
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol. , 11 July 2023
Sec. Neurorehabilitation
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1156987
This article is part of the Research Topic Combining a non-invasive transcranial stimulation technique with another therapeutic approach: mechanisms of action, therapeutic interest and tolerance View all 10 articles
Stroke is a central nervous system disease that causes structural lesions and functional impairments of the brain, resulting in varying types, and degrees of dysfunction. The bimodal balance-recovery model (interhemispheric competition model and vicariation model) has been proposed as the mechanism of functional recovery after a stroke. We analyzed how combinations of motor observation treatment approaches, transcranial electrical (TES) or magnetic (TMS) stimulation and peripheral electrical (PES) or magnetic (PMS) stimulation techniques can be taken as accessorial physical therapy methods on symptom reduction of stroke patients. We suggest that top-down and bottom-up stimulation techniques combined with action observation treatment synergistically might develop into valuable physical therapy strategies in neurorehabilitation after stroke. We explored how TES or TMS intervention over the contralesional hemisphere or the lesioned hemisphere combined with PES or PMS of the paretic limbs during motor observation followed by action execution have super-additive effects to potentiate the effect of conventional treatment in stroke patients. The proposed paradigm could be an innovative and adjunctive approach to potentiate the effect of conventional rehabilitation treatment, especially for those patients with severe motor deficits.
Stroke is a neurological syndrome caused by an acute vascular injury of the central nervous system. The syndrome incorporates the cerebral infarction, intracerebral hemorrhage, and subarachnoid hemorrhage (1). It is one of the primary causes of mortality and severe long-term disability. Among all causes of death, stroke ranks fifth following heart disease, cancer, chronic lower respiratory disease, and unintentional injuries/accidents (2). In 2019, the prevalence of stroke was 101 million cases and there were 6.55 million deaths in global (3). As a major concern of global health, stroke poses great social economic burden, for example, the overall expenses of stroke in US was $52.8 billion in 2017–2018, with mean direct expenses of $8,242 for each patient (4). Stroke ranks second among all the contributors to disability-adjust life-years globally (5). Long-term complications of stroke include pain syndromes, depression and anxiety, cognitive decline and dementia, as well as falls and fractures due to gait instability (2). Motor impairment of the contralateral limb (e.g., loss or limitation of muscle control, mobility, power, and dexterity) is one of the commonest and most detrimental consequences after stroke (6, 7). Dysfunctional motor control affects functional independence of activities of daily living, and thus reduces the quality of life.
Neurorehabilitation after a stroke includes multidisciplinary rehabilitation methods to compensate for the motor deficit, restore motor functions, and improve the life quality of patients (8, 9). Despite intensive therapeutic efforts during stroke rehabilitation, a relevant amount of stroke survivors failed to regain their motor functions that are important for activities of daily living completely (10). Therefore new/advanced approaches are required to optimize motor functions and reduce disability in stroke patients. Based on basic behavioral science and neuroscientific knowledge, novel rehabilitative approaches have been developed to ameliorate perceptual abilities and improve motor functions after stroke in the last few years (11, 12). These novel rehabilitative intervention modalities included action observation treatment (AOT), non-invasive brain stimulation (NIBS) as well as repetitive peripheral electrical or magnetic stimulation (13). These tools share features of targeted modulation of central nervous system activity, and neuroplasticity induction, and might hereby generate therapeutic benefits (11, 14). In this perspective paper, we aimed to discuss how combinations with these novel stimulation techniques and approaches can be taken as potential rehabilitation methods for stroke patients.
Brain structural damage of areas and connections, as well as inhibition of the ipsilesional primary motor and sensory cortex disrupts functional connectivity of the motor network and impairs functional network flexibility after stroke (15). A bimodal balance-recovery model has been proposed as the mechanism of functional recovery after a stroke. The extent of structural reserve of the lesioned hemisphere is related to functional reorganization and the involvement of the affected hemisphere in motor control (16). The interhemispheric competition model dominates in stroke patients with high structural reserve (less impairment) (16). Functional neuroimaging studies showed a dysbalance of motor cortex excitability in post-stroke, which is relative hypo-excitability in the ipsilesional hemisphere and hyper-excitability in the contralesional hemisphere (16–18). The hyperactive contralesional hemisphere inhibits cortical excitability of the ipsilesional hemisphere via transcallosal inhibition, and compromises motor output (19, 20). Based on the inter-hemispheric competition model, upregulating the excitability of the lesioned hemisphere and/or downregulating the excitability of the intact hemisphere may facilitate recovery in stroke patients (21). In patients with little structural reserve (more severe impairment), the vicariation model predicts stroke recovery. Activity in the contralesional hemisphere compensates for functional loss by the affected hemisphere (16). In this case, instead of predicting a worse outcome on the basis of the interhemispheric competition model, interhemispheric imbalance facilitates vicarious activity of the intact hemisphere, allowing substitutional plasticity (16). A recent longitudinal study by Lin et al. has verified this bimodal balance recovery hypothesis, indicating that the contralesional hemisphere modulates differently across chronic stroke patients with different levels of ipsilesional hemisphere reserve (22).
Neuroplasticity is an important physiological foundation for the neurorehabilitation of stroke patients. It refers to the life-long ability of the central nervous system for reorganization and adaptation, which includes strengthening and weakening synaptic connections, as well as the formation of new neural pathways. Neuroplasticity is a crucial foundation for learning and memory formation, and recovery of motor functions after neurological injuries (9). Modifying neural circuit function in response to external/environmental stimuli and subsequently affecting behavior, cognition, and motor function is a crucial property of the mammalian brain (23, 24). Functional plasticity and structural plasticity are two types of plasticity mechanisms (25). Functional plasticity refers to alterations in the strength of preexisting synaptic transmission, whereas structural plasticity incorporates the growth and deletion of synaptic connections (23, 25, 26). Synaptic plasticity can occur from the ultrastructure level to the brain network level along with short- and long-term alternations in Ca2+ dynamics, modulation of neurotransmission as well as expression of protein and gene (27). Synaptic plasticity is classified into Hebbian and homeostatic synaptic plasticity (25, 28). Hebbian synaptic plasticity is a positive feedback loop and unrestricted dynamics via strengthening (long-term potentiation, LTP) or weakening (long-term depression, LTD) of synaptic transmission (24, 26, 29). In contrast, homeostatic synaptic plasticity is a negative feedback loop and stabilized neural dynamics in which synaptic efficacy decreases in the case of high neuronal activities and increases when activities are low (25, 30). Animal studies largely contributed to our knowledge about physiological plasticity mechanisms and led to further investigations of neuroplasticity in humans. In the neocortex, studies in animal models demonstrated a close association between motor learning and LTP-like plasticity (31–33). In humans, LTP-like plasticity was explored in the primary motor cortex (M1) concerning use-dependent plasticity (34–37), its involvement in motor learning (38), and its relevance for compensation of motor cortex dysfunctions after brain lesions (39). Post-transcriptional modifications of pe-existing protein account for LTP in the early phase, whereas alternations in the expression of genes and protein relate to LTP in the late phase (27). It has been shown that for studying the plasticity of the human brain, sensory inputs and non-invasive brain stimulation (NIBS) are able to alter respective cortical properties such as the strength of neural network connections, and movement representations (40, 41). Beyond its relevance to the learning formation of the healthy brain, cortical reorganization and adaptive plasticity apply to the field of neurorehabilitation (42–44).
Action observation and execution networks were found first in macaque monkeys. These networks are based on mirror neurons which are all-important to comprehending the actions of other individuals (45). The notion of mirror mechanisms displays that individuals observing an action could not only activate an identical or similar motor or motor-related cortical network but also automatically promote execution and motor skill acquisition in an observer (46, 47). Functional neuroimaging studies showed an observation-execution-dependent cortical network in human brains and revealed the overlapping of motor observation and motor execution in some brain regions. These networks incorporate M1, the primary somatosensory cortex, the ventral premotor cortex, several parietal areas, and the inferior frontal gyrus (48–52).
Respective observation-related network activation via observing a goal-directed movement of others promotes motor skill learning abilities and attainment of observers (53–55). Since long-term potentiation-like (LTP) plasticity is elevated by enhanced task-dependent motor cortex excitability (31, 56), the underlying mechanism of acquisition of a new motor skill via action observation might include LTP-like plasticity of these specific brain regions and network (57–60). Motor cortex activation by action observation might thus have the potential to develop into an effective rehabilitative strategy. In healthy humans, action observation enhances motor skill learning (46, 61–63), and action-related motor capacity with the untrained hand (64). AOT, in which action observation followed by execution of an identical task, has been used to alleviate motor function deficits in patients with neurological disorders (65). A typical rehabilitation session of AOT consists of an observation phase and an execution phase. A video clip of an actor and an actress performing object-directed daily action from different perspectives is presented on a computer screen. Specific action can be divided into three to four motor acts. Patients need to observe the motor act and execute the observed act afterwards (65, 66). In patients with acute ischemic stroke, AOT for 10 days facilitates relearning of upper extremity motor skills (67). For patients diagnosed with cerebral ischemic or hemorrhagic stroke in the subacute phase, AOT potentiated upper extremity motor function recovery, improved manual dexterity, and increased quality of life (68). AOT for 4 weeks improved upper extremity function and daily living performance in chronic stroke patients, and AOT of first-person perspective showed more beneficial effects in comparison with AOT of third-person perspective (69). AOT for 4 weeks has also been shown to promote gait ability in chronic stroke patients, and functional AOT was more effective than general AOT (70).
