- 1Institute of Clinical Medical Sciences, China-Japan Friendship Hospital, Beijing, China
- 2Department of Neurology, China-Japan Friendship Hospital, Beijing, China
Backgrounds and objectives: Currently, no consensus has been reached on the therapeutic implications of monoclonal antibodies against amyloid-beta (Aβ) in Alzheimer's disease (AD). This study aimed to examine the effectiveness and safety of monoclonal antibodies against Aβ as a whole and also to determine the superiority of individual antibodies vis-à-vis placebo in mild or moderate AD.
Methods: Literature retrieval, article selection, and data abstraction were performed independently and in duplicate. Cognition and function were appraised by the Mini-Mental State Examination (MMSE), Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-Cog), Disability Assessment for Dementia (DAD), and Clinical Dementia Rating Scale-Sum of Boxes (CDR-SB). Effect sizes are expressed as standardized mean difference (SMD) with a 95% confidence interval (CI).
Results: Twenty-nine articles involving 108 drug-specific trials and 21,383 participants were eligible for synthesis. Of the four assessment scales, only CDR-SB was significantly reduced after using monoclonal antibodies against Aβ relative to placebo (SMD: −0.12; 95% CI: −0.2 to −0.03; p = 0.008). Egger's tests indicated a low likelihood of publication bias. At individual levels, bapineuzumab was associated with a significant increase in MMSE (SMD: 0.588; 95% CI: 0.226–0.95) and DAD (SMD: 0.919; 95% CI: 0.105–1.943), and a significant decrease in CDR-SB (SMD: −0.15; 95% CI: −0.282–0.018). Bapineuzumab can increase the significant risk of serious adverse events (OR: 1.281; 95% CI: 1.075–1.525).
Conclusion: Our findings indicate that monoclonal antibodies against Aβ can effectively improve instrumental activities of daily life in mild or moderate AD. In particular, bapineuzumab can improve cognition and function, as well as activities of daily life, and meanwhile, it triggers serious adverse events.
Introduction
Alzheimer's disease (AD) is a chronic neurodegenerative disease with insidious clinical presentation, and it is characterized by progressive impairment of memory and cognitive function. Approximately 50 million people are suffering from dementia globally, and the number elevates by 10 million annually, as per the 2020 report of the World Health Organization (WHO) (1). By 2050, the cases of dementia are expected to triple (2). As revealed by a systematical analysis in 2020, the overall prevalence of AD was 3.2% in Chinese individuals over 60 years, and its annual prevalence was predicted to increase from 3.81 to 6.17% within the next 5 years (3). In India, the incidence rate of AD per 1,000 person-years was 11.67 for those aged ≥ 55 years (4). Patients diagnosed with AD often experience slow and variable clinical courses, and their original survival ability gradually decreases, eventually leading to death due to complications (5). It is, hence, clinically meaningful to retard, prevent, or even reverse neurological and functional impairment through early and effective pharmacologic treatment.
Alzheimer's disease is a multifactorial disorder involving interactions among genetic, environmental, and lifestyle factors, which open new avenues for the development of tailored therapeutics in the era of precision medicine (6). It is widely recognized that dementia is the underlying cause of AD, and it accounts for 60% of cases (7). AD progresses rapidly, yet treatment options are very limited. Some approved drugs targeting AD, such as donepezil, galantamine, rivastigmine, and memantine, can only help relieve patients' symptoms and suppress the psychological and behavioral symptoms of dementia. Several theories existed for the pathophysiology of AD, including the amyloid cascade hypothesis, degeneration of neuronal cells, and aggregation of tau proteins within the cell (8, 9). Thereof, the amyloid cascade hypothesis is widely accepted, and it proposes that the neurodegeneration and resultant dementia of AD occur as a result of the formation and accumulation of toxic, soluble amyloid-beta (Aβ) oligomers, formed by the misfolding of Aβ monomers (10). In the literature, different therapeutic strategies to clear Aβ from the brain were developed, and monoclonal antibodies against amyloid-beta (Aβ) have aroused growing concerns (11). There is clinical evidence that immunotherapy with monoclonal antibodies is effective for the treatment of patients at earlier AD stages before the emergence of dementia (12). Bapineuzumab is the first N-terminus-directed anti-Aβ antibody tested in humans. Subsequently, several anti-Aβ monoclonal antibody drugs were tested by clinical trials (13). Moreover, aducanumab, a human Ig monoclonal antibody, is recognized as being “risen from the grave,” and it acts in Aβ clearance and curtailing calcium defects in AD (14). Other treatment potentials, such as the immune response generating active immunotherapy and passive immunotherapeutic approaches targeting monoclonal antibodies toward Aβ aggregates, were also proposed (10). Of all anti-Aβ regimens, passive immunization with anti-Aβ antibodies is recognized as being safe and well-tolerated (15), whereas no consensus has been reached upon the therapeutic implications of monoclonal antibodies against Aβ in AD. Fortunately, meta-analysis can provide an opportunity to help derive more reliable estimates.
