
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 17 April 2023
Sec. Endovascular and Interventional Neurology
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1159601
Background: Increasing evidence suggests that insulin resistance is linked to cardiovascular disease and atherosclerosis. The triglyceride–glucose (TyG) index has proven to be a convincing marker to quantitatively evaluate insulin resistance. However, there is no relevant information about the relationship between the TyG index and restenosis after carotid artery stenting.
Methods: A total of 218 patients were enrolled. Carotid ultrasound and computed tomography angiography were used to evaluate in-stent restenosis. A Kaplan–Meier analysis and Cox regression method were performed to analyze the correlation between TyG index and restenosis. Schoenfeld residuals were used to determine the proportional-hazards assumption. A restricted cubic spline method was used to model and visualize the dose–response relationship between the TyG index and the risk of in-stent restenosis. Subgroup analysis was also performed.
Results: Thirty-one participants (14.2%) developed restenosis. The preoperative TyG index had a time-varying effect on restenosis. Within 29 months post-surgery, an increasing preoperative TyG index was linked to a significant increased risk of restenosis (hazard ratio: 4.347; 95% confidence interval 1.886–10.023). However, after 29 months, the effect was decreased, although not statistically significant. The subgroup analysis showed that the hazard ratios tended to be higher in the age ≤ 71 years subgroup (p < 0.001) and participants with hypertension (p < 0.001).
Conclusion: The preoperative TyG index was significantly associated with the risk of short-term restenosis after CAS within 29 months post-surgery. The TyG index may be employed to stratify patients based on their risk of restenosis after carotid artery stenting.
A global investigation published in 2014 showed that an approximated 6 million people died from stroke worldwide in 2013, with ischemic stroke accounting for 51% of all deaths (1). Severe internal carotid artery (ICA) stenosis is associated with a higher risk of ischemic stroke, and carotid artery stenting (CAS) is a popular minimally invasive treatment option for carotid stenosis. Moreover, it has been demonstrated that CAS is comparable to carotid endarterectomy in terms of lowering the risk of stroke (2). In-stent restenosis (ISR) is a major complication that affects long-term safety and efficacy. The incidence of restenosis after CAS is reported to be around 5–20% (3, 4). Although restenosis is mostly asymptomatic, it may require a second intervention, and this increases the risk of ipsilateral ischemic stroke. Therefore, early identification of convenient and accurate biomarkers of ISR is critical for patients with CAS.
Increasing evidence suggests that insulin resistance (IR) is linked to cardiovascular disease and atherosclerosis (5, 6). A previous study also reported that IR is related to restenosis after coronary stenting (7). The homeostasis model assessment index was applied in this study to assess IR. However, although this index is considered the gold standard, it is expensive and clinically inconvenient. IR can lead to hyperglycemia and increased serum triglyceride (TG) levels. On this basis, the TyG index, calculated from serum fasting blood glucose (FBG) and TG, has proven to be a convincing indicator for quantitative evaluation of IR (8). Recent research has demonstrated that a higher TyG index increases the risk of ISR after coronary stenting (9). However, no relevant information regarding the effect of the TyG index on restenosis after CAS is known. Moreover, our previous research (10) showed that a higher TyG index can increase the burden of carotid plaque in patients with new-onset diabetes. Accordingly, we hypothesized that the TyG index may also contribute to restenosis after CAS. Therefore, the aim of our study was to investigate the relevance of the preoperative TyG index on the risk of ISR in participants who underwent CAS for carotid atherosclerotic stenosis. The results of our study may assist in the identification of individuals at high risk of restenosis after CAS.
This study was reviewed and approved by the Academic Ethics Committee of Shaoxing People’s Hospital (ID: 2020-K-Y-070-01). Written informed consent for participation was not required for this study in accordance with the National Legislation and the Institutional Requirements.
This was a retrospective cohort study. Eligible participants were patients who underwent internal CAS at Shaoxing People’s Hospital between January 2013 and February 2022, at age > 18 years and with a glomerular filtration rate ≥ 30 ml/min (n = 250). Some patients were ruled out due to stenting not for atherosclerotic stenosis (n = 1), and lack of preoperative serum lipid profiles (n = 3). In addition, considering that a follow-up period that was too short could lead to false negative results, we also removed patients with less than 6 months of follow-up (n = 28) (9, 11). The selection flowchart is shown in Figure 1. The date of surgery was considered the start of the follow-up period. The date of the patient’s last visit to our hospital for carotid ultrasound or CTA examination was considered the end of the follow-up period. The endpoint was when ISR was detected by Doppler ultrasound or CTA.
The absolute indication for CAS in our hospital was symptomatic stenosis with a stenosis degree ≥ 70% on non-invasive examination or stenosis degree ≥ 50% on digital subtraction angiography (DSA). The relative indications were as follows: (1) asymptomatic stenosis and stenosis degree ≥ 70% on non-invasive examination or stenosis degree ≥ 60% on DSA; (2) asymptomatic stenosis and stenosis degree <70% on non-invasive examination and CTA or other examinations showing unstable plaques; and (3) symptomatic stenosis and a degree of stenosis of 50–60%. All patients provided written informed consent before surgery. A self-expanding stent with an embolic protection device (Abbott Xact Carotid Stent System, Park IL, United States; RX Acculink, Abbott Vascular Solutions, Santa Clara, CA, United States; Protégé, ev3 Inc., Plymouth, MN, United States) was used on all patients. Patients were followed up at 1, 3, and 6 months, and then once a year after CAS.
An experienced neurologist who was not informed of the purpose of the study searched the electronic medical record system, and demographic and clinical data were collected. The collected data included age, sex, body mass index (BMI), blood pressure, current smoking status (yes or no), alcohol consumption (yes or no), symptomatic stenosis (yes or no), medical history (hypertension, dyslipidemia, diabetes mellitus, and coronary heart disease), fasting laboratory data [total cholesterol, TG, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), FBG, and high-sensitivity C-reactive protein (hs-CRP)], atherosclerotic stenosis (> 50% degree) detected by intraoperative DSA (contralateral ICA, intracranial artery, vertebral artery, subclavian artery), stent type and size, adverse events (transient ischemic attack, ipsilateral stroke, all ischemic strokes, myocardial infarction, and death) during the follow-up, the date of operation, and the date of the last follow-up.