TMS produces a time-varying magnetic field perpendicular to the stimulating coil, inducing electric currents in the cortical tissue beneath the scalp, and eliciting action potentials in targeted neuronal populations. As a neuromodulatory tool, repetitive TMS (rTMS) induces frequency-dependent after-effects. Low-frequency rTMS (LF-rTMS, ≤1 Hz) induces a prolonged decrease in cortical excitability, whereas high-frequency stimulation (HF-rTMS, ≥5 Hz) enhances cortical excitability (10, 71). Theta burst stimulation (TBS) is a subtype of rTMS, including intermittent (iTBS) and continuous (cTBS) stimulation that enhances and suppresses cortical excitability, respectively (72–74). HF-rTMS delivered to M1 concurrent with motor learning practice accelerated the rate of motor skill acquisition and improved motor performance in healthy individuals (75). It is assumed that the effect of this combined intervention is accomplished by the induction of LTP-like processes in the motor network, which promotes task-specific plasticity (75). In subacute hemorrhagic and ischemic stroke patients, delivery of HF-rTMS in the affected hemisphere facilitated motor function recovery of the paralytic hand (76). HF-rTMS over ipsilesional M1 promoted upper extremity motor recovery and daily living ability in acute stroke patients suffering from unilateral subcortical infarction in the middle cerebral artery (77). In subacute ischemic stroke patients, iTBS over the lesioned M1 prior to physiotherapy increased network connectivity between bilateral motor areas and M1, which is correlated with grip strength improvement (78). Resting-state interhemispheric motor network connectivity gradually decreases early after ischemic stroke and subsequently re-increases in the progress of motor function recovery (79). Application of iTBS facilitates reorganization of the motor network and induces neuronal plasticity, contributing to motor function recovery (78). It is proposed that HF-rTMS (76) and iTBS (78) over the ipsilesional M1 up-regulates the activity of the lesioned cortex. LF-rTMS applied over the unaffected motor cortex promoted motor function recovery and improved daily living ability in patients with cerebral infarction (80). LF-rTMS (80) and cTBS applied over the unaffected motor cortex down-regulates the excitability of the unaffected hemisphere and alleviates the interhemispheric inhibition imposed on the affected side. However, these approaches fail to induce beneficial effects in all stroke patients, and individuals respond differently to various stimulation parameters (81). Sankarasubramanian and co-workers demonstrated that upper limb reaching ability was facilitated by HF-rTMS over contralesional dorsal premotor cortex rather than standard stimulation approach (LF-rTMS over contralesional M1) in severely affected stroke patients (82). Therefore, classifying stroke patients into different subgroups (less affected vs. more affected) based on bimodal balance-recovery model is necessary for designing targeted and effective treatments.
Some studies showed that transcranial electrical stimulation (tES), including transcranial direct current (tDCS), transcranial random noise (tRNS), and transcranial alternating current (tACS) stimulation can increase the acquisition and retention of motor skills and improve motor functions in healthy humans, and rehabilitation (83, 84). These intervention tools elicit long-lasting augments or decrements of motor cortical excitability, and these effects are dependent on brain state and cognitive task performance before and/or during the intervention (85, 86).
tDCS modulates motor cortex excitability and/or activity via a weak electrical current (87), which de- or hyperpolarizes neuronal resting membrane potentials (86, 88). tDCS has a polarity-dependent influence on motor cortex excitability and/or activity. When the anode is positioned over M1, the amplitude of motor-evoked potentials (MEP) is increased (89, 90), whereas cathodal tDCS decreases MEPs with standard dosages (89, 91). Dependent on stimulation duration, tDCS can induce after-effects, which resemble LTP-like or LTD-like plasticity (85, 86, 92). In healthy humans, anodal tDCS over M1 during task execution improves motor learning (93–96). This effect is likely accomplished via modulation of LTP-like plasticity, and enhancement of functional connectivity of respective brain networks via anodal tDCS, resulting in motor performance improvement. Some studies reported that cathodal tDCS over M1 reduced motor performance speed (95, 97), but improved motor learning under specific conditions (98, 99). It is proposed that cathodal tDCS diminishes cortical excitability (“noise reduction”) via induction of LTD-like plasticity, thus focusing cortical activity on the neurons relevant to motor learning (93, 98, 99). In patient populations, this intervention has the potential to relieve maladaptive neuroplasticity and improve the neurophysiological state of the targeted brain regions as well as motor functions. The effects of tDCS on stroke patients were not consistently reported in different studies. Ojardias and co-authors reported that one session of anodal tDCS over ipsilesional M1 had a significant beneficial effect on gait endurance in chronic hemiplegic patients (100). In chronic ischemic and hemorrhagic stroke patients, two sessions of anodal tDCS applied over the lesioned M1 improved movement planning and preparation in a standing reaching task (101). Likewise, cathodal tDCS can also induce some positive effects in patients with stroke. Zimerman and co-works reported that cathodal tDCS applied to the non-lesioned M1 facilitated hand motor skill acquisition and retention in patients with subcortical ischemic stroke (102). Cathodal tDCS positioned over the unaffected motor cortex enhanced dual-task gait performance in chronic stroke patients (103). Seamon and co-works, however, indicated that neither anodal tDCS over the lesioned M1 nor cathodal tDCS over the non-lesioned M1 induced any significant effect on walking performance in chronic stroke patients (104). The variable effects of tDCS might be due to the inherent heterogeneity of the stroke patients, the variability of the stimulation parameters and the choice of motor paradigms (105). For stroke patients who benefit from tDCS, the interhemispheric balancing model has been proposed as the mechanism for motor function improvement. Anodal tDCS upregulates ipsilesional cortical excitability, improves network connectivity, and leads to alterations in interhemispheric balance (10). Cathodal tDCS over the contralesional M1 leads to downregulation of the contralesional cortical excitability and upregulation of the ipsilesional cortical excitability via reduced transcallosal inhibition (10, 106). Restoration of interhemispheric balance might be a relevant mechanism of tDCS-induced motor control improvement (107). As heterogeneity exists regarding the effect of tDCS on stroke patients, stratifying patients into different subgroups according to the etiology, the damage extent, and the phase of stroke is required to provide personalized therapeutic interventions.
tRNS is a relatively new neuromodulatory electrical stimulation method, which produces a white noise of a Gaussian or bell-shaped alternating current from 0.1 Hz to 640 Hz in a full-frequency spectrum or between 101 and 640 Hz in a high-frequency spectrum (108). Its random electrical oscillation spectrum in a full frequency spectrum or a high frequency spectrum applied to specific brain regions modulates neuronal membrane potentials, induces neuroplasticity, and results in an increase in motor cortex excitability (109–111). Proposed mechanisms of action are modulation of the neural signal-to-noise ratio via stochastic resonance (112, 113), and stimulation effects involve voltage-gated sodium channels (114–116). tRNS facilitates motor skill acquisition and consolidation in healthy humans (111, 117). Regarding the impact of tRNS in neurorehabilitation, Hayward and co-authors demonstrated that tRNS over ipsilesional M1 during reaching training improved clinical motor outcomes in chronic stroke patients suffering from severe arm dysfunction (118). tRNS combined with the Graded Arm Supplementary Program promoted upper extremity motor function recovery in ischemic stroke patients in the subacute phase (119). This implied that tRNS can boost functional adaptations of cortical tissue (118).
In tACS, another electrical non-invasive brain stimulation protocol, weak alternating sinusoidal currents over the cortical target region can entrain endogenous brain oscillations at some frequency brand (120). tACS enhanced either motor functions or cognitive functions via associated brain functions with stimulation frequencies matched to the natural dominant rhythm of the underlying brain area (121, 122). Antal et al. showed that tACS over M1 promoted motor learning in healthy humans (123). Beta-tACS over the lesioned M1 reduced the variance of sensorimotor beta-oscillations in stroke patients (124). With respect to motor rehabilitation, beta-tACS might be suitable for facilitating the specificity of brain self-regulation-based neurofeedback via interference with endogenous cortical rhythms and intrinsic brain oscillations in stroke patients (124).
In the human brain, regions are interconnected in complex functional networks, incorporating multiple anatomically remote but functionally interlinked areas (125–127). Some studies demonstrated that tES modulates brain activity and/or excitability in both local areas under the stimulation electrodes and remote interlinked brain regions (10, 128). Brain hubs have a critical impact on dynamic interactions between brain areas and integrate the information from different brain regions of the network (127, 129, 130). The effects of tES involving a node or hub of a specific cortical network can spread to functionally connected brain areas (128, 131, 132). Due to activity-dependent network models, tES-generated cortical activity and/or excitability alterations are furthermore sensitive to the specific state of brain networks, and dependent on the level of the ongoing activity of the stimulated cortical networks (128, 133). A wealth of studies has reported that tES can modulate behavior dependent on the neural activity level of brain networks involved in a task (98, 99, 134–137).
Beyond non-invasive brain stimulation, peripheral stimulation techniques are also explored for their ability to improve neurorehabilitation. Non-invasive peripheral stimulation uses external devices to generate muscle contractions and sensory afferents that can be used in clinical settings to reduce pain and promote recovery of sensorimotor functions (138). Successful goal-directed movements necessary for interaction with the environment rely on the integration of sensory and motor information (139). Stroke is a common neurological disorder leading to compromised sensorimotor integration (140). Accurate sensorimotor integration of afferent and efferent signals in the cerebral cortex contributes to precise motor control and efficient action execution, and plays a critical role in motor learning. To target sensorimotor integration in stroke patients, either enhancement of afferent input to M1 by peripheral electrical stimulation (PES) or peripheral magnetic stimulation (PMS) to modulate motor output, or reduction of sensory input by temporary deafferentation, might be the potential therapeutic interventions (139).
PES activates not only superficial cutaneous receptors but also somatosensory nerve fibers (141, 142). PES over a muscle belly or a nerve at motor threshold intensity induces muscle contractions by depolarization of motor axons and facilitates motor unit recruitment (143). Modulation of afferent input by PES at motor threshold induces neuroplastic alternations and organizational changes in the sensorimotor cortex, and increases cortical excitability that produces adaptations in central motor pathways (144–147). PES over a nerve at sensory threshold intensity enhances somatosensory input, improves corticomotor excitability (148), facilitates connectivity in sensorimotor regions (149), and induces reorganization of cortical maps (150). Some studies reported that PES improves motor learning (151), motor memory consolidation (152), and inter-limb transfer of motor skills (149) in healthy individuals. In stroke patients, PES at motor threshold increased wrist range of motion and hand muscle strength, improved muscle tone and muscle electrical activity, enhanced functional performance of the upper extremity, and promoted daily living capacity (153, 154). In patients with subacute and chronic stroke, PES at motor threshold decreased muscle spasticity, increased muscle strength, facilitated gait performance, and promoted motor function recovery of the lower extremity (155, 156). One session of PES at sensory threshold reduced muscle spasticity, enhanced muscle strength and proprioception, and improved balance and gait ability in chronic stroke patients (157–159).
In comparison to PES, PMS is deemed to stimulate deeper tissue regions and induce strong muscle contractions for neuromuscular stimulation, with less pain, and fewer side effects with respect to stimulation of the spinal root, muscle belly, or nerve (160, 161). PMS increases peripheral venous blood flow (162), induces muscle contractions with minimal cutaneous sensations (138), and reduces spasticity and muscle hyperreflexia (163). PMS effects depend on the induction of the activity of proprioceptive afferents to the central nervous system, which results in modulation of the excitability of specific spinal circuits and the motor cortex (142, 164–166). PMS improved motor functions in healthy humans (167). In stroke patients, PMS can also induce some beneficial effects. It is reported that in patients with severe upper extremity paresis during the early acute and subacute phase of stroke, PMS prior to standard care promoted upper limb functions, improved daily living abilities, and accelerated the progress rate of motor function recovery (168, 169). In chronic stroke patients with ankle impairment, PMS improved ankle joint mobility and muscle strength, increased M1 transsynaptic excitability in the contralesional hemisphere, and decreased short-interval intracortical inhibition in both hemispheres (170, 171). It is hypothesized that proprioceptive afferents generated by PMS reduce GABAergic inhibition, and the induction of brain plasticity in the sensorimotor cortex may contribute to the increase of muscle strength (171). Furthermore, a single session of PMS significantly reduced spasticity along with decreased event-related desynchronization of mu rhythm in the contralesional hemisphere in subacute or chronic stroke patients (172). It is proposed that the reduction of spasticity might be related to cortical activity alternations in the contralesional hemisphere (172).
