stimulation combined with feeding training on patients with dysphagia after stroke. Chin J Integr Med Cardio-Cerebrovasc Dis. (2020) 18:337–40. doi: 10.12102/j.issn.1672-1349.2020.02.038
19. Chen XX, Chen QG, Gu LH, Zhao YL, Cui T, Lin L, et al. Clinical observation of percutaneous acupoint electrical stimulation combined with swallowing training on patients with swallowing dysfunction after stroke. Chin Man Rehabil Med. (2021) 12:49–50+52. doi: 10.19787/j.issn.1008-1879.2021.01.016
20. Chen XX. Effect of neuromuscular electrical stimulation combined with swallowing train on the survival quality of patients with dysphagia after cerebral infarction. Clin Res Pract. (2017) 2:19–20. doi: 10.19347/j.cnki.2096-1413.201736008
21. Cui YQ, Song QQ, Ye HM, Mo JM, Lu HY. Influence of nerve and muscle electrical stimulation on swallowing function and living quality in stroke patients with dysphagia. Tianjin J Nurs. (2018) 26:421–4. doi: 10.3969/j.issn.1006-9143.2018.04.013
22. Du YB, Shao K. To study and analyze the feasibility and effectiveness of neuromuscular electrical stimulation combined with rehabilitation therapy in the treatment of swallowing dysfunction after stroke. Reflexol Rehabil Med. (2020) 29:129–31.
23. Geng XZ. Effects of neuromuscular electrical stimulation combined with early swallowing management on swallowing function and nerve function in patients with swallowing disorder after craniocerebral injury. Reflexol Rehabil Med. (2021) 2:163–5.
24. Gong XQ. Efficacy of neuromuscular electrical stimulation combined with swallowing training for dysphagia after stroke. Clin Res Pract. (2018) 3:34–5. doi: 10.19347/j.cnki.2096-1413.201807016
25. Gu YW, Shu J. Effects of surface electromyogram biofeedback and neuromuscular electrical stimulation on dysphagia after stroke and quality of life. Chin J Rehabil. (2021) 36:599–603. doi: 10.3870/zgkf.2021.10.005
26. Lei C, Sun ZM, Wang YF, Ming WW, Li HY. Application of pharyngeal ice stimulation combined with low-frequency pulsed electrical stimulation in patients with post-stroke swallowing disorders. J Clin Pathol. (2020) 40:116–23. doi: 10.3978/j.issn.2095-6959.2020.01.020
27. Li Q. Effect of neuromuscular electrical stimulation combined with swallowing rehabilitation training on swallowing function in patients with dysphagia after stroke. Reflexol Rehabil Med. (2021) 2:154–6.
28. Liang ZL, Huang L, Huang TZ. Observation on treating dysphagia by low-frequency electrical stimulation plus swallowing training. Clin J Chin Med. (2019) 11:75–7. doi: 10.3969/j.issn.1674-7860.2019.29.029
29. Mo SJ, Chen SY, Tan YH. Application of pharyngeal muscle electrical stimulation combined with direct feeding training in swallowing function rehabilitation of patients with indwelling gastric tube in stroke. J Qilu Nurs. (2022) 28:110–3.
30. Shi XW, Yang WL, Jie WJ. Effect of neuromuscular electrical stimulation in the treatment of neurological dysphagia after stroke. Pract Clin J Integr Tradit Chin West Med. (2021) 21:53–5. doi: 10.13638/j.issn.1671-4040.2021.10.024
31. Tian T, Li JR, Li SH. Observation of curative effect of low frequency electric stimulation swallowing disorder training instrument on swallowing dysfunction after stroke. J Clin Neurol. (2016) 29:378–80.