The formula for calculating the TyG index was Ln [fasting triglyceride (mg/dL) × fasting blood glucose (mg/dL)/2] (12), and BMI was calculated as weight/height2 (kg/m2). Hypertension was defined as a blood pressure ≥ 140/90 mmHg or reception of antihypertensive therapy. Diabetes mellitus was defined as an FBG > 7.0 mmol/l, random blood glucose > 11.1 mmol/l, or reception of hypoglycemic drugs (13). Dyslipidemia was defined as fasting total cholesterol > 6.22 mmol/l, TG ≥ 2.26 mmol/l, LDL-C ≥ 4.14, HDL < 1.04 mmol/l, or reception of lipid-lowering drugs (14). Symptomatic stenosis was defined as neurological dysfunction due to cerebral or retinal ischemia in the ipsilateral carotid region within the previous 6 months (15). ISR was defined as ≥ 50% restenosis inside the stent or involving 5-mm margins. Adverse events were defined as those resulting in readmission during the follow-up period, and included transient ischemic attack, ipsilateral stroke, ischemic stroke, myocardial infarction, and death.
ISR was evaluated based on the carotid ultrasound or CTA results. Due to the absence of ultrasound velocity criteria for evaluating ISR, an ISR cutoff of 50% or greater was classified as the peak systolic velocity in an ICA greater than 150 cm/s, based on a previous study (16). The North American Symptomatic Carotid Endarterectomy Trial method was applied to determine the degree of restenosis on CTA images. If a patient underwent both Doppler ultrasound and CTA, ISR was considered if either examination met the diagnostic criteria. All images were reevaluated by two experienced radiologists who were unaware of the relevant patient data.
Normally or nearly normally distributed data such as age, blood pressure, total cholesterol, TG, HDL-C, and LDL-C are presented as the mean ± standard deviation. The distribution of CRP and the follow-up period was skewed, and therefore expressed as median (interquartile range). Qualitative variables are expressed as numbers (percentages). According to the mean value of the TyG index, the participants were assigned to two groups: group A (TyG index ≤ 8.62) and group B (TyG index > 8.62). A Student’s t-test was used for the comparison of normally-distributed parameters between the two groups, the Mann–Whitney U test was used for skewed parameters, and the Chi-square test or Fisher’s exact test was used for qualitative variables. We opted for mean imputation for patients with missing BMI values (n = 23).
The frequency of restenosis of the two groups was estimated using Kaplan–Meier analysis. The log-rank test was performed to calculate any differences in survival. The Cox regression method was used to analyze the correlation between the continuous TyG index and ISR. The correlation was evaluated by hazard ratio (HR) and 95% confidence interval (CI). Schoenfeld residuals were used to determine the proportional-hazards (PH) assumption. Because the continuous TyG index violated the PH assumption, we added a time interaction term to the model. To select independent variables, candidate variables (p < 0.2) in the univariate Cox analysis were included in the backward stepwise regression model. The independent variables (TyG index and minimum stent diameter) identified by the above procedure were then included in the multivariate model, along with variables with potential clinical relevance, including age, sex, symptomatic stenosis, hypertension, diabetes mellitus, dyslipidemia, smoking status, and stent length. We also used restricted cubic splines with four knots at the 5th, 35th, 65th, and 95th centiles to model and visualize the dose–response relationship of the TyG index with the risk of ISR. Subgroup analysis was also performed according to age, sex, BMI, symptomatic stenosis, smoking status, hypertension, and diabetes mellitus. For qualitative data, subgroups were divided according to yes and no. For quantitative data, namely BMI and age, subgroups were divided according to mean values. All statistical analyses were conducted using SAS 9.4 (Cary, NC, United States) software. p < 0.05 was considered statistically significant. Statistical power was calculated using PASS 11 software.
As shown in Table 1, the mean age of the participants was 70.69 years (range: 53–85 years) and 182 (83.5%) participants were males. The prevalence of symptomatic stenosis, hypertension, diabetes mellitus, dyslipidemia, and coronary heart disease was 104 (47.7%), 180 (82.6%), 95 (43.6%), 126 (57.8%), and 27 (12.4%), respectively. In the sample, 92 (42.2%) participants reported consuming alcohol, and 78 (35.8%) were smokers. In terms of stent type, the Abbott Xact Carotid Stent System was used in the majority of participants (87.6%). Based on intraoperative CTA, 69 (31.7%) participants had contralateral ICA stenosis (≥ 50%). The stenosis (≥ 50%) of the intracranial, vertebral, and subclavian arteries were 30 (13.8%), 49 (22.5%), and 12 (5.5%), respectively. Thirty-one participants (14.2%) developed ISR at the end of the study.
The participants were assigned to two groups according to the mean TyG index value (M = 8.62): 108 to group A (TyG index ≤ 8.62) and 210 to group B (TyG index > 8.62). Diabetes mellitus (56.4 vs. 30.6%, p < 0.001) and dyslipidemia (79.1 vs. 46.3%, p = 0.001) were more prevalent in group B. Within the laboratory data, total cholesterol (4.15 ± 1.07 vs. 3.56 ± 0.83 mmol/l, p < 0.001), TG (1.80 ± 0.83 vs. 0.93 ± 0.26 mmol/l, p < 0.001), LDL-C (2.58 ± 0.83 vs. 2.12 ± 0.71 mmol/l, p < 0.001), and FBG (6.72 ± 2.32 vs. 5.04 ± 1.04 mmol/l, p < 0.001) levels were higher in group B, but HDL-C (1.01 ± 0.25 vs. 1.14 ± 0.31 mmol/l, p = 0.001) levels were lower. However, no difference was observed in hs-CRP levels. Other baseline variables, including age, sex, BMI, symptomatic stenosis, prevalence of current smoking and hypertension, and stent-related parameters were not statistically different. Meanwhile, we observed no significant differences in stenosis of the contralateral ICA, intracranial artery, vertebral artery, or subclavian artery.
According to the tests on Schoenfeld residuals, the continuous TyG index violated the PH assumption (p = 0.031). If the PH assumption holds, the change in Schoenfeld residuals over time should fluctuate randomly above and below the zero horizontal line in the Schoenfeld residual plot. However, sometimes it is difficult to evaluate the trend of scatter. We can use the local-weighted scatterplot smoothing function to plot the smooth curve of the Schoenfeld residual, which helps us to make judgments. Theoretically, under the null assumption of PHs, this function would have a slope of zero (17). Figure 2A shows that the curve fluctuates up and down with time, which does not meet the PH assumption. Therefore, we added a time interaction term to the model. In this study, combined the extreme and zero points of the curve with the results of model construction (optimally satisfied the PH assumption), we finally chose 29 months as the proper cutoff time point (18). Figure 2B shows that after adding 29 months as the time interaction term, the smooth curve lies on the zero horizontal line, satisfying the PH assumption (p > 0.05). The tests on Schoenfeld residuals of all variables and the entire model are presented in Supplementary Table S1. All variables and the entire model were in accordance with the PH assumption after including the time interaction term (p > 0.05).