Action observation treatment, transcranial electrical or magnetic stimulation, and peripheral electrical or magnetic stimulation are important components for the development of new treatment methods in the field of neurorehabilitation.
The combined intervention of NIBS and action observation can modulate neuroplasticity and motor functions in both healthy and stroke patients. Our previous studies showed that tRNS over M1 paired with mirror-matching action observation enhances observation-dependent motor cortex excitability, and then this effect promotes execution-dependent motor cortex excitability (137). Some studies reported that action observation improves connectivity between the ventral premotor cortex and M1, and movement execution promotes connections either between the dorsal premotor cortex and M1 or the supplementary motor region and M1 (55, 173). tRNS and motor observation might have synergistic effects in improving cortical excitability via premotor mirror neurons to directly and/or indirectly activate M1 neurons. Vice versa, 20 Hz tACS with target electrode over the left M1 and return electrode over the contralateral supraorbital region during movement observation inhibits motor cortex excitability and subsequently inhibits action execution-dependent cortical excitability (174). As a neurophysiological biomarker of functional reorganization, suppression of beta power oscillations is associated with motor learning and consolidation (175). These findings indicated that action observation combined with TES resulted in changes of task-dependent motor cortex activity, which could be advantageous to prevent pathological alterations in stroke sickness (65). In stroke patients with ideomotor apraxia, AOT combined with LF-TMS over the intact hemisphere increased motor cortex excitability and facilitated the recovery of hand motor function (176). LF-TMS over contralesional M1 during observation of complex hand movements improved distal upper extremity functions in the subacute phase following stroke (177). Action observation coupled with PES induced a long-lasting increase in primary motor cortex excitability (178) and improved spontaneous movement tempo (179) in healthy persons. It is proposed that PES paired with action observation might be a promising treatment technique in neurorehabilitation. PES is thought to provide movement-related afferent stimulation to consolidate the kinematic information learned from action observation and lead to neuroplastic adaptations (179).
Some studies explored the effects of transcranial magnetic or electrical stimulation combined with peripheral electrical or magnetic stimulation techniques in neurorehabilitation. In healthy individuals, the effects of combined brain and peripheral stimulation were inconsistently reported. Anodal tDCS (1 mA) alone for 5 min transiently increased cortical excitability, whereas anodal tDCS paired with PES prolonged the facilitating effect for up to 60 min (180). Likewise, cathodal tDCS (1 mA) alone for 5 min decreased the cortical excitability immediately after the stimulation, and the changes were prolonged for up to 60 min when combined with PES (180). The proposed mechanism is that anodal tDCS paired with PES induces LTP-like plasticity and cathodal tDCS combined with PES evoked LTD-like plasticity (180). Schabrun and co-authors, however, failed to find any summative effects after concurrent application of 1 mA tDCS and peripheral nerve electrical stimulation for 20 min, which might be explained by the homeostatic plasticity mechanism (181). In another study, 2 mA anodal tDCS significantly increased MEP amplitude, whereas tDCS combined with PES did not induce any changes in MEP amplitude, indicating a suppression effect following combined stimulation (182). In patients within the first few days following a stroke, anodal tDCS over the ipsilesional M1 coupled with PES of the paretic hand for 5 consecutive days promoted hand motor function recovery (183). In chronic stroke patients, tDCS over the ipsilesional M1 combined with PES prior to motor training potentiated the beneficial effects of motor learning beyond levels reached with tDCS or PES alone (184). This might be that tDCS paired with PES produces additive effects on motor functions through different pathways where anodal tDCS depolarizes neuronal membrane potential and modulates Glutamate as well as GABA concentrations (86, 185), whereas PES modulates GABAergic interneurons activity (184, 186). In contrast, Menezes and co-authors reported that one session of combined stimulation (PES of the paretic arm and tDCS over the ipsilesional M1) prior to motor training did not facilitate training effects on range of motion, gasp and pinch strength in chronic stroke patients with moderate to severe upper extremity motor deficits (187). As discrepancy exists, more studies are needed to optimize the simulation parameters to induce the beneficial effects of this combined intervention. Paired associative stimulation (PAS) modulates motor cortex excitability based on associative LTP/LTD mechanism governed by Hebbian principles (188–190). When PES was applied 10 ms prior to TMS, motor cortex excitability was increased (facilitatory PAS), whereas motor cortex excitability was inhibited when PES is delivered 25 ms preceding TMS (inhibitory PAS) (190). Facilitatory PAS enhanced motor learning in healthy humans (191). It is suggested that PAS induces LTP-like plasticity, and triggers alterations in synaptogenesis and structure connectivity, leading to the facilitation of motor learning (191). Furthermore, facilitatory PAS can promote motor functions in stroke patients via the upregulation of motor cortex excitability in the ipsilesional hemisphere (192). Other forms of associative stimulation, though with limited investigations, showed some promise in treating neurological diseases. Kumru et al. reported that repetitive TMS at 0.1 Hz combined with rPMS at 10 Hz increased motor cortex excitability and reduced intracortical inhibition that might be mediated by GABA-ergic inhibition, but repetitive TMS at 0.1 Hz or rPMS at 10 Hz, respectively, did not improve motor cortex excitability (193).
Both central and peripheral stimulation protocols modulate cortical activity in a state-dependent manner (194–197). The cortical activity in action observation and execution network can be modulated by AOT and synchronously central and peripheral stimulation techniques. Combined top-down with bottom-up stimulation approaches could synergistically modulate cortical activity, spinal networks as well as motor unit recruitment in muscle, reduce spasticity and muscle hyperreflexia, and develop into physical therapy strategies in neurorehabilitation of stroke patients (Figure 1).
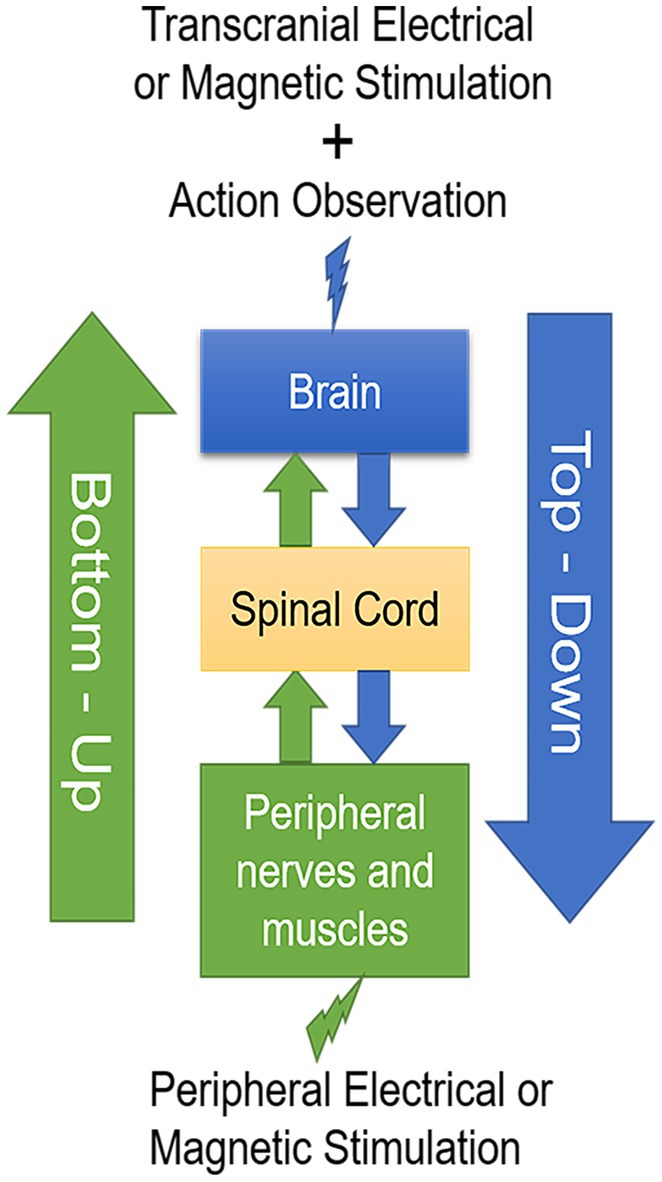
Figure 1. A schematic drawing of the two-stage stimulation model. Transcranial electrical or magnetic stimulation during action observation in a top-down manner combined with peripheral electrical or magnetic stimulation in a bottom-up manner develop into valuable physical therapy strategies in neurorehabilitation after stroke.
Both top-down and bottom-up stimulation techniques have shown some promise in promoting stroke recovery. However, as studies vary in the extent of the structural reserve, the simulation parameters, the phase of stroke, the duration of follow-up, and the outcome measurements, the therapeutic efficacy of different simulation techniques are inconsistently reported. The existing evidence is insufficient to make clinical recommendations in different phases post-stroke, and the way to appropriately apply these techniques in the clinical setting remains to be clarified. Top-down and bottom-up stimulation combined with AOT may have synergistic effects to reach a clinically meaningful level in stroke patients, which need to be investigated in well-designed randomized controlled trial studies with prolonged follow-up.
There are a few limitations that should be mentioned in this perspective. First, we did not differentiate the results following the time windows post-stroke. The neuromodulating effect of variable techniques may change in different stages of stroke. In addition, we did not discuss other neurorehabilitation approaches such as mirror therapy, motor imagery and constraint-induced movement therapy. Last, we did not include other new forms of neuromodulation techniques for instance vagal nerve stimulation and extremely low-frequency magnetic fields (11).