We aimed to examine the effectiveness and safety profiles of monoclonal antibodies against Aβ as a whole and also to determine the superiority of individual monoclonal antibodies against Aβ vis-à-vis placebo in the treatment of patients with mild or moderate AD.
Methods
Guidelines
The conduct of this meta-analysis conformed to the statement in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (16). The PRISMA checklist is provided in Supplementary Table 1.
Search strategy
Potential clinical trials were searched from PubMed, Excerpta Medica Database (EMBASE), and Web of Science, and the last search was conducted on 31 March 2022. The keywords used for the literature search are expressed in the Boolean form, that is (Alzheimer's* OR dementia*), in the Title/Abstract AND (Aducanumab* OR aduhelm OR BIIB-037 OR BIIB037 OR Solanezumab* OR LY 2062430 OR LY2062430 OR LY-2062430 OR Bapineuzumab* OR AAB-001 OR AAB 001 OR Gantenerumab* OR RG-1450 OR R-1450 OR R1450 OR RG1450 OR R04909832 OR R-04909832 OR RO-4909832 OR Crenezumab* OR MABT5102A OR MABT-5102A OR RG7412 OR RG-7412 OR Ponezumab* OR RN-1219 OR PF-04360365) in the Title/Abstract AND (clinical AND trial OR random*) in the Title/Abstract. In addition, the bibliographies of identified trials were scanned for additional references. All trials were conducted in humans and reported in English. Trials were searched independently by two authors (Y.H. and M.D.), and any disagreement was resolved by discussion with a third author (W.N.).
Inclusion/exclusion criteria
Trials were eligible for inclusion if they met the following criteria simultaneously: (i) participants: patients with mild or moderate AD; (ii) intervention: monoclonal antibodies against Aβ and placebo; (iii) comparator: control; (iv) clinical outcomes: changes in one of the four scales adopted to assess the cognition and function aspects of AD, including the Mini-Mental State Examination (MMSE), Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-Cog), Disability Assessment for Dementia (DAD), and Clinical Dementia Rating Scale-Sum of Boxes (CDR-SB); (v) study design: randomized controlled trials; and (vi) formal publication in peer-review journals.
Trials were excluded if one or more of the following criteria were satisfied: (i) publication type: narrative or systematic review, meta-analysis, case report, case series, conference abstract, comment, correspondence, or editorial; (ii) duplication publication; (iii) lack of comparator; (iv) control rather than placebo; and (v) clinical outcomes rather than four assessment scales mentioned earlier. In the case of more than one article was published using the overlapped study participants, the article with the largest sample size was retained in this meta-analysis.
The eligibility assessment of each retrieved trial was made by two authors (Y.H. and M.D.) independently. Any discrepancy was solved by discussion, and if necessary, was adjudicated by a third author (W.N.).
Data collection
Data from each qualified article were separately abstracted from each qualified article by two reviewers (Y.H. and M.D.) and were typed into a predesigned Excel file, including the surname of the first author, year of publication, ethnicity, and country where participants were enrolled, study design, trial phase, intervention drugs and doses, degree of AD, intervention period, sample size of each arm, number of responses, and dropouts during regimen treatment, and assessment scales for AD, as well as some baseline characteristics, including age, gender, weight, height, body mass index, duration of AD, use of AChEI (acetylcholinesterase inhibitors) or memantine, four assessment scales associated with the risk of AD and adverse reactions, when available.
The process of data collection was completed independently and in duplicate (Y.H. and M.D.), and the consistency of the two datasets was tested by the kappa statistic. In the case of kappa statistics less than unity, original data were checked, and if necessary, a third author (W.N.) was involved.