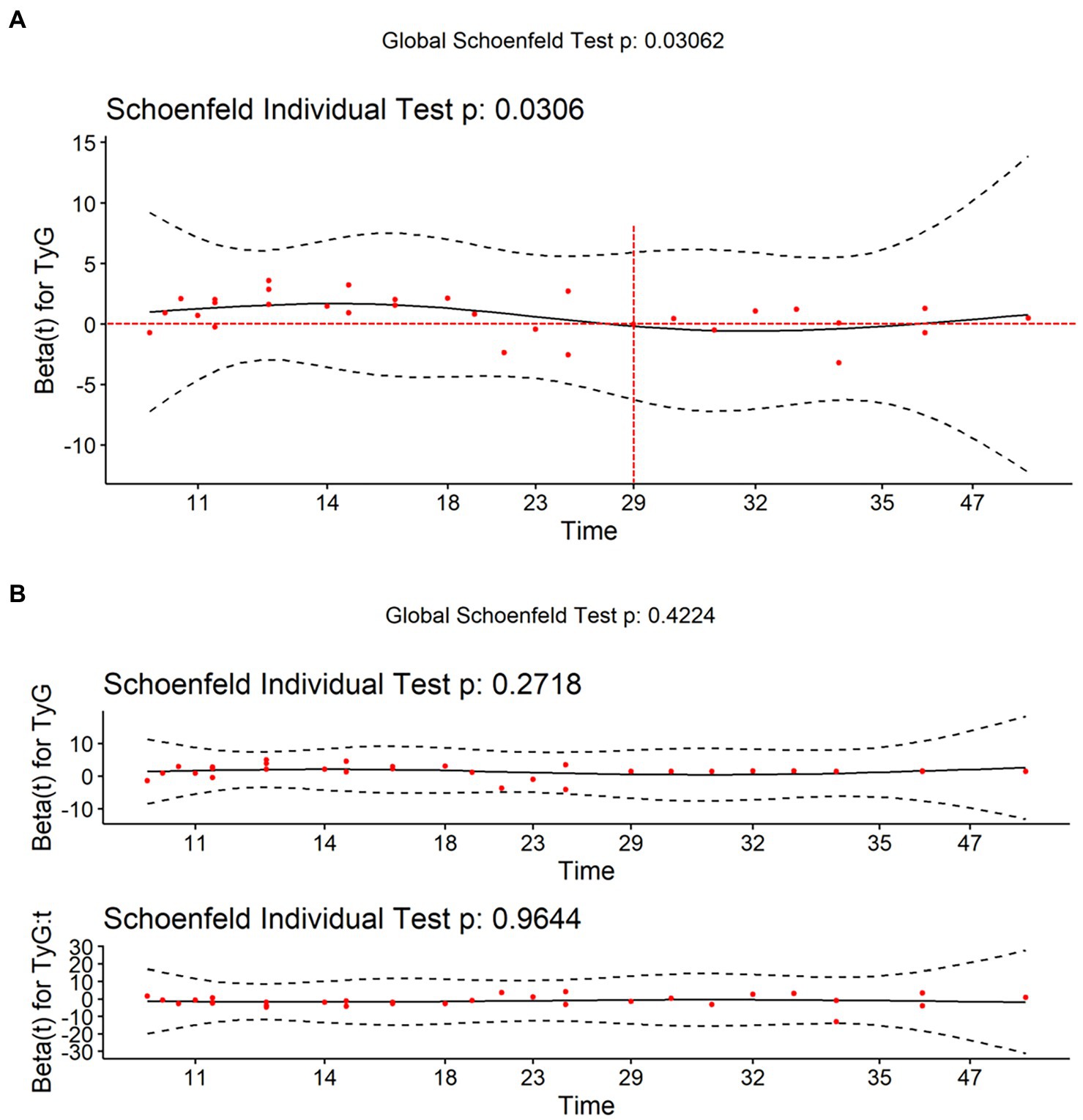
Figure 2. Visualization of Schoenfeld residuals. The red points represent the Schoenfeld residuals. The black curve represents the smooth curve of Schoenfeld residuals against time. The black dashed line represents the confidence interval of the smooth curve. The red dashed line represents zero horizontal line. (A) The smooth curve fluctuates up and down the zero horizontal line over time, which does not meet the PH assumption. (B) After adding 29 months as the time interaction term, the smooth curve lies on the zero horizontal line, satisfying the proportional-hazards assumption (p > 0.05).
The number of participants with restenosis was 23 (20.9%) in group B and eight (7.4%) in group A, and this difference was significant (p = 0.004; Figure 3A). Meanwhile, the TyG index was significantly higher in the restenosis group than in the non-restenosis group (8.55 ± 0.53 vs. 9.02 ± 0.65, p < 0.001; Figure 3B). The 12- and 24-month cumulative restenosis-free rates in group A were 99.0 and 91.2%, respectively versus 94.7 and 78.3%, respectively in group B. The log-rank test showed that the cumulative restenosis-free rate in group B was significantly lower than that in group A (p = 0.005; Figure 4).