32. Wang JL, Ding DQ, Tan F, Wang SY, Wu HK, Huang T, et al. Clinical study of neuromuscular electrical stimulation combined with electroacupuncture in the treatment of acute cerebral infarction pharyngeal disorder. Chongqing Med. (2014) 43:4071–3. doi: 10.3969/j.issn.1671-8348.2014.30.030
33. Wang L. Effects of neuromuscular electrical stimulation combined with swallowing training on swallowing function and quality of life in patients with dysphagia after acute cerebral infarction. Reflexol Rehabil Med. (2021) 2:125–7.
34. Wang L, Ye FL. Observation on curative effects of lemone ice stimulation in combination with low frequency electrical stimulation in the treatment of SD after stroke. West J Tradit Chin Med. (2020) 33:131–5. doi: 10.12174/j.issn.1004-6852.2020.05.37
35. Wang XJ. Observation of the effect of neuromuscular electrical stimulation combined with swallowing function training on swallowing function and quality of survival after cerebral infarction. Reflexol Rehabili Med. (2021) 2:119–21, 162.
36. Wang YC, Luo L, Li P, Wen XM, Xiang T, Wu J. Effect of early comprehensive rehabilitation therapy on dysphagia in stroke patients. Chin J Phys Med Rehabil. (2009) 31:839–42. doi: 10.3760/cma.j.issn.0254-1424.2009.12.016
37. Wen JY, Wu GH. Effect of neuromuscular electrical stimulation therapy combined with rehabilitation exercise on the recovery of swallowing function in patients with dysphagia after cerebral infarction. Med Theory Pract. (2020) 33:3738–40. doi: 10.19381/j.issn.1001-7585.2020.22.017
38. Zhan Y, Liu YY, Wang SS, Xun YJ, Zhai HY, Zhang X, et al. Efficacy of neuromuscular electrical stimulation on the rehabilitation of post-stroke pharyngeal dysphagia. Chin J Rehabil. (2016) 31:372–4. doi: 10.3870/zgkf.2016.05.016
39. Zhang Q, Wu S, Zhou TF, Chen Y, Shi YM. Effects of synchronous neuromuscular electrical stimulation in feeding training in patients with post-stroke swallowing disorders. Chin J Phys Med Rehabil. (2022) 44:415–8. doi: 10.3760/cma.j.issn.0254-1424.2022.05.007
40. Zhang T, Fu Y, Yu SH. Analysis of the effect of neuromuscular electrical stimulation on dysphagia in patients with cerebral infarction. Chin Community Doctors. (2017) 33:152–3. doi: 10.3969/j.issn.1007-614x.2017.23.92
41. Guo YX, Zhang Y. Clinical study of the treatment for swallowing dysfunction following acute cerebral infarction by neuromuscular electrical stimulation combined with swallowing training. Chin J Pract Intern Med. (2019) 39:1084–7. doi: 10.19538/j.nk2019120115
42. Zheng CJ, Xia WG, Zhang YP, Hua Q, Xu T. Neuromuscular electrical stimulation combined with swallowing training for dysphagia after stroke. Chin J Phys Med Rehabil. (2013) 35:201–4. doi: 10.3760/cma.j.issn.0254-1424.2013.03.011
43. Zhou WZ, Wang HB, Yang SH. Efficacy of NMES therapy and swallowing training alone for dysphagia after stroke. J Brain Nerv Dis. (2016) 24:75–8.