Functional recovery after a stroke depends on the extent of structural reserve of the lesioned hemisphere. The interhemispheric competition model dominates in stroke patients with high structural reserve, whereas the vicariation model dominates in those with little structural reserve. In line with this bimodal balance-recovery model, future studies should explore the effects of (1) anodal tDCS, beta tACS, high-frequency tRNS, HF-TMS, or iTBS over the contralesional hemisphere combined with PES or PMS of the paretic limbs during motor observation followed by motor execution of an identical task on subsequent motor execution-dependent motor cortex excitability in the stroke patients of the severe lesioned hemisphere; (2) anodal tDCS, beta tACS, HF-tRNS or HF-TMS over the lesioned hemisphere and cathodal tDCS, LF-TMS, or cTBS over the non-lesioned hemisphere combined with PES or PMS of the paretic limbs during motor observation followed by motor execution of an identical task on subsequent motor execution-dependent motor cortex excitability in stroke patients with high structural reserve of the lesioned hemisphere. Further research also considers its feasibility for recovery of motor functions in upper and lower limbs in stroke patients. The combination of these techniques followed by motor execution may have a synergic effect to optimize neuroplastic changes and improve motor recovery. The task-dependent neuronal network might be efficiently connected when participants observed the correspondingly complex movement under the combination stimulation techniques, which then promoted task-dependent network activity during performance of the identical task. The proposed paradigms are an innovative approach and could be an adjunctive therapy to potentiate the effect of conventional rehabilitation treatment, especially for those patients with severe motor deficit. Future studies are required to improve the efficacy of the respective interventions, and to validate these results in larger multicenter clinical trials.
FQ, MN, LW, and DW contributed to the conception and design. FQ, LW, and DW drafted the paper. MN and XR revised it critically for important intellectual content. All authors have read and agreed to the published version of the manuscript.
This research was funded by the Special Fund for Fundamental Research Funds of the Central Universities (Sports Rehabilitation Science Laboratory), the Fundamental Research Funds for the Central Universities (2022QN001), and the Research Foundation for Advanced Talents of Beijing Sport University.
MN is member of the scientific advisory board of Neuroelectrics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sacco, RL, Kasner, SE, Broderick, JP, Caplan, LR, Connors, JJ, Culebras, A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2013) 44:2064–89. doi: 10.1161/STR.0b013e318296aeca
2. Virani, SS, Alonso, A, Aparicio, HJ, Benjamin, EJ, Bittencourt, MS, Callaway, CW, et al. Heart disease and stroke Statistics-2021 update: a report from the American Heart Association. Circulation. (2021) 143:e254–743. doi: 10.1161/CIR.0000000000000950
3. Leigh, J, John, O, Fischer, F, Rabiee, N, and Mirzaei Alavijeh, M. Global burden of 369 diseases and injuries In 204 countries and territories 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1223–49. doi: 10.1016/S0140-6736(20)30752-2
4. Tsao, CW, Aday, AW, Almarzooq, ZI, Alonso, A, Beaton, AZ, Bittencourt, MS, et al. Heart disease and stroke Statistics-2022 update: a report from the American Heart Association. Circulation. (2022) 145:e153–639. doi: 10.1161/CIR.0000000000001052
5. Feigin, VL, Krishnamurthi, RV, Parmar, P, Norrving, B, Mensah, GA, Bennett, DA, et al. Update on the global burden of ischemic and hemorrhagic stroke in 1990-2013: the GBD 2013 study. Neuroepidemiology. (2015) 45:161–76. doi: 10.1159/000441085
6. Pollock, A, Baer, G, Campbell, P, Choo, PL, Forster, A, Morris, J, et al. Physical rehabilitation approaches for the recovery of function and mobility following stroke. New York: John Wiley & Sons, Ltd (2014).
7. Ward, N . Assessment of cortical reorganisation for hand function after stroke. J Physiol. (2011) 589:5625–32. doi: 10.1113/jphysiol.2011.220939
8. Angerova, Y, Svestkova, O, Sussova, J, Vele, F, Sladkova, P, and Lippertova-Grunerova, M. Neurorehabilitation. Ceska A Slovenska Neurologie a Neurochirurgie, No. 73, pp. 131–135. (2010).
9. Krupinski, J, Secades, J, and Shiraliyeva, R. Towards effective neurorehabilitation for stroke patients. Int J Phys Med Rehabil. (2014) 2:183. doi: 10.4172/2329-9096.1000183
10. Liew, SL, Santarnecchi, E, Buch, ER, and Cohen, LG. Non-invasive brain stimulation in neurorehabilitation: local and distant effects for motor recovery. Front Hum Neurosci. (2014) 8:378. doi: 10.3389/fnhum.2014.00378
11. Motolese, F, Capone, F, and Di Lazzaro, V. New tools for shaping plasticity to enhance recovery after stroke. Handb Clin Neurol. (2022) 184:299–315. doi: 10.1016/B978-0-12-819410-2.00016-3
12. Taub, E, Uswatte, G, and Elbert, T. New treatments in neurorehabiliation founded on basic research. Nat Rev Neurosci. (2002) 3:228. doi: 10.1038/nrn754
13. Abboud, H, Hill, E, Siddiqui, J, Serra, A, and Walter, B. Neuromodulation in multiple sclerosis. Mult Scler J. (2017) 23:1663–76. doi: 10.1177/1352458517736150
14. Jackson, A, and Zimmermann, JB. Neural interfaces for the brain and spinal cord—restoring motor function. Nat Rev Neurol. (2012) 8:690. doi: 10.1038/nrneurol.2012.219
15. Lariviere, S, Ward, NS, and Boudrias, MH. Disrupted functional network integrity and flexibility after stroke: relation to motor impairments. Neuroimage Clin. (2018) 19:883–91. doi: 10.1016/j.nicl.2018.06.010
16. Di Pino, G, Pellegrino, G, Assenza, G, Capone, F, Ferreri, F, Formica, D, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol. (2014) 10:597–608. doi: 10.1038/nrneurol.2014.162
17. Rehme, AK, and Grefkes, C. Cerebral network disorders after stroke: evidence from imaging-based connectivity analyses of active and resting brain states in humans. J Physiol. (2013) 591:17–31. doi: 10.1113/jphysiol.2012.243469
18. Xerri, C, Zennou-Azogui, Y, Sadlaoud, K, and Sauvajon, D. Interplay between intra- and interhemispheric remodeling of neural networks as a substrate of functional recovery after stroke: adaptive versus maladaptive reorganization. Neuroscience. (2014) 283:178–201. doi: 10.1016/j.neuroscience.2014.06.066
19. Coppens, MJM, Staring, WHA, Nonnekes, J, Geurts, ACH, and Weerdesteyn, V. Offline effects of transcranial direct current stimulation on reaction times of lower extremity movements in people after stroke: a pilot cross-over study. J Neuroeng Rehabil. (2019) 16:10. doi: 10.1186/s12984-019-0604-y
20. Murase, N, Duque, J, Mazzocchio, R, and Cohen, LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. (2004) 55:400. doi: 10.1002/ana.10848
21. Di Lazzaro, V, Dileone, M, Capone, F, Pellegrino, G, Ranieri, F, Musumeci, G, et al. Immediate and late modulation of interhemipheric imbalance with bilateral transcranial direct current stimulation in acute stroke. Brain Stimul. (2014) 7:841–8. doi: 10.1016/j.brs.2014.10.001
22. Lin, YL, Potter-Baker, KA, Cunningham, DA, Li, M, Sankarasubramanian, V, Lee, J, et al. Stratifying chronic stroke patients based on the influence of contralesional motor cortices: an inter-hemispheric inhibition study. Clin Neurophysiol. (2020) 131:2516–25. doi: 10.1016/j.clinph.2020.06.016
23. Citri, A, and Malenka, RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. (2008) 33:18. doi: 10.1038/sj.npp.1301559
24. Karabanov, A, Ziemann, U, Hamada, M, George, MS, Quartarone, A, Classen, J, et al. Consensus paper: probing homeostatic plasticity of human cortex with non-invasive transcranial brain stimulation. Brain Stimul. (2015) 8:442–54. doi: 10.1016/j.brs.2015.01.404
25. Fauth, M, and Tetzlaff, C. Opposing effects of neuronal activity on structural plasticity. Front Neuroanat. (2016) 10:75. doi: 10.3389/fnana.2016.00075
26. Malenka, RC, and Bear, MF. LTP and LTD: an embarrassment of riches. Neuron. (2004) 44:5–21. doi: 10.1016/j.neuron.2004.09.012
27. Cirillo, G, Di Pino, G, Capone, F, Ranieri, F, Florio, L, Todisco, V, et al. Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul. (2017) 10:1–18. doi: 10.1016/j.brs.2016.11.009
28. Yee, AX, Hsu, YT, and Chen, L. A metaplasticity view of the interaction between homeostatic and Hebbian plasticity. Philos Trans R Soc Lond Ser B Biol Sci. (2017) 372:20160155. doi: 10.1098/rstb.2016.0155