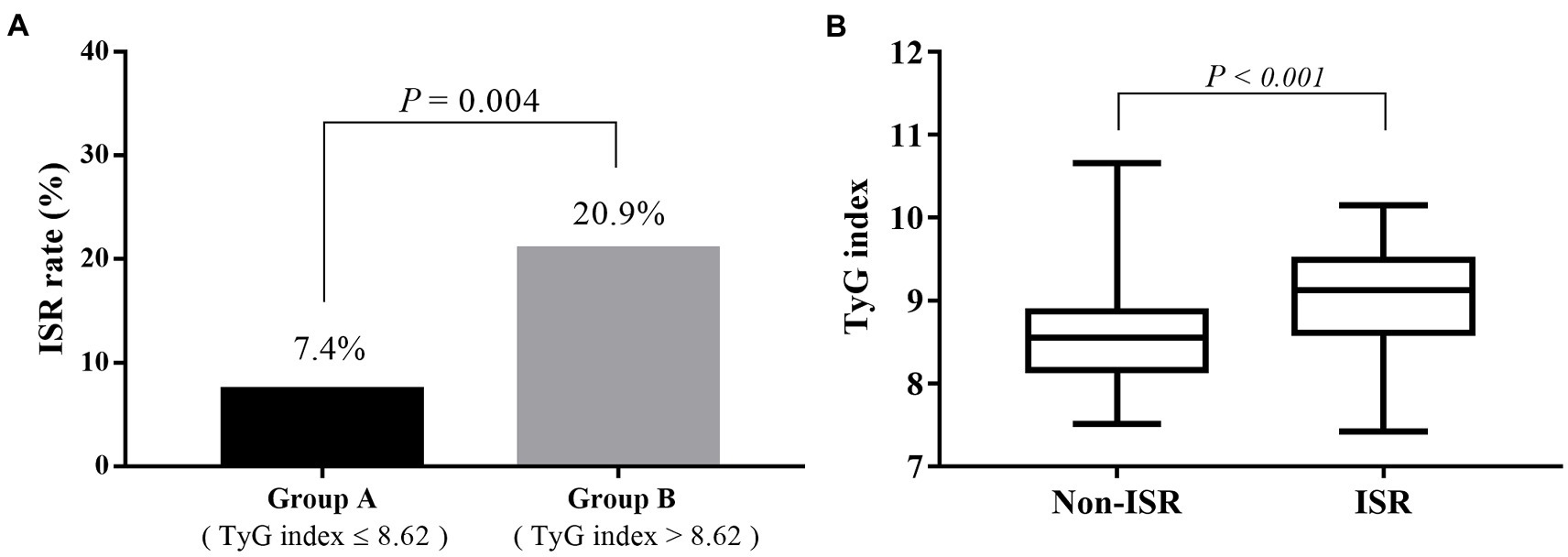
Figure 3. The relevance of the preoperative TyG index on the risk of restenosis. (A) The restenosis rate of participants in group B was significantly higher than that in group A (20.9 vs. 7.4%, p = 0.004). (B) The TyG index was significantly higher in the restenosis group than in the non-restenosis group (8.55 ± 0.53 vs. 9.02 ± 0.65, p < 0.001). TyG, triglyceride–glucose.
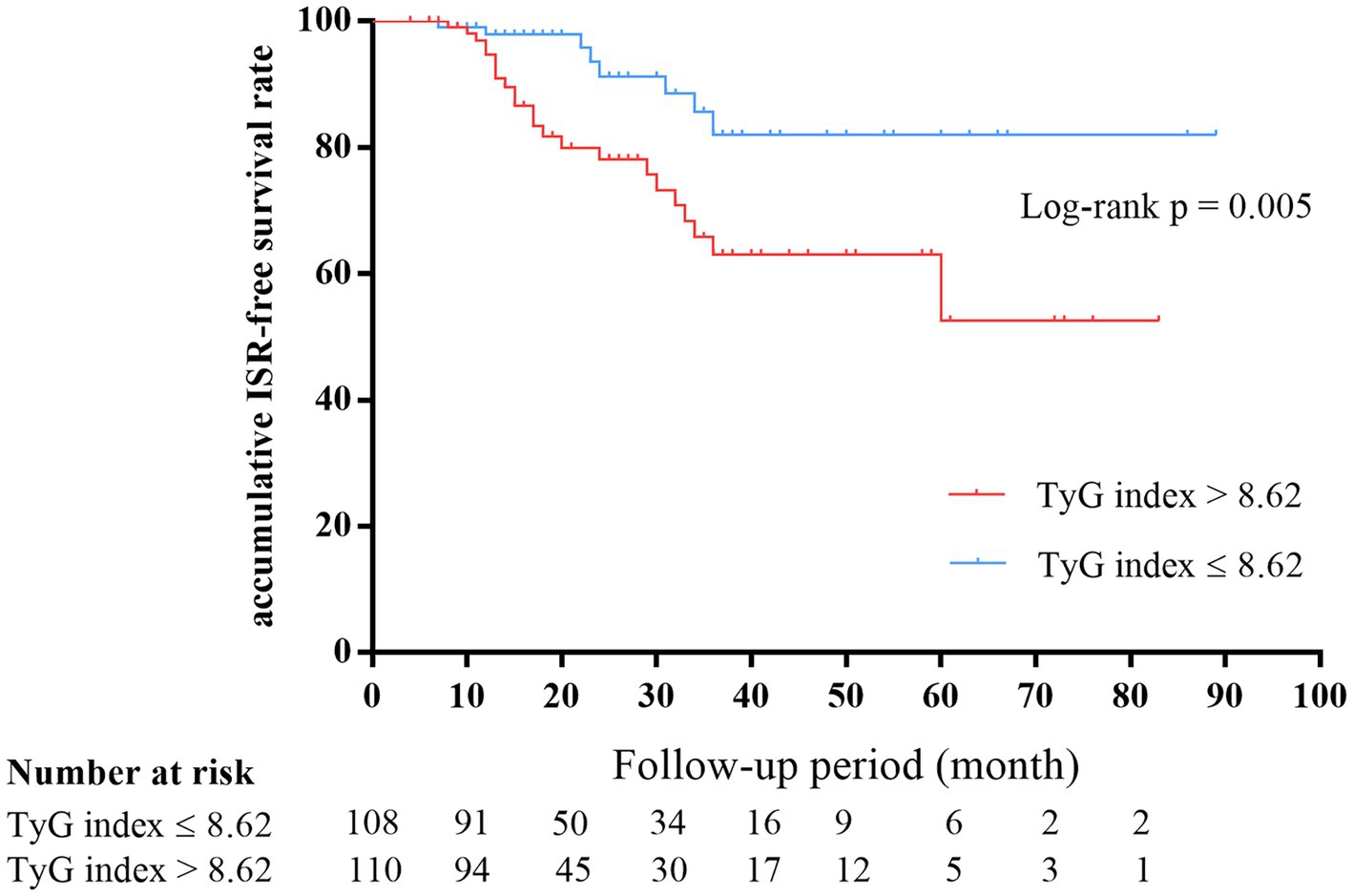
Figure 4. Kaplan–Meier survival estimates showed that the cumulative restenosis-free rate in the high TyG index group was significantly lower than that in the low group (p = 0.005). TyG, triglyceride–glucose.