44. Zhu SW, Jiang PY, Dang HM, Zhang JL, Zhu YL. Effect of neuromuscular electrical stimulation and swallowing training on dysphasia after stroke. Chin J Rehabil Theory Pract. (2011) 17:730–2. doi: 10.3969/j.issn.1006-9771.2011.08.010
45. Dong JZ. Efficacy of neuromuscular electrical stimulation therapy in patients with swallowing disorders after cerebral infarction. Chin J Pract Nerv Dis. (2017) 20:49–51. doi: 10.3969/j.issn.1673-5110.2017.10.016
46. Liu YS. Effect of neuromuscular electrical stimulation combined with swallowing rehabilitation training on post-stroke dysphagia and its effect on movement of the hyolaryngeal complex. Chin J Gen Med. (2017) 15:1782–5. doi: 10.16766/j.cnki.issn.1674-4152.2017.10.042
47. Wu AC, Zhang ZH. Neuromuscular electrical stimulation combined with swallowing function training for the treatment of post-stroke swallowing dysfunction. Chin J Rehabil. (2010) 25:453–4. doi: 10.3870/zgkf.2010.06.022
48. Xu MX, Wang Q, Meng PP, Jiang L. Electrical stimulation and swallowing training in treating post-stroke dysphagia. Chin J Phys Med Rehabil. (2014) 36:274–7. doi: 10.3760/cma.j.issn.0254-1424.2014.04.008
49. Deng HQ. Study on Acute Ischemic Stroke Patients with Swallowing Disorder Treated by Early Combinational Stimulations Therapy [Dissertation/master's thesis]. Jinan, Shandong: Taishan Medical University (2014).
50. Zhang XL. The Study of Efficacy of Vitalstim Electrical Stimulation-swallowing Training on Dysphagia After Stroke [Dissertation/master's thesis]. Jinan, Shandong: Taishan Medical University (2014).
51. Li LJ, Li YM, Wu XH, Wang GH, Yi XJ, Zhao YC, et al. The value of adding transcutaneous neuromuscular electrical stimulation (VitalStim) to traditional therapy for poststroke dysphagia: a randomized controlled trial. Top Geriatr Rehabil. (2018) 34:200–6. doi: 10.1097/TGR.0000000000000195
52. Sproson L, Pownall S, Enderby P, Freeman J. Combined electrical stimulation and exercise for swallow rehabilitation post-stroke: a pilot randomized control trial. Int J Lang Commun Disord. (2018) 53:405-17. doi: 10.1111/1460-6984.12359
53. Simonelli M, Ruoppolo G, Iosa M, Morone G, Fusco A, Grasso MG, et al. A stimulus for eating. The use of neuromuscular transcutaneous electrical stimulation in patients affected by severe dysphagia after subacute stroke: a pilot randomized controlled trial. NeuroRehabilitation. (2019) 44:103–10. doi: 10.3233/NRE-182526
54. Meng P, Zhang S, Wang Q, Wang P, Han C, Gao J, et al. The effect of surface neuromuscular electrical stimulation on patients with post-stroke dysphagia. J Back Musculoskelet Rehabil. (2018) 31:363–70. doi: 10.3233/BMR-170788
55. Park JS, Oh DH, Hwang NK, Lee JH. Effects of neuromuscular electrical stimulation combined with effortful swallowing on post-stroke oropharyngeal dysphagia: a randomised controlled trial. J Oral Rehabil. (2016) 43:426–34. doi: 10.1111/joor.12390
56. Arreola V, Ortega O, Álvarez-Berdugo D, Rofes L, Tomsen N, Cabib C, et al. Effect of transcutaneous electrical stimulation in chronic poststroke patients with oropharyngeal dysphagia: 1-year results of a randomized controlled trial. Neurorehabil Neural Repair. (2021) 35:778–89. doi: 10.1177/15459683211023187
57. Carnaby GD, LaGorio L, Silliman S, Crary M. Exercise-based swallowing intervention (McNeill Dysphagia Therapy) with adjunctive NMES to treat dysphagia post-stroke: a double-blind placebo-controlled trial. J Oral Rehabil. (2020) 47:501–10. doi: 10.1111/joor.12928