29. Hebb, DO . The organization of behavior: A neuropsychological theory. London: Psychology Press (2005).
30. Toyoizumi, T, Kaneko, M, Stryker, MP, and Miller, KD. Modeling the dynamic interaction of Hebbian and homeostatic plasticity. Neuron. (2014) 84:497–510. doi: 10.1016/j.neuron.2014.09.036
31. Rioult-Pedotti, MS, Friedman, D, and Donoghue, JP. Learning-induced LTP in neocortex. Science. (2000) 290:533–6. doi: 10.1126/science.290.5491.533
32. Rioult-Pedotti, MS, Friedman, D, Hess, G, and Donoghue, JP. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci. (1998) 1:230–4. doi: 10.1038/678
33. Rosenzweig, ES, and Barnes, CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol. (2003) 69:143–79. doi: 10.1016/S0301-0082(02)00126-0
34. Butefisch, CM, Davis, BC, Wise, SP, Sawaki, L, Kopylev, L, Classen, J, et al. Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci U S A. (2000) 97:3661–5. doi: 10.1073/pnas.97.7.3661
35. Classen, J, Liepert, J, Wise, SP, Hallett, M, and Cohen, LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol. (1998) 79:1117–23. doi: 10.1152/jn.1998.79.2.1117
36. Cohen, LG, Ziemann, U, Chen, R, Classen, J, Hallett, M, Gerloff, C, et al. Studies of neuroplasticity with transcranial magnetic stimulation. J Clin Neurophysiol. (1998) 15:305–24. doi: 10.1097/00004691-199807000-00003
37. Ziemann, U, Muellbacher, W, Hallett, M, and Cohen, LG. Modulation of practice-dependent plasticity in human motor cortex. Brain. (2001) 124:1171–81. doi: 10.1093/brain/124.6.1171
38. Pascual-Leone, A, Nguyet, D, Cohen, LG, Brasil-Neto, JP, Cammarota, A, and Hallett, M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol. (1995) 74:1037–45. doi: 10.1152/jn.1995.74.3.1037
39. Bütefisch, CM . Neurobiological bases of rehabilitation. Neurol Sci. (2006) 27:s18–23. doi: 10.1007/s10072-006-0540-z
40. Berardelli, A, Inghilleri, M, Rothwell, J, Romeo, S, Curra, A, Gilio, F, et al. Facilitation of muscle evoked responses after repetitive cortical stimulation in man. Exp Brain Res. (1998) 122:79–84. doi: 10.1007/s002210050493
41. Brasil-Neto, J, Cohen, L, Pascual-Leone, A, Jabir, F, Wall, R, and Hallett, M. Rapid reversible modulation of human motor outputs after transient deafferentation of the forearm: a study with transcranial magnetic stimulation. Neurology. (1992) 42:1302–2. doi: 10.1212/WNL.42.7.1302
42. Duffau, H . Brain plasticity: from pathophysiological mechanisms to therapeutic applications. J Clin Neurosci. (2006) 13:885–97. doi: 10.1016/j.jocn.2005.11.045
43. Kuo, H-I, Qi, F-X, Paulus, W, Kuo, M-F, and Nitsche, MA. Noradrenergic enhancement of motor learning, attention, and working memory in humans. Int J Neuropsychopharmacol. (2021) 24:490–8. doi: 10.1093/ijnp/pyab006
44. Ziemann, U, Hallett, M, and Cohen, LG. Mechanisms of deafferentation-induced plasticity in human motor cortex. J Neurosci. (1998) 18:7000–7. doi: 10.1523/JNEUROSCI.18-17-07000.1998
45. Huntley, MK, Muller, S, and Vallence, AM. Corticospinal excitability is modulated by distinct movement patterns during action observation. Exp Brain Res. (2018) 236:1067–75. doi: 10.1007/s00221-018-5199-1
46. Mattar, A, and Gribble, PL. Motor learning by observing. Neuron. (2005) 46:153–60. doi: 10.1016/j.neuron.2005.02.009
47. Rizzolatti, G, and Craighero, L. The mirror-neuron system. Annu Rev Neurosci. (2004) 27:169–92. doi: 10.1146/annurev.neuro.27.070203.144230
48. Gazzola, V, and Keysers, C. The observation and execution of actions share motor and somatosensory voxels in all tested subjects: single-subject analyses of unsmoothed fMRI data. Cereb Cortex. (2008) 19:1239–55. doi: 10.1093/cercor/bhn181
49. Hari, R, Forss, N, Avikainen, S, Kirveskari, E, Salenius, S, and Rizzolatti, G. Activation of human primary motor cortex during action observation: a neuromagnetic study. Proc Natl Acad Sci U S A. (1998) 95:15061–5. doi: 10.1073/pnas.95.25.15061
50. Kilner, JM, and Lemon, RN. What we know currently about Mirror neurons. Curr Biol. (2013) 23:R1057–62. doi: 10.1016/j.cub.2013.10.051
51. Molenberghs, P, Cunnington, R, and Mattingley, JB. Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neurosci Biobehav Rev. (2012) 36:341–9. doi: 10.1016/j.neubiorev.2011.07.004
52. Shmuelof, L, and Zohary, E. A mirror representation of others' actions in the human anterior parietal cortex. J Neurosci. (2006) 26:9736–42. doi: 10.1523/JNEUROSCI.1836-06.2006
53. Heyes, CM, and Foster, CL. Motor learning by observation: evidence from a serial reaction time task. Q J Exp Psychol A. (2002) 55:593–607. doi: 10.1080/02724980143000389
54. Nielsen, JB, and Cohen, LG. The olympic brain. Does corticospinal plasticity play a role in acquisition of skills required for high-performance sports? J Physiol. (2008) 586:65–70. doi: 10.1113/jphysiol.2007.142661
55. Stefan, K, Classen, J, Celnik, P, and Cohen, LG. Concurrent action observation modulates practice-induced motor memory formation. Eur J Neurosci. (2008) 27:730–8. doi: 10.1111/j.1460-9568.2008.06035.x
56. Spampinato, D, and Celnik, P. Temporal dynamics of cerebellar and motor cortex physiological processes during motor skill learning. Sci Rep. (2017) 7:40715. doi: 10.1038/srep40715
57. Celnik, P, Stefan, K, Hummel, F, Duque, J, Classen, J, and Cohen, LG. Encoding a motor memory in the older adult by action observation. NeuroImage. (2006) 29:677–84. doi: 10.1016/j.neuroimage.2005.07.039
58. Loporto, M, Mcallister, C, Williams, J, Hardwick, R, and Holmes, P. Investigating central mechanisms underlying the effects of action observation and imagery through transcranial magnetic stimulation. J Mot Behav. (2011) 43:361–73. doi: 10.1080/00222895.2011.604655
59. Rioult-Pedotti, M-S, Donoghue, JP, and Dunaevsky, A. Plasticity of the synaptic modification range. J Neurophysiol. (2007) 98:3688–95. doi: 10.1152/jn.00164.2007
60. Sanes, JN, and Donoghue, JP. Plasticity and primary motor cortex. Annu Rev Neurosci. (2000) 23:393–415. doi: 10.1146/annurev.neuro.23.1.393
61. Bazzini, MC, Nuara, A, Scalona, E, De Marco, D, Rizzolatti, G, Avanzini, P, et al. The proactive synergy between action observation and execution in the Acquisition of new Motor Skills. Front Hum Neurosci. (2022) 16:793849. doi: 10.3389/fnhum.2022.793849
62. Mcgregor, HR, and Gribble, PL. Functional connectivity between somatosensory and motor brain areas predicts individual differences in motor learning by observing. J Neurophysiol. (2017) 118:1235–43. doi: 10.1152/jn.00275.2017
63. Stefan, K, Cohen, LG, Duque, J, Mazzocchio, R, Celnik, P, Sawaki, L, et al. Formation of a motor memory by action observation. J Neurosci. (2005) 25:9339–46. doi: 10.1523/JNEUROSCI.2282-05.2005
64. Bähr, F, Ritter, A, Seidel, G, Puta, C, Gabriel, HH, and Hamzei, F. Boosting the motor outcome of the untrained hand by action observation: Mirror visual feedback, video therapy, or both combined–what is more effective? Neural Plast 2018. (2018) 2018:1–10. doi: 10.1155/2018/8369262
65. Buccino, G . Action observation treatment: a novel tool in neurorehabilitation. Philos Trans R Soc Lond Ser B Biol Sci. (2014) 369:20130185. doi: 10.1098/rstb.2013.0185
66. Rossi, F, Savi, F, Prestia, A, Mongardi, A, Demarchi, D, and Buccino, G. Combining action observation treatment with a brain-computer Interface system: perspectives on neurorehabilitation. Sensors (Basel). (2021) 21:8504. doi: 10.3390/s21248504
67. Gaowgeh, RAM, Neelam, S, Subramanian, SS, Regan, R, Selvaraj, SK, and Anandan, AD. Efficacy of action observation for upper limb motor deficit in acute stroke participants. J Pharm Res Int. (2021) 33:227–33. doi: 10.9734/jpri/2021/v33i56A33905
68. Hsieh, YW, Lin, YH, Zhu, JD, Wu, CY, Lin, YP, and Chen, CC. Treatment effects of upper limb action observation therapy and Mirror therapy on rehabilitation outcomes after subacute stroke: a pilot study. Behav Neurol. (2020) 2020:6250524. doi: 10.1155/2020/6250524
69. Yu, JA, and Park, J. The effect of first-person perspective action observation training on upper extremity function and activity of daily living of chronic stroke patients. Brain Behav. (2022) 12:e2565. doi: 10.1002/brb3.2565
70. Oh, SJ, Lee, JH, and Kim, DH. The effects of functional action-observation training on gait function in patients with post-stroke hemiparesis: a randomized controlled trial. Technol Health Care. (2019) 27:159–65. doi: 10.3233/THC-181388
71. Klomjai, W, Katz, R, and Lackmy-Vallee, A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann Phys Rehabil Med. (2015) 58:208–13. doi: 10.1016/j.rehab.2015.05.005
72. Demirtas-Tatlidede, A, Vahabzadeh-Hagh, AM, Bernabeu, M, Tormos, JM, and Pascual-Leone, A. Noninvasive brain stimulation in traumatic brain injury. J Head Trauma Rehabil. (2012) 27:274. doi: 10.1097/HTR.0b013e318217df55
73. Di Lazzaro, V, Pilato, F, Saturno, E, Oliviero, A, Dileone, M, Mazzone, P, et al. Theta-burst repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol. (2005) 565:945–50. doi: 10.1113/jphysiol.2005.087288
74. Huang, YZ, Edwards, MJ, Rounis, E, Bhatia, KP, and Rothwell, JC. Theta burst stimulation of the human motor cortex. Neuron. (2005) 45:201–6. doi: 10.1016/j.neuron.2004.12.033
75. Narayana, S, Zhang, W, Rogers, W, Strickland, C, Franklin, C, Lancaster, JL, et al. Concurrent TMS to the primary motor cortex augments slow motor learning. NeuroImage. (2014) 85:971–84. doi: 10.1016/j.neuroimage.2013.07.024
76. Hosomi, K, Morris, S, Sakamoto, T, Taguchi, J, Maruo, T, Kageyama, Y, et al. Daily repetitive transcranial magnetic stimulation for Poststroke upper limb paresis in the subacute period. J Stroke Cerebrovasc Dis. (2016) 25:1655–64. doi: 10.1016/j.jstrokecerebrovasdis.2016.02.024
77. Guan, YZ, Li, J, Zhang, XW, Wu, S, Du, H, Cui, LY, et al. Effectiveness of repetitive transcranial magnetic stimulation (rTMS) after acute stroke: a one-year longitudinal randomized trial. CNS Neurosci Ther. (2017) 23:940–6. doi: 10.1111/cns.12762