The univariate Cox regression analysis results are presented in Table 2. The TyG index (p < 0.001), BMI (p = 0.037), TG (p = 0.002), FBG (p = 0.007), and rate of diabetes mellitus (p = 0.012) were significantly different between the groups. Variables with p < 0.2 were entered into the backward stepwise regression model. TyG index and minimum stent diameter were entered as independent predictors of ISR, as shown in Table 3. At < 29 months of follow-up, the risk of restenosis increased by 5.135 (2.463–10.709) times for every 1-unit increase in the TyG index. Participants with a minimum stent diameter of ≥ 7 mm were 0.463 (0.221–0.969) times more likely to develop restenosis than those with a minimum stent diameter of 6 mm. After further adjustment for potential clinical relevance, the TyG index (4.347, 1.886–10.023) was still a robust predictor of ISR. Figure 5 shows the dose–response relationship of the TyG index with ISR. There was a trend towards an increased risk of restenosis with higher TyG indexes.
The subgroup analysis within 29 months post-surgery is presented in Figure 6. The association between the TyG index and ISR was still consistent (P for interactions >0.05) in the subgroups of sex (male or female), BMI (≤ 23 or > 23 kg/m2), symptomatic stenosis (yes or no), current smoking (yes or no), and diabetes mellitus (yes or no). However, there was a significant difference in the hypertension subgroups (yes or no; p for interactions = 0.013) and a marginally significant difference in the age subgroups (≤ 71 or > 71 years; p = 0.061). HRs tended to be higher in the age ≤ 71 years subgroup (p < 0.001) and participants with hypertension (p < 0.001).
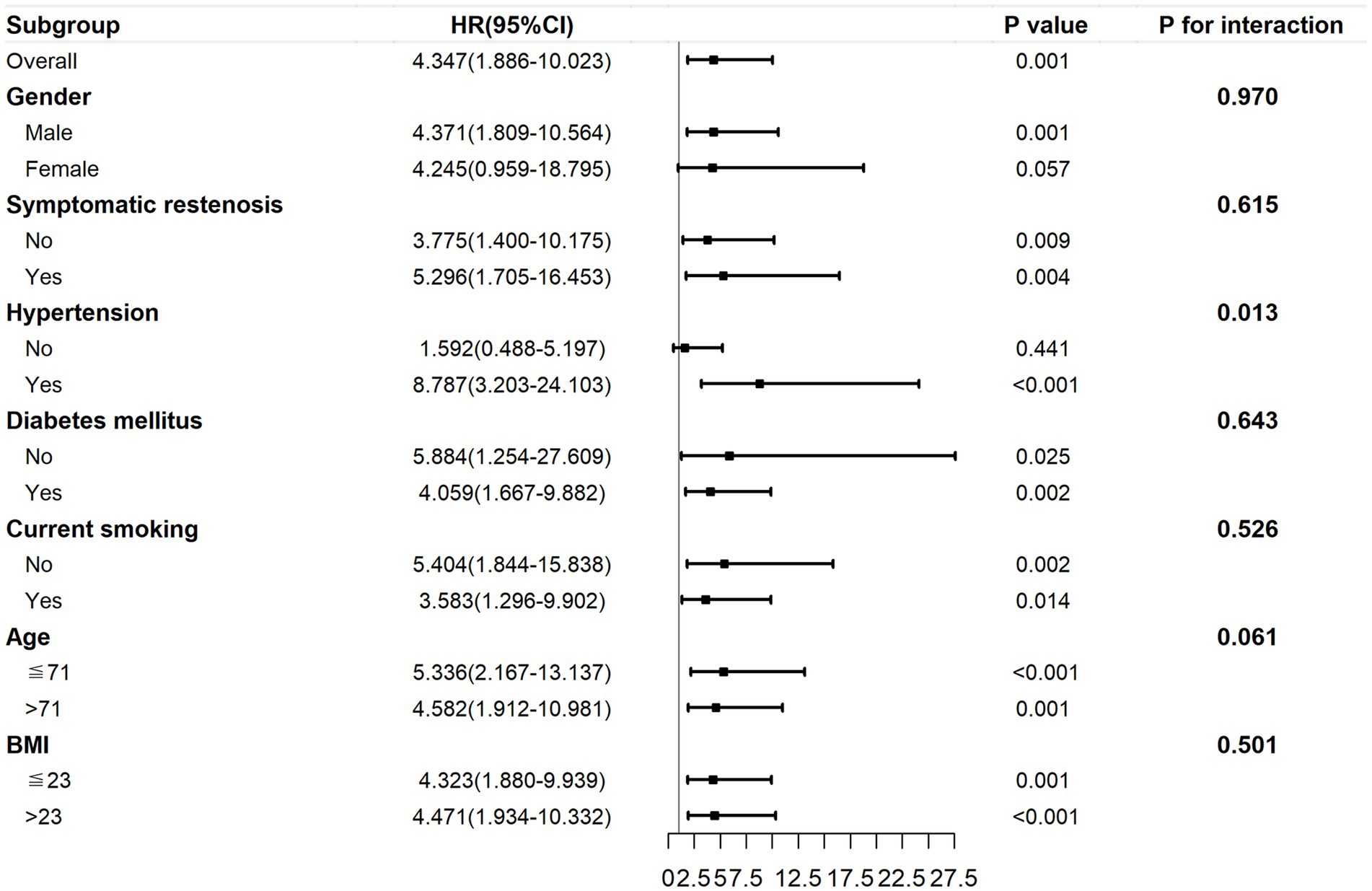
Figure 6. The subgroup analysis of the association between the preoperative TyG index and ISR within 29 months post-surgery. The TyG index was adjusted for variables with p < 0.2 in univariate Cox regression and variables with potential clinical relevance. TyG, triglyceride–glucose; ISR, in-stent restenosis.
During the follow-up period, adverse events occurred in 27 (12.4%) participants. The prevalence of transient ischemic attack, ipsilateral stroke, all ischemic stroke, myocardial infarction, and death were one (0.5%), five (2.3%), 15 (6.9%), five (2.3%), and one (0.5%), respectively. The participants in group B had a higher incidence of total adverse events (17.3% vs. 7.4%, p = 0.027). In addition, the median follow-up period was 17 months (range: 6–89 months). The follow-up period was not significantly different in the two groups[18.00 (13.0, 34.00) vs. 15.50 (12.00, 33.25) months, p = 0.616] (Table 4).