58. Huang KL, Liu TY, Huang YC, Leong CP, Lin WC, Pong YP. Functional outcome in acute stroke patients with oropharyngeal dysphagia after swallowing therapy. J Stroke Cerebrovasc Dis. (2014) 23:2547–53. doi: 10.1016/j.jstrokecerebrovasdis.2014.05.031
59. Lee KW, Kim SB, Lee JH, Lee SJ, Ri JW, Park JG. The effect of early neuromuscular electrical stimulation therapy in acute/subacute ischemic stroke patients with dysphagia. Ann Rehabil Med. (2014) 38:153–9. doi: 10.5535/arm.2014.38.2.153
60. Lim KB, Lee HJ, Yoo J, Kwon YG. Effect of low-frequency rTMS and NMES on subacute unilateral hemispheric stroke with dysphagia. Ann Rehabil Med. (2014) 38:592–602. doi: 10.5535/arm.2014.38.5.592
61. Zhang Q, Wu S. Effects of synchronized neuromuscular electrical stimulation (NMES) on the submental muscles during ingestion of a specified volume of soft food in patients with mild-to-moderate dysphagia following stroke. Med Sci Monit Int Med J Exp Clin Res. (2021) 27:e928988–1. doi: 10.12659/MSM.928988
62. Guillén-Solà A, Messagi Sartor M, Bofill Soler N, Duarte E, Barrera MC, Marco E. Respiratory muscle strength training and neuromuscular electrical stimulation in subacute dysphagic stroke patients: a randomized controlled trial. Clin Rehabil. (2017) 31:761–71. doi: 10.1177/0269215516652446
63. Zhang M, Tao T, Zhang ZB, Zhu X, Fan WG, Pu LJ, et al. Effectiveness of neuromuscular electrical stimulation on patients with dysphagia with medullary infarction. Arch Phys Med Rehabil. (2016) 97:355–62. doi: 10.1016/j.apmr.2015.10.104
64. Hamdy S, Rothwell JC, Aziz Q, Thompson DG. Organization and reorganization of human swallowing motor cortex: implications for recovery after stroke. Clin Sci. (2000) 99:151–7. doi: 10.1042/cs0990151
65. Hamdy S, Aziz Q, Rothwell JC, Power M, Thompson DG. Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology. (1998) 115:1104–12. doi: 10.1016/S0016-5085(98)70081-2
66. Steele CM, Miller AJ. Sensory input pathways and mechanisms in swallowing: a review. Dysphagia. (2010) 25:323–33. doi: 10.1007/s00455-010-9301-5
67. Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci. (2007) 25:123–9.
68. Doeltgen SH, Dalrymple-Alford J, Ridding MC, Huckabee ML. Differential effects of neuromuscular electrical stimulation parameters on submental motor-evoked potentials. Neurorehabil Neural Repair. (2010) 24:519–27. doi: 10.1177/1545968309360417
69. Fraser C, Power M, Hamdy S, Rothwell J, Hobday D, Hollander I, et al. Driving plasticity in human adult motor cortex is associated with improved motor function after brain injury. Neuron. (2002) 34:831–40. doi: 10.1016/S0896-6273(02)00705-5
70. Hamdy S, Rothwell JC, Aziz Q, Singh KD, Thompson DG. Long-term reorganization of human motor cortex driven by short-term sensory stimulation. Nat Neurosci. (1998) 1:64–8. doi: 10.1038/264
71. Wirth R, Dziewas R, Beck AM, Clavé P, Hamdy S, Heppner HJ, et al. Oropharyngeal dysphagia in older persons–from pathophysiology to adequate intervention: a review and summary of an international expert meeting. Clin Interv Aging. (2016) 11:189–208. doi: 10.2147/CIA.S97481
72. Kletzien H, Russell JA, Leverson G, Connor NP. Effect of neuromuscular electrical stimulation frequency on muscles of the tongue. Muscle Nerve. (2018) 58:441–8. doi: 10.1002/mus.26173
73. Blumenfeld L, Hahn Y, LePage A, Leonard R, Belafsky PC. Transcutaneous electrical stimulation versus traditional dysphagia therapy: a nonconcurrent cohort study. Otolaryngol Head Neck Surg. (2006) 135:754–7. doi: 10.1016/j.otohns.2006.04.016