Quality assessment
Risk of bias for each clinical trial was assessed using the “Revised Cochrane risk-of-bias tool for randomized trials” (RoB 2) (17) from the following five aspects, that is, randomization process, bias due to deviations of intended interventions, bias due to missing outcome data, bias in outcome measurements, and bias in the selection of reported results. Individual domains of risk of bias can be categorized as “low risk,” “some concerns,” or “high risk.” Quality assessment was performed by two authors (Y.H. and M.D.), and any disagreement was solved by a third author (W.N.).
Statistical analyses
Data were imported from Excel to STATA software version 16 (Stata Corp, College Station, Texas, USA), which was used to handle statistical analyses in this meta-analysis. Effect-size estimates from individual trials were pooled under random-effect models, irrespective of the presence or absence of statistical heterogeneity across trials (18). Statistical heterogeneity was measured by the I2 metric, which ranges from 0 to 100%, with higher values representing greater degree of heterogeneity.
The changes in assessment scales for AD before and after intervention are expressed as a standardized mean difference (SMD) with a 95% confidence interval (95% CI) because different rating subscales were used, and the changes in adverse events after intervention are expressed as odds ratio (OR) with a 95% CI.
Cumulative analyses were used to measure the influence of first published trials on subsequent publications and the evolution of accumulated estimates over time. Sensitivity analyses were used to assess the influence of any single trial on pooled effect-size estimates by removing one trial at a time.
Publication bias was inspected using Begg's funnel plots and Egger's tests. The significance of Egger's tests was set at 10%. In addition, to yield more information, the Duval and Tweedie non-parametric “trim and fill” method was employed to estimate the number of theoretically missing trials and derive “unbiased” effect-size estimates.
Results
Eligible articles
By using the prespecified key terms, the literature search of three public databases retrieved a total of 140 publications. After applying predesigned inclusion and exclusion criteria, only 29 articles published in English from 2009 to 2021 were eligible for the final analysis (19–47), involving 108 drug-specific trials and 21,383 participants. Figure 1 illustrates the process of article selection for this meta-analysis.

Figure 1. PRISMA flowchart illustrates the selection process of qualified articles with specific reasons for exclusion in this meta-analysis.
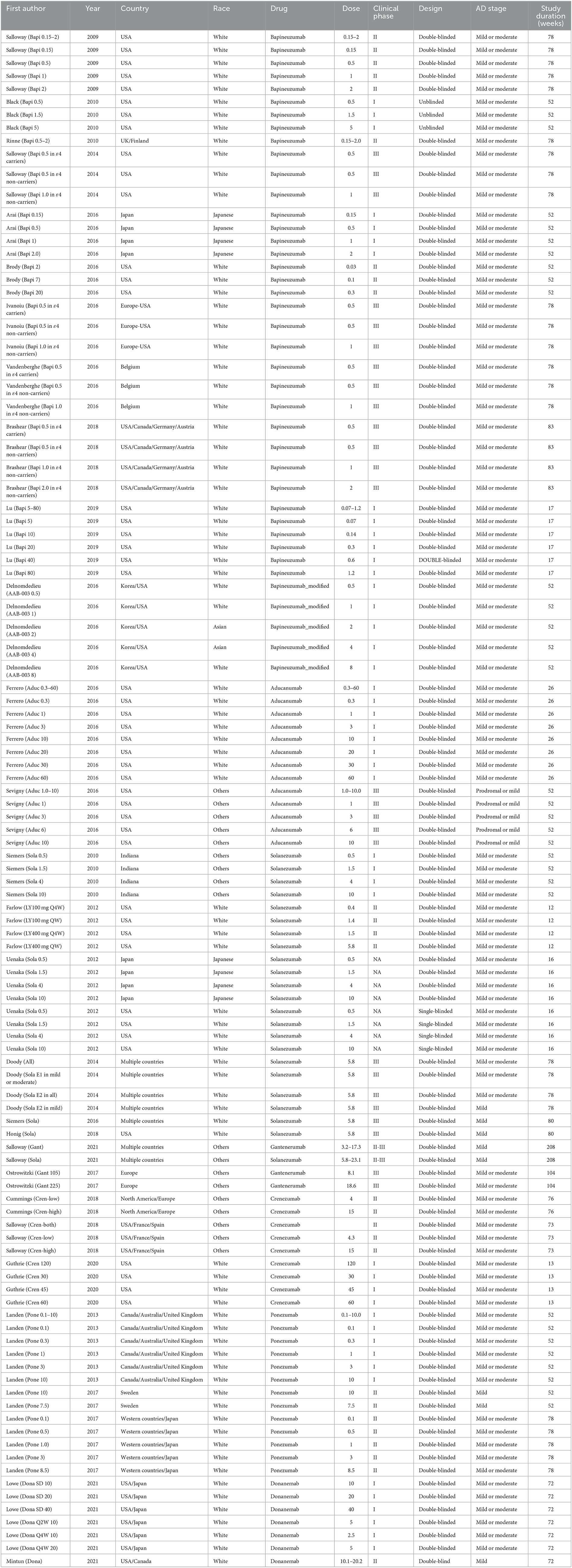
Trial characteristics
Table 1 shows the trial characteristics in this meta-analysis. Five trials involved patients with mild AD, 95 trials involved patients with mild or moderate AD, and five trials involved patients with prodromal or mild AD. Forty-six trials were in phase I, 26 in phase II, two in phase II–III, 26 in phase III, and eight in unreported phases. Trial duration ranged from 12 to 208 weeks. In terms of risk of bias, all clinical trials involved in this meta-analysis were classified as “low risk” or “having some concerns” due to missing necessary information.