A Cox regression of the log hazard ratio on a covariate with a standard deviation of 0.5690 based on a sample of 220 observations achieves 90.3% power at a 0.05 significance level to detect a regression coefficient equal to 1.1560. The sample size was adjusted since a multiple regression of the variable of interest on the other covariates in the Cox regression is expected to have an R-squared of 0.215. The sample size was adjusted for an anticipated event rate of 0.142. The results showed that the statistical power of the sample size of 218 was sufficient. A detailed report of the statistical power calculations is provided in the Supplementary material.
The Cox regression model is most commonly used for time-to-event analyses. However, it has to satisfy the PH assumption that the HRs in the two groups remain constant over time. In our study, we demonstrated that the preoperative TyG index violated the PH assumption, implying that it had a time-varying effect on ISR. Within 29 months post-surgery, an increasing preoperative TyG index was linked to a significant increased risk of restenosis. However, after 29 months, the effect was decreased, although not statistically significant. This may imply that we should be alert to the possibility of short-term restenosis in patients with a higher preoperative TyG index. The hazard reduction over time may be due to the instability of the TyG index during long-term follow-up. The TyG index can be influenced by medication and disease progression, thus leading to a weakening of the association. In addition, inadequate sample size may also have contributed to this result. To further clarify the relationship between TyG index and restenosis, restricted cubic spline was also used. Restricted cubic spline is one of the commonly used methods to study nonlinear relationships. This method can fit the curve relationship of independent variables and realize the association analysis between continuous exposure and outcome (19, 20). In this study, the risk of restenosis increased with the increase in the TyG index. Moreover, we stratified baseline variables and demonstrated that a higher TyG index posed greater risks to younger patients or those with hypertension.
There are some new studies about the relationship between the TyG index and carotid atherosclerosis. Wang et al. (21) determined that a higher TyG index was linked to the occurrence of carotid plaques in non-diabetic participants through a large sample study of 24,895 urban workers. Li et al. (22) discovered that the TyG index and carotid plaques were significantly associated in participants with coronary heart disease. Li et al. (23) investigated the relationship between the TyG index and carotid atherosclerosis in a general population aged ≥ 40 years from five cities and discovered that the TyG index had a significant relationship with the progression of carotid intima-media thickness, plaques, and stenosis. These findings indirectly support our research finding that the TyG index may contribute to restenosis after CAS for atherosclerotic carotid stenosis. Additionally, the TyG index has been confirmed to be related to hypertension (24) and diabetes mellitus (25, 26), which are recognized as risk factors for ISR. However, the relationship between TyG index and ISR has rarely been studied. Zhu et al. (9) retrospectively enrolled participants who underwent percutaneous coronary stenting. They discovered that a higher TyG index increased the risk of ISR through multivariate logistic regression. Since the pathogenesis of restenosis is similar between coronary and carotid stents (27, 28), we hypothesized that the TyG index might also contribute to ISR after CAS. In contrast to the aforementioned research, the participants were followed up over a much longer period of time (6–89 months) than were those in the Zhu et al. study (6–24 months). Moreover, we used survival analysis and multivariate Cox regression and demonstrated that the effect of preoperative TyG index on ISR varied with time. Notably, during the follow-up, we also observed a higher prevalence of adverse events in participants with a high TyG index. This result is in line with the previous findings. In patients with acute coronary syndrome, for instance, a higher TyG index can increase the prevalence of myocardial infarction, stroke, and other cardiovascular events (29, 30).
The mechanism underlying the positive correlation between the TyG index and ISR remains unclear. We suspected that this might be related to pathophysiological changes caused by IR. First, it has been established that neointimal hyperplasia is the primary cause of ISR in its early stages (within 2 years after stenting) (31). IR can induce proliferation and migration of vascular smooth muscle cells, leading to endointimal hyperplasia (32). Second, long-term restenosis after stenting may be associated with recurrent atherosclerosis and plaque formation (33, 34). IR can lead to endothelial dysfunction by reducing nitric oxide production and increasing the release of procoagulant factors, thus leading to recurrent atherosclerosis (35, 36). Previous studies have reported that some perioperative acute-phase reactants such as hs-CRP (16) and leukocytes (37) are related to ISR. Short-term elevation of these inflammatory indicators is often associated with endothelial injury caused by stent implantation. However, the development of ISR is a long-term process. Unlike these inflammatory indicators, the TyG index has a persistent effect on the human body.
It is well known that advanced age, female sex, hypertension, diabetes mellitus, smoking, and symptomatic stenosis are positively associated with restenosis after CAS (38–40). Interestingly, in our subgroup analysis, younger patients were at a higher risk of TyG-related restenosis. The reason for this phenomenon remains unclear. The results of our study indicate that more attention should be paid to the control of the TyG index in young patients. Our result was consistent with that of Li et al. (23), in which middle-aged participants had a higher prevalence of intima-media thickness and plaque formation than elderly participants. Li et al. (22) also reported that the correlation between the TyG index and carotid atherosclerosis was stronger in middle-aged participants. However, findings reported by Zhu et al. (9) were contrary to our findings. In their study, the association between the TyG index and ISR after coronary stenting was higher in participants older than 65 years. The reason for this may lie in the different study populations.
This study had some limitations. First, it was a retrospective cohort study. As a result, the follow-up intervals were not strictly set, which could have led to inaccurate endpoint times in some participants. Second, the sample size was small, particularly with respect to the number of participants with ISR. Although this is partly due to the low incidence rate of ISR, a prospective study with a larger sample size could provide more evidence. Third, Doppler ultrasound and CTA were used to assess ISR in our study. However, ultrasound velocity criteria for evaluating ISR are lacking. This is one of the reasons why restenosis rates vary widely among studies. Fourth, the TyG index was obtained only from preoperative laboratory data, whereas serum TG and FBG levels are dynamic parameters that can be influenced by medication and disease progression. Therefore, the association between the trajectory of the TyG index in follow-up and the risk of ISR needs further study.
In conclusion, the preoperative TyG index had a positive effect on the risk of short-term restenosis after CAS within 29 months post-surgery. As a simple and low-cost index, the TyG index may be employed to stratify patients based on their risk of restenosis after carotid artery stenting.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Academic Ethics Committee of Shaoxing People’s Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
BW and S-sZ were responsible for collecting the data of the patients. X-tL and J-bZ contributed to the study design. S-sZ wrote the manuscript. J-bZ and S-sZ performed the data analysis. All authors contributed to the article and approved the submitted version.