78. Volz, LJ, Rehme, AK, Michely, J, Nettekoven, C, Eickhoff, SB, Fink, GR, et al. Shaping early reorganization of neural networks promotes motor function after stroke. Cereb Cortex. (2016) 26:2882–94. doi: 10.1093/cercor/bhw034
79. Park, C-H, Chang, WH, Ohn, SH, Kim, ST, Bang, OY, Pascual-Leone, A, et al. Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke. (2011) 42:1357–62. doi: 10.1161/STROKEAHA.110.596155
80. Meng, ZY, and Song, WQ. Low frequency repetitive transcranial magnetic stimulation improves motor dysfunction after cerebral infarction. Neural Regen Res. (2017) 12:610–3. doi: 10.4103/1673-5374.205100
81. Kindred, JH, Wonsetler, EC, Charalambous, CC, Srivastava, S, Marebwa, BK, Bonilha, L, et al. Individualized responses to Ipsilesional high-frequency and Contralesional low-frequency rTMS in chronic stroke: a pilot study to support the individualization of neuromodulation for rehabilitation. Front Hum Neurosci. (2020) 14:578127. doi: 10.3389/fnhum.2020.578127
82. Sankarasubramanian, V, Machado, AG, Conforto, AB, Potter-Baker, KA, Cunningham, DA, Varnerin, NM, et al. Inhibition versus facilitation of contralesional motor cortices in stroke: deriving a model to tailor brain stimulation. Clin Neurophysiol. (2017) 128:892–902. doi: 10.1016/j.clinph.2017.03.030
83. Reis, J, and Fritsch, B. Modulation of motor performance and motor learning by transcranial direct current stimulation. Curr Opin Neurol. (2011) 24:590–6. doi: 10.1097/WCO.0b013e32834c3db0
84. Tanaka, S, Sandrini, M, and Cohen, LG. Modulation of motor learning and memory formation by non-invasive cortical stimulation of the primary motor cortex. Neuropsychol Rehabil. (2011) 21:650–75. doi: 10.1080/09602011.2011.605589
85. Polania, R, Nitsche, MA, and Ruff, CC. Studying and modifying brain function with non-invasive brain stimulation. Nat Neurosci. (2018) 21:174–87. doi: 10.1038/s41593-017-0054-4
86. Stagg, CJ, Antal, A, and Nitsche, MA. Physiology of transcranial direct current stimulation. J ECT. (2018) 34:144–52. doi: 10.1097/YCT.0000000000000510
87. Nitsche, MA, and Paulus, W. Transcranial direct current stimulation - update 2011. Restor Neurol Neurosci. (2011) 29:463–92. doi: 10.3233/RNN-2011-0618
88. Stagg, CJ, and Nitsche, MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. (2011) 17:37–53. doi: 10.1177/1073858410386614
89. Nitsche, MA, and Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. (2000) 527:633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x
90. Nitsche, MA, and Paulus, W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. (2001) 57:1899–901. doi: 10.1212/WNL.57.10.1899
91. Nitsche, MA, Nitsche, MS, Klein, CC, Tergau, F, Rothwell, JC, and Paulus, W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol. (2003) 114:600–4. doi: 10.1016/S1388-2457(02)00412-1
92. Qi, F, Nitsche, MA, and Zschorlich, VR. Modulating observation-execution-related motor cortex activity by cathodal transcranial direct current stimulation. J Brain Sci. (2019) 9:121. doi: 10.3390/brainsci9050121
93. Nitsche, MA, Schauenburg, A, Lang, N, Liebetanz, D, Exner, C, Paulus, W, et al. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci. (2003) 15:619–26. doi: 10.1162/089892903321662994
94. Reis, J, Schambra, HM, Cohen, LG, Buch, ER, Fritsch, B, Zarahn, E, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A. (2009) 106:1590–5. doi: 10.1073/pnas.0805413106
95. Stagg, C, Jayaram, G, Pastor, D, Kincses, Z, Matthews, P, and Johansen-Berg, H. Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia. (2011) 49:800–4. doi: 10.1016/j.neuropsychologia.2011.02.009
96. Waters, S, Wiestler, T, and Diedrichsen, J. Cooperation not competition: bihemispheric tDCS and fMRI show role for ipsilateral hemisphere in motor learning. J Neurosci. (2017) 37:7500–12. doi: 10.1523/JNEUROSCI.3414-16.2017
97. Schmidt, S, Fleischmann, R, Bathe-Peters, R, Irlbacher, K, and Brandt, SA. Evolution of premotor cortical excitability after cathodal inhibition of the primary motor cortex: a sham-controlled serial navigated TMS study. PLoS One. (2013) 8:e57425. doi: 10.1371/journal.pone.0057425
98. Focke, J, Kemmet, S, Krause, V, Keitel, A, and Pollok, B. Cathodal transcranial direct current stimulation (tDCS) applied to the left premotor cortex (PMC) stabilizes a newly learned motor sequence. Behav Brain Res. (2017) 316:87–93. doi: 10.1016/j.bbr.2016.08.032
99. Zhu, FF, Yeung, AY, Poolton, JM, Lee, TM, Leung, GK, and Masters, RS. Cathodal transcranial direct current stimulation over left dorsolateral prefrontal cortex area promotes implicit motor learning in a golf putting task. Brain Stimul. (2015) 8:784–6. doi: 10.1016/j.brs.2015.02.005
100. Ojardias, E, Aze, OD, Luneau, D, Mednieks, J, Condemine, A, Rimaud, D, et al. The effects of anodal transcranial direct current stimulation on the walking performance of chronic hemiplegic patients. Neuromodulation. (2020) 23:373–9. doi: 10.1111/ner.12962
101. Yang, CL, Gad, A, Creath, RA, Magder, L, Rogers, MW, and Waller, SM. Effects of transcranial direct current stimulation (tDCS) on posture, movement planning, and execution during standing voluntary reach following stroke. J Neuroeng Rehabil. (2021) 18:5. doi: 10.1186/s12984-020-00799-8
102. Zimerman, M, Heise, KF, Hoppe, J, Cohen, LG, Gerloff, C, and Hummel, FC. Modulation of training by single-session transcranial direct current stimulation to the intact motor cortex enhances motor skill acquisition of the paretic hand. Stroke. (2012) 43:2185–91. doi: 10.1161/STROKEAHA.111.645382
103. Wong, PL, Yang, YR, Tang, SC, Huang, SF, and Wang, RY. Comparing different montages of transcranial direct current stimulation on dual-task walking and cortical activity in chronic stroke: double-blinded randomized controlled trial. BMC Neurol. (2022) 22:119. doi: 10.1186/s12883-022-02644-y
104. Seamon, BA, Bowden, MG, Kindred, JH, Embry, AE, and Kautz, SA. Transcranial direct current stimulation electrode montages may differentially impact variables of walking performance in individuals Poststroke: a preliminary study. J Clin Neurophysiol. (2021) 40:71–8. doi: 10.1097/WNP.0000000000000848
105. Lefebvre, S, and Liew, SL. Anatomical parameters of tDCS to modulate the motor system after stroke: a review. Front Neurol. (2017) 8:29. doi: 10.3389/fneur.2017.00029
106. Hummel, FC, and Cohen, LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. (2006) 5:708–12. doi: 10.1016/S1474-4422(06)70525-7
107. Bolognini, N, Pascual-Leone, A, and Fregni, F. Using non-invasive brain stimulation to augment motor training-induced plasticity. J Neuroeng Rehabil. (2009) 6:8. doi: 10.1186/1743-0003-6-8
108. Antal, A, Alekseichuk, I, and Paulus, W. The new modalities of transcranial electric stimulation: tACS, tRNS, and other approaches In:. Transcranial direct current stimulation in neuropsychiatric disorders. Berlin: Springer (2016). 21–8.
109. Moliadze, V, Atalay, D, Antal, A, and Paulus, W. Close to threshold transcranial electrical stimulation preferentially activates inhibitory networks before switching to excitation with higher intensities. Brain Stimul. (2012) 5:505–11. doi: 10.1016/j.brs.2011.11.004
110. Moliadze, V, Fritzsche, G, and Antal, A. Comparing the efficacy of excitatory transcranial stimulation methods measuring motor evoked potentials. Neural Plast. (2014) 2014:837141–1. doi: 10.1155/2014/837141
111. Terney, D, Chaieb, L, Moliadze, V, Antal, A, and Paulus, W. Increasing human brain excitability by transcranial high-frequency random noise stimulation. J Neurosci. (2008) 28:14147–55. doi: 10.1523/JNEUROSCI.4248-08.2008
112. Antal, A, Ambrus, GG, and Chaieb, L. The impact of electrical stimulation techniques on behavior. Wiley Interdiscip Rev Cogn Sci. (2014) 5:649–59. doi: 10.1002/wcs.1319
113. Pavan, A, Ghin, F, Contillo, A, Milesi, C, Campana, G, and Mather, G. Modulatory mechanisms underlying high-frequency transcranial random noise stimulation (hf-tRNS): a combined stochastic resonance and equivalent noise approach. Brain Stimul. (2019) 12:967–77. doi: 10.1016/j.brs.2019.02.018
114. Antal, A, Chaieb, L, Moliadze, V, Monte-Silva, K, Poreisz, C, Thirugnanasambandam, N, et al. Brain-derived neurotrophic factor (BDNF) gene polymorphisms shape cortical plasticity in humans. Brain Stimul. (2010) 3:230–7. doi: 10.1016/j.brs.2009.12.003
115. Chaieb, L, Antal, A, and Paulus, W. Transcranial random noise stimulation-induced plasticity is NMDA-receptor independent but sodium-channel blocker and benzodiazepines sensitive. Front Neurosci. (2015) 9:125. doi: 10.3389/fnins.2015.00125
116. Remedios, L, Mabil, P, Flores-Hernandez, J, Torres-Ramírez, O, Huidobro, N, Castro, G, et al. Effects of short-term random noise electrical stimulation on dissociated pyramidal neurons from the cerebral cortex. Neuroscience. (2019) 417:107–8. doi: 10.1016/j.neuroscience.2019.07.034
117. Prichard, G, Weiller, C, Fritsch, B, and Reis, J. Effects of different electrical brain stimulation protocols on subcomponents of motor skill learning. Brain Stimul. (2014) 7:532–40. doi: 10.1016/j.brs.2014.04.005
118. Hayward, KS, Brauer, SG, Ruddy, KL, Lloyd, D, and Carson, RG. Repetitive reaching training combined with transcranial random noise stimulation in stroke survivors with chronic and severe arm paresis is feasible: a pilot, triple-blind, randomised case series. J Neuroeng Rehabil. (2017) 14:46. doi: 10.1186/s12984-017-0253-y
119. Arnao, V, Riolo, M, Carduccio, F, Tuttolomondo, A, D'amelio, M, Brighina, F, et al. Effects of transcranial random noise stimulation combined with graded repetitive arm supplementary program (GRASP) on motor rehabilitation of the upper limb in sub-acute ischemic stroke patients: a randomized pilot study. J Neural Transm (Vienna). (2019) 126:1701–6. doi: 10.1007/s00702-019-02087-9