Monoclonal antibodies against Aβ
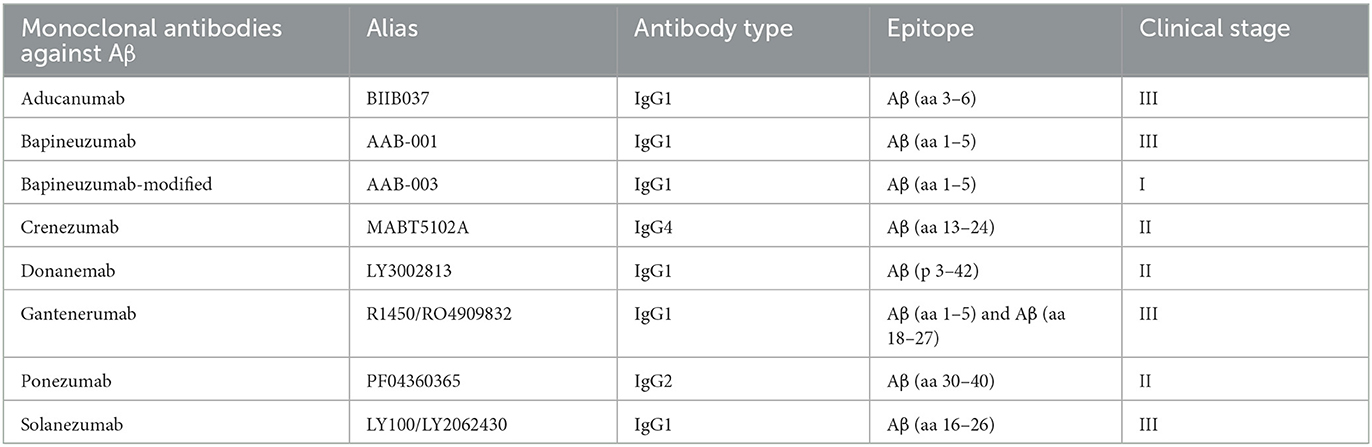
Table 2 shows the detailed targeting information of monoclonal antibodies against Aβ under evaluation. Specifically, eight monoclonal antibodies against Aβ were available, including aducanumab (BIIB037), bapineuzumab (AAB-001), bapineuzumab modified (AAB-003), crenezumab (MABT5102A), donanemab (LY3002813), gantenerumab (R1450/RO4909832), ponezumab (PF04360365), and solanezumab (LY100/LY2062430). Comparison with placebo was available for aducanumab in 13 trials, for bapineuzumab in 35 trials, for bapineuzumab modified in five trials, for crenezumab in nine trials, for donanemab in seven trials, for gantenerumab in three trials, for ponezumab in 13 trials, and for solanezumab in 23 trials.
Overall estimation
Figure 2 provides the forest plots of four assessment scales for monoclonal antibodies against Aβ vis-à-vis placebo in the treatment of AD. Of four assessment scales, only CDR-SB was significantly reduced after using monoclonal antibodies against Aβ relative to placebo (SMD: −0.12; 95% CI: −0.2 to −0.03; p = 0.008), indicating that monoclonal antibodies against Aβ can effectively improve instrumental activities of daily life. Statistical heterogeneity across trials for each assessment scale was significant (I2 > 90%; p < 0.001).