This study was supported by the General Project of Zhejiang Health Science, Technology Plan (Nos. 2021KY1147 and 2023KY1248), Shaoxing People’s Hospital Youth Research Fund (2022YB06), and Shaoxing Medical Key Discipline (2019SZD05).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1159601/full#supplementary-material
1. Ng, M, Fleming, T, Robinson, M, Thomson, B, Graetz, N, Margono, C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the global burden of disease study 2013. Lancet (London, England). (2014) 384:766–81. doi: 10.1016/s0140-6736(14)60460-8
2. Halliday, A, Bulbulia, R, Bonati, LH, Chester, J, Cradduck-Bamford, A, Peto, R, et al. Second asymptomatic carotid surgery trial (ACST-2): a randomised comparison of carotid artery stenting versus carotid endarterectomy. Lancet (London, England). (2021) 398:1065–73. doi: 10.1016/s0140-6736(21)01910-3
3. Lal, BK, Beach, KW, Roubin, GS, Lutsep, HL, Moore, WS, Malas, MB, et al. Restenosis after carotid artery stenting and endarterectomy: a secondary analysis of CREST, a randomised controlled trial. Lancet Neurol. (2012) 11:755–63. doi: 10.1016/S1474-4422(12)70159-X
4. Bonati, LH, Ederle, J, McCabe, DJ, Dobson, J, Featherstone, RL, Gaines, PA, et al. Long-term risk of carotid restenosis in patients randomly assigned to endovascular treatment or endarterectomy in the carotid and vertebral artery transluminal angioplasty study (CAVATAS): long-term follow-up of a randomised trial. Lancet Neurol. (2009) 8:908–17. doi: 10.1016/S1474-4422(09)70227-3
5. Ormazabal, V, Nair, S, Elfeky, O, Aguayo, C, Salomon, C, and Zuñiga, FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. (2018) 17:122. doi: 10.1186/s12933-018-0762-4
6. O'Hagan, R, Gonzalez-Cantero, A, Patel, N, Hong, CG, Berg, AR, Li, H, et al. Association of triglyceride glucose index with insulin resistance and subclinical atherosclerosis in psoriasis: an observational cohort study. J Am Acad Dermatol. (2022): S0190-9622(22)02551-8. doi: 10.1016/j.jaad.2022.08.027 [Online ahead of print].
7. Zhao, LP, Xu, WT, Wang, L, Li, H, Shao, CL, Gu, HB, et al. Influence of insulin resistance on in-stent restenosis in patients undergoing coronary drug-eluting stent implantation after long-term angiographic follow-up. Coron Artery Dis. (2015) 26:5–10. doi: 10.1097/MCA.0000000000000170
8. Minh, HV, Tien, HA, Sinh, CT, Thang, DC, Chen, CH, Tay, JC, et al. Assessment of preferred methods to measure insulin resistance in Asian patients with hypertension. J Clin Hypertens (Greenwich). (2021) 23:529–37. doi: 10.1111/jch.14155
9. Zhu, Y, Liu, K, Chen, M, Liu, Y, Gao, A, Hu, C, et al. Triglyceride-glucose index is associated with in-stent restenosis in patients with acute coronary syndrome after percutaneous coronary intervention with drug-eluting stents. Cardiovasc Diabetol. (2021) 20:137. doi: 10.1186/s12933-021-01332-4
10. Jiang, ZZ, Zhu, JB, Shen, HL, Zhao, SS, Tang, YY, Tang, SQ, et al. A high triglyceride-glucose index value is associated with an increased risk of carotid plaque burden in subjects with prediabetes and new-onset type 2 diabetes: a real-world study. Front Cardiovasc Med. (2022) 9:832491. doi: 10.3389/fcvm.2022.832491
11. Miura, Y, Kanamaru, H, Yasuda, R, Toma, N, and Suzuki, H. Nonfasting triglyceride as an independent predictor of carotid restenosis after carotid endarterectomy or carotid artery stenting. World Neurosurg. (2021) 156:e415–25. doi: 10.1016/j.wneu.2021.09.091
12. Guerrero-Romero, F, Simental-Mendía, LE, González-Ortiz, M, Martínez-Abundis, E, Ramos-Zavala, MG, Hernández-González, SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. (2010) 95:3347–51. doi: 10.1210/jc.2010-0288
13. Classification and Diagnosis of Diabetes . Standards of medical care in diabetes-2020. Diabetes Care. (2020) 43:S14–s31. doi: 10.2337/dc20-S002
14. Yan, Y, Wang, D, Sun, Y, Ma, Q, Wang, K, Liao, Y, et al. Triglyceride-glucose index trajectory and arterial stiffness: results from Hanzhong adolescent hypertension cohort study. Cardiovasc Diabetol. (2022) 21:33. doi: 10.1186/s12933-022-01453-4
15. Sacco, RL, Kasner, SE, Broderick, JP, Caplan, LR, Connors, JJ, Culebras, A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2013) 44:2064–89. doi: 10.1161/STR.0b013e318296aeca
16. Schillinger, M, Exner, M, Mlekusch, W, Rumpold, H, Ahmadi, R, Sabeti, S, et al. Acute-phase response after stent implantation in the carotid artery: association with 6-month in-stent restenosis. Radiology. (2003) 227:516–21. doi: 10.1148/radiol.2272020183
17. Therneau, G. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. (1994) 81:515–26. doi: 10.1093/biomet/81.3.515
18. Klein, JP, and Moeschberger, ML. Survival Analysis: Techniques for Censored and Truncated Data. New York, NY: Springer (2003).