120. Lee, TL, Lee, H, and Kang, N. A meta-analysis showing improved cognitive performance in healthy young adults with transcranial alternating current stimulation. NPJ Sci Learn. (2023) 8:1. doi: 10.1038/s41539-022-00152-9
121. Vosskuhl, J, Struber, D, and Herrmann, CS. Non-invasive brain stimulation: a paradigm shift in understanding brain oscillations. Front Hum Neurosci. (2018) 12:211. doi: 10.3389/fnhum.2018.00211
122. Wischnewski, M, Schutter, D, and Nitsche, MA. Effects of beta-tACS on corticospinal excitability: a meta-analysis. Brain Stimul. (2019) 12:1381–9. doi: 10.1016/j.brs.2019.07.023
123. Antal, A, Boros, K, Poreisz, C, Chaieb, L, Terney, D, and Paulus, W. Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimul. (2008) 1:97–105. doi: 10.1016/j.brs.2007.10.001
124. Naros, G, and Gharabaghi, A. Physiological and behavioral effects of beta-tACS on brain self-regulation in chronic stroke. Brain Stimul. (2017) 10:251–9. doi: 10.1016/j.brs.2016.11.003
125. Fornito, A, Zalesky, A, and Breakspear, M. Graph analysis of the human connectome: promise, progress, and pitfalls. NeuroImage. (2013) 80:426–44. doi: 10.1016/j.neuroimage.2013.04.087
126. To, W.TDe Ridder, D, Hart, J, and Vanneste, S. Changing brain networks through non-invasive neuromodulation. Front Hum Neurosci. (2018) 12:128. doi: 10.3389/fnhum.2018.00128
127. Van Den Heuvel, MP, and Sporns, O. Rich-club organization of the human connectome. J Neurosci. (2011) 31:15775–86. doi: 10.1523/JNEUROSCI.3539-11.2011
128. Fertonani, A, and Miniussi, C. Transcranial electrical stimulation: what we know and do not know about mechanisms. Neuroscientist. (2017) 23:109–23. doi: 10.1177/1073858416631966
129. Cocchi, L, Sale, MV, Lord, A, Zalesky, A, Breakspear, M, and Mattingley, JB. Dissociable effects of local inhibitory and excitatory theta-burst stimulation on large-scale brain dynamics. J Neurophysiol. (2015) 113:3375–85. doi: 10.1152/jn.00850.2014
130. Van Den Heuvel, MP, and Sporns, O. An anatomical substrate for integration among functional networks in human cortex. J Neurosci. (2013) 33:14489–500. doi: 10.1523/JNEUROSCI.2128-13.2013
131. Polania, R, Paulus, W, and Nitsche, MA. Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Hum Brain Mapp. (2012) 33:2499–508. doi: 10.1002/hbm.21380
132. Polanía, R, Paulus, W, and Nitsche, MA. Reorganizing the intrinsic functional architecture of the human primary motor cortex during rest with non-invasive cortical stimulation. PLoS One. (2012) 7:e30971. doi: 10.1371/journal.pone.0030971
133. Polania, R, Nitsche, MA, and Paulus, W. Modulating functional connectivity patterns and topological functional organization of the human brain with transcranial direct current stimulation. Hum Brain Mapp. (2011) 32:1236–49. doi: 10.1002/hbm.21104
134. Benwell, CS, Learmonth, G, Miniussi, C, Harvey, M, and Thut, G. Non-linear effects of transcranial direct current stimulation as a function of individual baseline performance: evidence from biparietal tDCS influence on lateralized attention bias. Cortex. (2015) 69:152–65. doi: 10.1016/j.cortex.2015.05.007
135. Bortoletto, M, Pellicciari, MC, Rodella, C, and Miniussi, C. The interaction with task-induced activity is more important than polarization: a tDCS study. Brain Stimul. (2015) 8:269–76. doi: 10.1016/j.brs.2014.11.006
136. Gill, J, Shah-Basak, PP, and Hamilton, R. It's the thought that counts: examining the task-dependent effects of transcranial direct current stimulation on executive function. Brain Stimul. (2015) 8:253–9. doi: 10.1016/j.brs.2014.10.018
137. Qi, FX, Nitsche, MA, and Zschorlich, VR. Interaction between transcranial random noise stimulation and observation-execution matching activity promotes motor cortex excitability. Front Neurosci. (2019) 13:10. doi: 10.3389/fnins.2019.00069
138. Beaulieu, LD, and Schneider, C. Repetitive peripheral magnetic stimulation to reduce pain or improve sensorimotor impairments: a literature review on parameters of application and afferents recruitment. Neurophysiol Clin. (2015) 45:223–37. doi: 10.1016/j.neucli.2015.08.002
139. Edwards, LL, King, EM, Buetefisch, CM, and Borich, MR. Putting the "sensory" into sensorimotor control: the role of sensorimotor integration in goal-directed hand movements after stroke. Front Integr Neurosci. (2019) 13:16. doi: 10.3389/fnint.2019.00016
140. Rubakova, AA, Ivanova, GE, and Bulatova, MA. Activation of sensorimotor integration processes with a brain-computer interface. Moscow: Bulletin of Russian State Medical University (2021).
141. Masse-Alarie, H, Beaulieu, L-D, Preuss, R, and Schneider, C. Repetitive peripheral magnetic neurostimulation of multifidus muscles combined with motor training influences spine motor control and chronic low back pain. Clin Neurophysiol. (2017) 128:442–53. doi: 10.1016/j.clinph.2016.12.020
142. Struppler, A, Binkofski, F, Angerer, B, Bernhardt, M, Spiegel, S, Drzezga, A, et al. A fronto-parietal network is mediating improvement of motor function related to repetitive peripheral magnetic stimulation: a PET-H2O15 study. NeuroImage. (2007) 36:T174–86. doi: 10.1016/j.neuroimage.2007.03.033
143. Bergquist, AJ, Wiest, MJ, and Collins, DF. Motor unit recruitment when neuromuscular electrical stimulation is applied over a nerve trunk compared with a muscle belly: triceps surae. J Appl Physiol. (2012) 113:78. doi: 10.1152/japplphysiol.00074.2011
144. Everaert, DG, Thompson, AK, Su, LC, and Stein, RB. Does functional electrical stimulation for foot drop strengthen corticospinal connections? Neurorehabil Neural Repair. (2010) 24:168–77. doi: 10.1177/1545968309349939
145. Mang, CS, Clair, JM, and Collins, DF. Neuromuscular electrical stimulation has a global effect on corticospinal excitability for leg muscles and a focused effect for hand muscles. Exp Brain Res. (2011) 209:355–63. doi: 10.1007/s00221-011-2556-8
146. Ridding, MC, Brouwer, B, Miles, TS, Pitcher, JB, and Thompson, PD. Changes in muscle responses to stimulation of the motor cortex induced by peripheral nerve stimulation in human subjects. Exp Brain Res. (2000) 131:135–43. doi: 10.1007/s002219900269
147. Ridding, MC, Mckay, DR, Thompson, PD, and Miles, TS. Changes in corticomotor representations induced by prolonged peripheral nerve stimulation in humans. Clin Neurophysiol. (2001) 112:1461–9. doi: 10.1016/S1388-2457(01)00592-2
148. Kaelin-Lang, A, Luft, AR, Sawaki, L, Burstein, AH, Sohn, YH, and Cohen, LG. Modulation of human corticomotor excitability by somatosensory input. J Physiol. (2010) 540:623–33. doi: 10.1113/jphysiol.2001.012801
149. Veldman, MP, Maurits, NM, Inge, Z, Maffiuletti, NA, Stella, VM, Chris, MJ, et al. Somatosensory electrical stimulation improves skill acquisition, consolidation, and transfer by increasing sensorimotor activity and connectivity. J Neurophysiol. (2018) 120:281–90. doi: 10.1152/jn.00860.2017
150. Meesen, RLJ, Cuypers, K, Rothwell, JC, Swinnen, SP, and Levin, O. The effect of long-term TENS on persistent neuroplastic changes in the human cerebral cortex. Hum Brain Mapp. (2011) 32:872–82. doi: 10.1002/hbm.21075
151. Veldman, MP, Zijdewind, I, Solnik, S, Maffiuletti, NA, Berghuis, KM, Javet, M, et al. Direct and crossed effects of somatosensory electrical stimulation on motor learning and neuronal plasticity in humans. Eur J Appl Physiol. (2015) 115:2505–19. doi: 10.1007/s00421-015-3248-z
152. Veldman, MP, Zijdewind, I, Maffiuletti, NA, and Hortobágyi, T. Motor skill acquisition and retention after somatosensory electrical stimulation in healthy humans. Front Hum Neurosci. (2016) 10:115. doi: 10.3389/fnhum.2016.00115
153. Huang, S, Liu, P, Chen, Y, Gao, B, Li, Y, Chen, C, et al. Effectiveness of Contralaterally controlled functional electrical stimulation versus neuromuscular electrical stimulation on upper limb motor functional recovery in subacute stroke patients: a randomized controlled trial. Neural Plast. (2021) 2021:1987662. doi: 10.1155/2021/1987662
154. Sentandreu-Mañó, T, Tomás, JM, and Ricardo Salom Terrádez, J. A randomised clinical trial comparing 35 Hz versus 50 Hz frequency stimulation effects on hand motor recovery in older adults after stroke. Sci Rep. (2021) 11:9131. doi: 10.1038/s41598-021-88607-8
155. Shen, Y, Chen, L, Zhang, L, Hu, S, Su, B, Qiu, H, et al. Effectiveness of a novel Contralaterally controlled neuromuscular electrical stimulation for restoring lower limb motor performance and activities of daily living in stroke survivors: a randomized controlled trial. Neural Plast. (2022) 2022:5771634. doi: 10.1155/2022/5771634
156. Yang, YR, Mi, PL, Huang, SF, Chiu, SL, Liu, YC, and Wang, RY. Effects of neuromuscular electrical stimulation on gait performance in chronic stroke with inadequate ankle control - a randomized controlled trial. PLoS One. (2018) 13:e0208609. doi: 10.1371/journal.pone.0208609
157. Cho, HY, In, TS, Cho, KH, and Song, CH. A single trial of transcutaneous electrical nerve stimulation (TENS) improves spasticity and balance in patients with chronic stroke. Tohoku J Exp Med. (2013) 229:187–93. doi: 10.1620/tjem.229.187
158. Kwong, PWH, Chan, KL, Choi, HY, Guo, H, Tam, YF, Tao, SC, et al. Immediate effects of transcutaneous electrical nerve stimulation on gait patterns in chronic stroke survivors: a single group, pretest-posttest clinical trial. Hum Mov Sci. (2022) 83:102948. doi: 10.1016/j.humov.2022.102948
159. Tyson, SF, Sadeghi-Demneh, E, and Nester, CJ. The effects of transcutaneous electrical nerve stimulation on strength, proprioception, balance and mobility in people with stroke: a randomized controlled cross-over trial. Clin Rehabil. (2013) 27:785–91. doi: 10.1177/0269215513478227