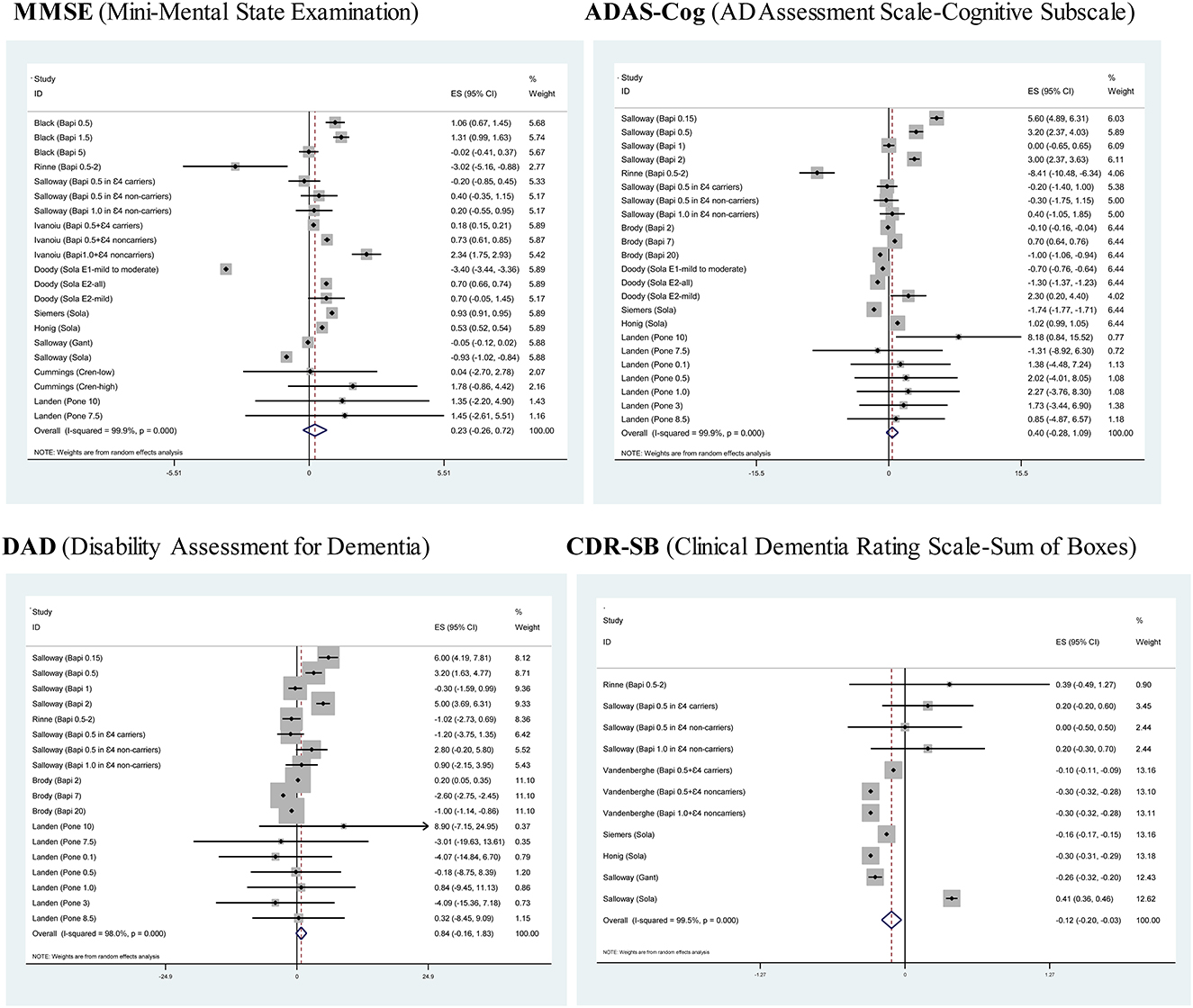
Figure 2. Forest plots of four assessment scales for monoclonal antibodies against Aβ vis-à-vis placebo in the treatment of mild or moderate Alzheimer's disease.
Cumulative and influential analyses
Supplementary Figures 1, 2 separately show the cumulative and influential analyses of four assessment scales for monoclonal antibodies against Aβ vis-à-vis placebo in the treatment of AD.