19. Roshani, D, and Ghaderi, E. Comparing smoothing techniques for fitting the nonlinear effect of covariate in cox models. Acta Inform Med. (2016) 24:38–41. doi: 10.5455/aim.2016.24.38-41
20. Durrleman, S, and Simon, R. Flexible regression models with cubic splines. Stat Med. (1989) 8:551–61. doi: 10.1002/sim.4780080504
21. Wang, A, Li, Y, Zhou, L, Liu, K, Li, S, Song, B, et al. Triglyceride-glucose index is related to carotid plaque and its stability in nondiabetic adults: a cross-sectional study. Front Neurol. (2022) 13:823611. doi: 10.3389/fneur.2022.823611
22. Li, Z, He, Y, Wang, S, Li, L, Yang, R, Liu, Y, et al. Association between triglyceride glucose index and carotid artery plaque in different glucose metabolic states in patients with coronary heart disease: a RCSCD-TCM study in China. Cardiovasc Diabetol. (2022) 21:38. doi: 10.1186/s12933-022-01470-3
23. Li, W, Chen, D, Tao, Y, Lu, Z, and Wang, D. Association between triglyceride-glucose index and carotid atherosclerosis detected by ultrasonography. Cardiovasc Diabetol. (2022) 21:137. doi: 10.1186/s12933-022-01570-0
24. Cai, Q, Xing, CY, Zhu, J, Wang, Y, Lu, F, and Peng, J. Associations between triglyceride-glucose index and different hypertension subtypes: a population-based study in China. Front Cardiovasc Med. (2022) 9:901180. doi: 10.3389/fcvm.2022.901180
25. Song, B, Zhao, X, Yao, T, Lu, W, Zhang, H, Liu, T, et al. Triglyceride glucose-body mass index and risk of incident type 2 diabetes mellitus in Japanese people with Normal glycemic level: a population-based longitudinal cohort study. Front Endocrinol. (2022) 13:907973. doi: 10.3389/fendo.2022.907973
26. Li, X, Sun, M, Yang, Y, Yao, N, Yan, S, Wang, L, et al. Predictive effect of triglyceride glucose-related parameters, obesity indices, and lipid ratios for diabetes in a Chinese population: a prospective cohort study. Front Endocrinol. (2022) 13:862919. doi: 10.3389/fendo.2022.862919
27. Giustino, G, Colombo, A, Camaj, A, Yasumura, K, Mehran, R, Stone, GW, et al. Coronary in-stent restenosis: JACC state-of-the-art review. J Am Coll Cardiol. (2022) 80:348–72. doi: 10.1016/j.jacc.2022.05.017
28. Dai, Z, and Xu, G. Restenosis after carotid artery stenting. Vascular. (2017) 25:576–86. doi: 10.1177/1708538117706273
29. Akbar, MR, Pranata, R, and Wibowo, A. Irvan, Sihite TA, Martha JW. The association between triglyceride-glucose index and major adverse cardiovascular events in patients with acute coronary syndrome - dose-response meta-analysis. Nutr Metab Cardiovasc Dis. (2021) 31:3024–30. doi: 10.1016/j.numecd.2021.08.026
30. Guo, Q, Feng, X, Zhang, B, Zhai, G, Yang, J, Liu, Y, et al. Influence of the triglyceride-glucose index on adverse cardiovascular and cerebrovascular events in prediabetic patients with acute coronary syndrome. Front Endocrinol. (2022) 13:843072. doi: 10.3389/fendo.2022.843072
31. Hunter, GC, and Edgar, J, Poth Memorial/W.L. Gore and Associates, Inc. Lectureship. The clinical and pathological spectrum of recurrent carotid stenosis. Am J Surg. (1997) 174:583–8. doi: 10.1016/S0002-9610(97)80927-0
32. Qi, W, Li, Q, Liew, CW, Rask-Madsen, C, Lockhart, SM, Rasmussen, LM, et al. SHP-1 activation inhibits vascular smooth muscle cell proliferation and intimal hyperplasia in a rodent model of insulin resistance and diabetes. Diabetologia. (2017) 60:585–96. doi: 10.1007/s00125-016-4159-1
33. Tokunaga, K, Tokunaga, S, Hara, K, Yasaka, M, Okada, Y, Kitazono, T, et al. Intraplaque high-intensity signal on time-of-flight magnetic resonance angiography and restenosis after carotid artery stenting. J Neurosurg. (2022) 136:1029–34. doi: 10.3171/2021.4.JNS21546
34. AbuRahma, AF, Abu-Halimah, S, Hass, SM, Nanjundappa, A, Stone, PA, Mousa, A, et al. Carotid artery stenting outcomes are equivalent to carotid endarterectomy outcomes for patients with post-carotid endarterectomy stenosis. J Vasc Surg. (2010) 52:1180–7. doi: 10.1016/j.jvs.2010.06.074
35. Tousoulis, D, Simopoulou, C, Papageorgiou, N, Oikonomou, E, Hatzis, G, Siasos, G, et al. Endothelial dysfunction in conduit arteries and in microcirculation. Novel therapeutic approaches. Pharmacol Ther. (2014) 144:253–67. doi: 10.1016/j.pharmthera.2014.06.003
36. Galluccio, E, Piatti, P, Citterio, L, Lucotti, PC, Setola, E, Cassina, L, et al. Hyperinsulinemia and impaired leptin-adiponectin ratio associate with endothelial nitric oxide synthase polymorphisms in subjects with in-stent restenosis. Am J Physiol Endocrinol Metab. (2008) 294:E978–86. doi: 10.1152/ajpendo.00003.2008
37. Wasser, K, Schnaudigel, S, Wohlfahrt, J, Psychogios, MN, Knauth, M, and Gröschel, K. Inflammation and in-stent restenosis: the role of serum markers and stent characteristics in carotid artery stenting. PLoS One. (2011) 6:e22683. doi: 10.1371/journal.pone.0022683
38. Zapata-Arriaza, E, Moniche, F, González, A, Bustamante, A, Escudero-Martínez, I, De la Torre Laviana, FJ, et al. Predictors of restenosis following carotid angioplasty and stenting. Stroke. (2016) 47:2144–7. doi: 10.1161/STROKEAHA.116.012650
39. Khan, MA, Liu, MW, Chio, FL, Roubin, GS, Iyer, SS, and Vitek, JJ. Predictors of restenosis after successful carotid artery stenting. Am J Cardiol. (2003) 92:895–7. doi: 10.1016/S0002-9149(03)00912-3
Keywords: TyG index, insulin resistance, carotid artery stenting, in-stent restenosis, adverse event
Citation: Zhao S-s, Jiang Z-z, Wei B, Zhu J-b and Liu X-t (2023) The preoperative triglyceride-glucose index has a positive effect on predicting the risk of short-term restenosis after carotid artery stenting: a retrospective cohort study. Front. Neurol. 14:1159601. doi: 10.3389/fneur.2023.1159601
Received: 06 February 2023; Accepted: 27 March 2023;
Published: 17 April 2023.
Edited by:
Osama O. Zaidat, Northeast Ohio Medical University, United StatesReviewed by:
Kecheng Yao, The People's Hospital of China Three Gorges University, ChinaCopyright © 2023 Zhao, Jiang, Wei, Zhu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xia-tian Liu, bHh0MjAxNUAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.