160. Sato, A, Torii, T, Iwahashi, M, and Iramina, K. Alterations in motor cortical excitability induced by peripheral stimulation with magnetic stimulation. IEEE Trans Magn. (2018) 54:1–4. doi: 10.1109/TMAG.2018.2851358
161. Szecsi, J, Schiller, M, Straube, A, and Gerling, D. A comparison of functional electrical and magnetic stimulation for propelled cycling of paretic patients. Arch Phys Med Rehabil. (2009) 90:564–70. doi: 10.1016/j.apmr.2008.09.572
162. Okudera, Y, Matsunaga, T, Sato, M, Chida, S, Hatakeyama, K, Watanabe, M, et al. The impact of high-frequency magnetic stimulation of peripheral nerves: muscle hardness, venous blood flow, and motor function of upper extremity in healthy subjects. Biomed Res. (2015) 36:81–7. doi: 10.2220/biomedres.36.81
163. Zschorlich, VR, Hillebrecht, M, Tanjour, T, Qi, FX, Behrendt, F, Kirschstein, T, et al. Repetitive peripheral magnetic nerve stimulation (rPMS) as adjuvant therapy reduces skeletal muscle reflex activity. Front Neurol. (2019) 10:8. doi: 10.3389/fneur.2019.00930
164. Flamand, VH, Beaulieu, L-D, Nadeau, L, and Schneider, C. Peripheral magnetic stimulation to decrease spasticity in cerebral palsy. Pediatr Neurol. (2012) 47:345–8. doi: 10.1016/j.pediatrneurol.2012.07.005
165. Krause, P, and Straube, A. Peripheral repetitive magnetic stimulation induces intracortical inhibition in healthy subjects. Neurol Res. (2008) 30:690–4. doi: 10.1179/174313208X297959
166. Nielsen, JF, and Sinkjær, T. Long-lasting depression of soleus motoneurons excitability following repetitive magnetic stimuli of the spinal cord in multiple sclerosis patients. Mult Scler J. (1997) 3:18–30. doi: 10.1177/135245859700300103
167. Jia, Y, Liu, X, Wei, J, Li, D, and Liu, H. Modulation of the Corticomotor excitability by repetitive peripheral magnetic stimulation on the median nerve in healthy subjects. Front Neural Circuits. (2021) 15:616084. doi: 10.3389/fncir.2021.616084
168. Jiang, YF, Zhang, D, Zhang, J, Hai, H, Zhao, YY, and Ma, YW. A randomized controlled trial of repetitive peripheral magnetic stimulation applied in early subacute stroke: effects on severe upper-limb impairment. Clin Rehabil. (2022) 36:693–702. doi: 10.1177/02692155211072189
169. Obayashi, S, and Takahashi, R. Repetitive peripheral magnetic stimulation improves severe upper limb paresis in early acute phase stroke survivors. NeuroRehabilitation. (2020) 46:569–75. doi: 10.3233/NRE-203085
170. Beaulieu, LD, Masse-Alarie, H, Brouwer, B, and Schneider, C. Noninvasive neurostimulation in chronic stroke: a double-blind randomized sham-controlled testing of clinical and corticomotor effects. Top Stroke Rehabil. (2015) 22:8–17. doi: 10.1179/1074935714Z.0000000032
171. Beaulieu, LD, Masse-Alarie, H, Camire-Bernier, S, Ribot-Ciscar, E, and Schneider, C. After-effects of peripheral neurostimulation on brain plasticity and ankle function in chronic stroke: the role of afferents recruited. Neurophysiol Clin. (2017) 47:275–91. doi: 10.1016/j.neucli.2017.02.003
172. Chen, S, Li, Y, Shu, X, Wang, C, Wang, H, Ding, L, et al. Electroencephalography mu rhythm changes and decreased spasticity after repetitive peripheral magnetic stimulation in patients following stroke. Front Neurol. (2020) 11:546599. doi: 10.3389/fneur.2020.546599
173. Cantarero, G, Galea, JM, Ajagbe, L, Salas, R, Willis, J, and Celnik, P. Disrupting the ventral premotor cortex interferes with the contribution of action observation to use-dependent plasticity. J Cogn Neurosci. (2011) 23:3757–66. doi: 10.1162/jocn_a_00051
174. Wang, L, Nitsche, MA, Zschorlich, VR, Liu, H, Kong, Z, and Qi, F. 20 Hz transcranial alternating current stimulation inhibits observation-execution-related motor cortex excitability. J Pers Med. (2021) 11:979. doi: 10.3390/jpm11100979
175. Pollok, B, Latz, D, Krause, V, Butz, M, and Schnitzler, A. Changes of motor-cortical oscillations associated with motor learning. Neuroscience. (2014) 275:47–53. doi: 10.1016/j.neuroscience.2014.06.008
176. Yang, BI, Ma, SR, and Song, BK. Effects of action observation training with 1 Hz low frequency repeated transcranial magnetic stimulation on cerebral cortex activity and hand function in patients with Ideomotor apraxia after stroke. J Magn. (2019) 24:770–5. doi: 10.4283/JMAG.2019.24.4.770
177. Noh, JS, Lim, JH, Choi, TW, Jang, SG, and Pyun, SB. Effects and safety of combined rTMS and action observation for recovery of function in the upper extremities in stroke patients: a randomized controlled trial. Restor Neurol Neurosci. (2019) 37:219–30. doi: 10.3233/RNN-180883
178. Bisio, A, Avanzino, L, Gueugneau, N, Pozzo, T, Ruggeri, P, and Bove, M. Observing and perceiving: a combined approach to induce plasticity in human motor cortex. Clin Neurophysiol. (2015) 126:1212–20. doi: 10.1016/j.clinph.2014.08.024
179. Bisio, A, Avanzino, L, Lagravinese, G, Biggio, M, Ruggeri, P, and Bove, M. Spontaneous movement tempo can be influenced by combining action observation and somatosensory stimulation. Front Behav Neurosci. (2015) 9:228. doi: 10.3389/fnbeh.2015.00228
180. Rizzo, V, Terranova, C, Crupi, D, Sant'angelo, A, Girlanda, P, and Quartarone, A. Increased transcranial direct current stimulation after effects during concurrent peripheral electrical nerve stimulation. Brain Stimul. (2014) 7:113–21. doi: 10.1016/j.brs.2013.10.002
181. Schabrun, SM, Chipchase, LS, Zipf, N, Thickbroom, GW, and Hodges, PW. Interaction between simultaneously applied neuromodulatory interventions in humans. Brain Stimul. (2013) 6:624–30. doi: 10.1016/j.brs.2012.09.009
182. Tsuiki, S, Sasaki, R, Miyaguchi, S, Kojima, S, Saito, K, Inukai, Y, et al. The effect of combined transcranial direct current stimulation and peripheral nerve electrical stimulation on corticospinal excitability. PLoS One. (2019) 14:e0214592. doi: 10.1371/journal.pone.0214592
183. Sattler, V, Acket, B, Raposo, N, Albucher, JF, Thalamas, C, Loubinoux, I, et al. Anodal tDCS combined with radial nerve stimulation promotes hand motor recovery in the acute phase after ischemic stroke. Neurorehabil Neural Repair. (2015) 29:743–54. doi: 10.1177/1545968314565465
184. Celnik, PA, Paik, N-J, Vandermeeren, Y, Dimyan, MA, and Cohen, LG. Effects of combined peripheral nerve stimulation and brain polarization on performance of a motor sequence task after chronic stroke. Stroke. (2009) 40:1764–71. doi: 10.1161/STROKEAHA.108.540500
185. Nitsche, MA, Fricke, K, Henschke, U, Schlitterlau, A, Liebetanz, D, Lang, N, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. (2003) 553:293–301. doi: 10.1113/jphysiol.2003.049916
186. Kaelin-Lang, A, Luft, AR, Sawaki, L, Burstein, AH, Sohn, YH, Cohen, LG, et al. Modulation of human corticomotor excitability by somatosensory input. J Physiol. (2002, 2002) 540:623–33. doi: 10.1113/jphysiol.2001.012801
187. Menezes, IS, Cohen, LG, Mello, EA, Machado, AG, Peckham, PH, Anjos, SM, et al. Combined brain and peripheral nerve stimulation in chronic stroke patients with moderate to severe motor impairment. Neuromodulation. (2018) 21:176–83. doi: 10.1111/ner.12717
188. Carson, RG, and Kennedy, NC. Modulation of human corticospinal excitability by paired associative stimulation. Front Hum Neurosci. (2013) 7:823. doi: 10.3389/fnhum.2013.00823
189. Stefan, K . Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. (2000) 123:572–84. doi: 10.1093/brain/123.3.572
190. Wolters, A . A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. (2003) 89:2339–45. doi: 10.1152/jn.00900.2002
191. Rajji, TK, Liu, SK, Frantseva, MV, Mulsant, BH, Thoma, J, Chen, R, et al. Exploring the effect of inducing long-term potentiation in the human motor cortex on motor learning. Brain Stimul. (2011) 4:137–44. doi: 10.1016/j.brs.2010.09.007
192. Palmer, JA, Wolf, SL, and Borich, MR. Paired associative stimulation modulates corticomotor excitability in chronic stroke: a preliminary investigation. Restor Neurol Neurosci. (2018) 36:183–94. doi: 10.3233/RNN-170785
193. Kumru, H, Albu, S, Rothwell, J, Leon, D, Flores, C, Opisso, E, et al. Modulation of motor cortex excitability by paired peripheral and transcranial magnetic stimulation. Clin Neurophysiol. (2017) 128:2043–7. doi: 10.1016/j.clinph.2017.06.041
194. Feurra, M, Blagovechtchenski, E, Nikulin, VV, Nazarova, M, Lebedeva, A, Pozdeeva, D, et al. State-dependent effects of transcranial oscillatory currents on the motor system during action observation. Sci Rep. (2019) 9:12858. doi: 10.1038/s41598-019-49166-1
195. Kraus, D, Naros, G, Guggenberger, R, Leao, MT, Ziemann, U, and Gharabaghi, A. Recruitment of additional corticospinal pathways in the human brain with state-dependent paired associative stimulation. J Neurosci. (2018) 38:1396–407. doi: 10.1523/JNEUROSCI.2893-17.2017
196. Mrachacz-Kersting, N, Stevenson, AJT, Jorgensen, HRM, Severinsen, KE, Aliakbaryhosseinabadi, S, Jiang, N, et al. Brain state-dependent stimulation boosts functional recovery following stroke. Ann Neurol. (2019) 85:84–95. doi: 10.1002/ana.25375
Keywords: transcranial direct current stimulation, transcranial random noise stimulation, transcranial alternating current stimulation, transcranial magnetic stimulation, peripheral electrical stimulation, peripheral magnetic stimulation, action observation
Citation: Qi F, Nitsche MA, Ren X, Wang D and Wang L (2023) Top-down and bottom-up stimulation techniques combined with action observation treatment in stroke rehabilitation: a perspective. Front. Neurol. 14:1156987. doi: 10.3389/fneur.2023.1156987
Received: 03 February 2023; Accepted: 26 June 2023;
Published: 11 July 2023.
Edited by:
Julien Nizard, Université de Nantes, FranceReviewed by:
Vincenzo Di Lazzaro, Campus Bio-Medico University, ItalyCopyright © 2023 Qi, Nitsche, Ren, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijuan Wang, d2FuZ2xpanVhbkBic3UuZWR1LmNu; Duanwei Wang, d3JlbjkyNUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.