74. Ahlborn P, Schachner M, Irintchev A. One hour electrical stimulation accelerates functional recovery after femoral nerve repair. Exp Neurol. (2007) 208:137–44. doi: 10.1016/j.expneurol.2007.08.005
75. Haastert-Talini K, Schmitte R, Korte N, Klode D, Ratzka A, Grothe C. Electrical stimulation accelerates axonal and functional peripheral nerve regeneration across long gaps. J Neurotrauma. (2011) 28:661–74. doi: 10.1089/neu.2010.1637
76. Johnson AM, Connor NP. Effects of electrical stimulation on neuromuscular junction morphology in the aging rat tongue. Muscle Nerve. (2011) 43:203–11. doi: 10.1002/mus.21819
77. Langdon PC, Lee AH, Binns CW. High incidence of respiratory infections in ‘nil by mouth' tube-fed acute ischemic stroke patients. Neuroepidemiology. (2009) 32:107–13. doi: 10.1159/000177036
78. Ishida R, Palmer JB, Hiiemae KM. Hyoid motion during swallowing: factors affecting forward and upward displacement. Dysphagia. (2002) 17:262–72. doi: 10.1007/s00455-002-0064-5
79. Kim Y, McCullough GH. Maximum hyoid displacement in normal swallowing. Dysphagia. (2008) 23:274–9. doi: 10.1007/s00455-007-9135-y
80. Paik NJ, Kim SJ, Lee HJ, Jeon JY, Lim JY, Han TR. Movement of the hyoid bone and the epiglottis during swallowing in patients with dysphagia from different etiologies. J Electromyogr Kinesiol. (2008) 18:329–35. doi: 10.1016/j.jelekin.2006.09.011
81. Ueda N, Nohara K, Kotani Y, Tanaka N, Okuno K, Sakai T. Effects of the bolus volume on hyoid movements in normal individuals. J Oral Rehabil. (2013) 40:491–9. doi: 10.1111/joor.12060
82. van der Kruis JG, Baijens LW, Speyer R, Zwijnenberg I. Biomechanical analysis of hyoid bone displacement in videofluoroscopy: a systematic review of intervention effects. Dysphagia. (2011) 26:171–82. doi: 10.1007/s00455-010-9318-9
83. Byeon H. Combined effects of NMES and Mendelsohn maneuver on the swallowing function and swallowing–Quality of life of patients with stroke-induced sub-acute swallowing disorders. Biomedicines. (2020) 8:12. doi: 10.3390/biomedicines8010012
84. Oh DH, Park JS, Kim WJ. Effect of neuromuscular electrical stimulation on lip strength and closure function in patients with dysphagia after stroke. J Phys Ther Sci. (2017) 29:1974–5. doi: 10.1589/jpts.29.1974
85. Lee KW, Kim SB, Lee JH, Lee SJ, Park JG, Jang KW. Effects of neuromuscular electrical stimulation for masseter muscle on oral dysfunction after stroke. Ann Rehabil Med. (2019) 43:11–8. doi: 10.5535/arm.2019.43.1.11
Keywords: stroke, dysphagia, neuromuscular electrical stimulation, meta-analysis, evidence-based medicine
Citation: Wang Y, Xu L, Wang L, Jiang M and Zhao L (2023) Effects of transcutaneous neuromuscular electrical stimulation on post-stroke dysphagia: a systematic review and meta-analysis. Front. Neurol. 14:1163045. doi: 10.3389/fneur.2023.1163045
Received: 10 February 2023; Accepted: 05 April 2023;
Published: 09 May 2023.
Edited by:
Pierluigi Zoccolotti, Sapienza University of Rome, ItalyReviewed by:
Marco Iosa, Sapienza University of Rome, ItalyRoberta Gonçalves Silva, São Paulo State University, Brazil
Copyright © 2023 Wang, Xu, Wang, Jiang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Zhao, emhhb2xpbmdAY2R1dGNtLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Yuhan Wang
Yuhan Wang Lu Xu2†
Lu Xu2† Linjia Wang
Linjia Wang Ling Zhao
Ling Zhao