Publication bias
Figure 3 presents the filled funnel plots of four assessment scales for monoclonal antibodies against Aβ vis-à-vis placebo in the treatment of AD. There were separately one, six, five, and four theoretically missing studies required to make the funnel plots symmetrical for MMSE, ADAS-Cog, DAD, and CDR-SB. Egger's test indicated a low likelihood of publication bias, with the corresponding probabilities being 0.687, 0.434, 0.880, and 0.282.
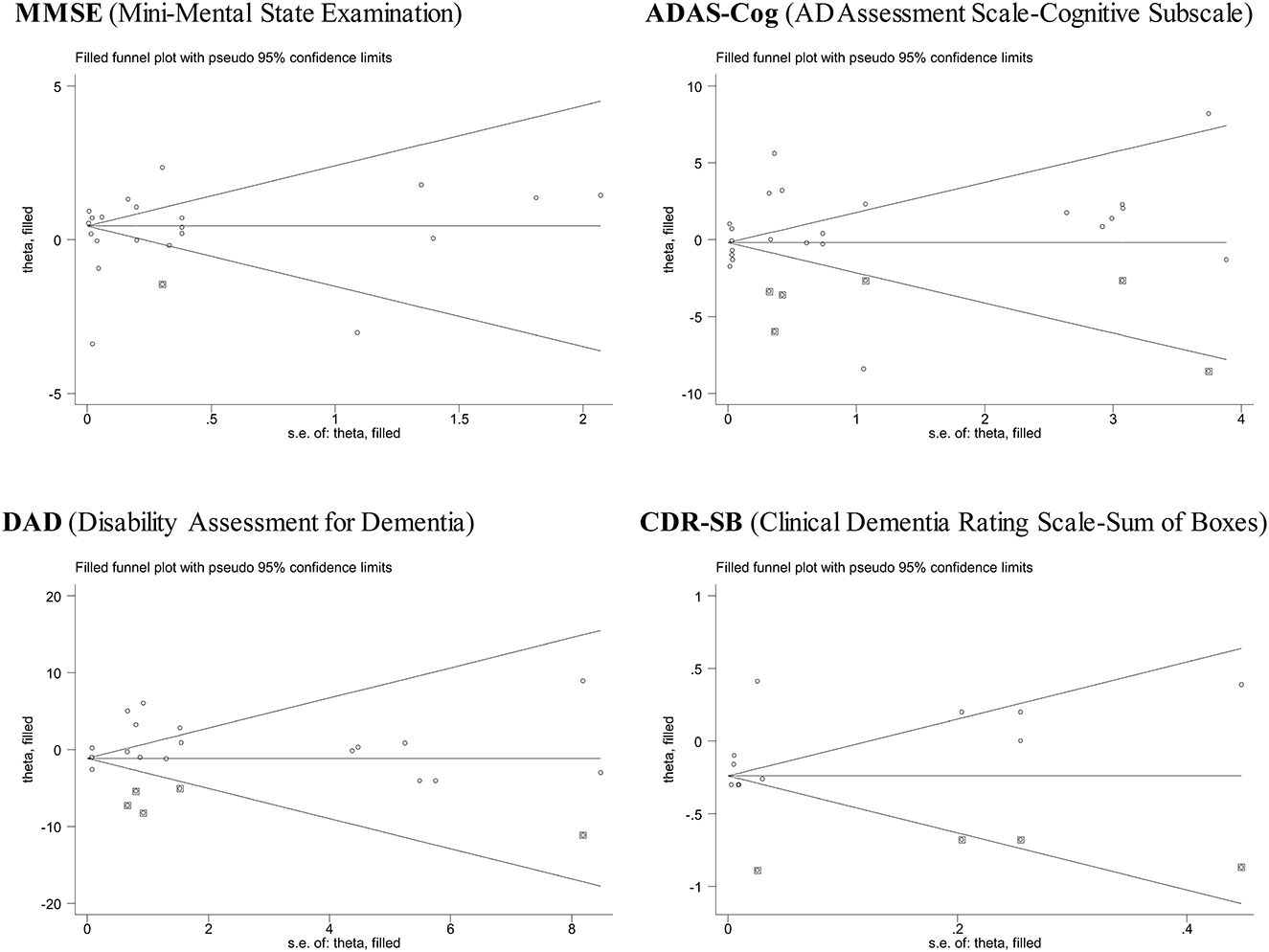
Figure 3. Funnel plots of four assessment scales for monoclonal antibodies against Aβ vis-à-vis placebo in the treatment of mild or moderate Alzheimer's disease.
Subsidiary estimation
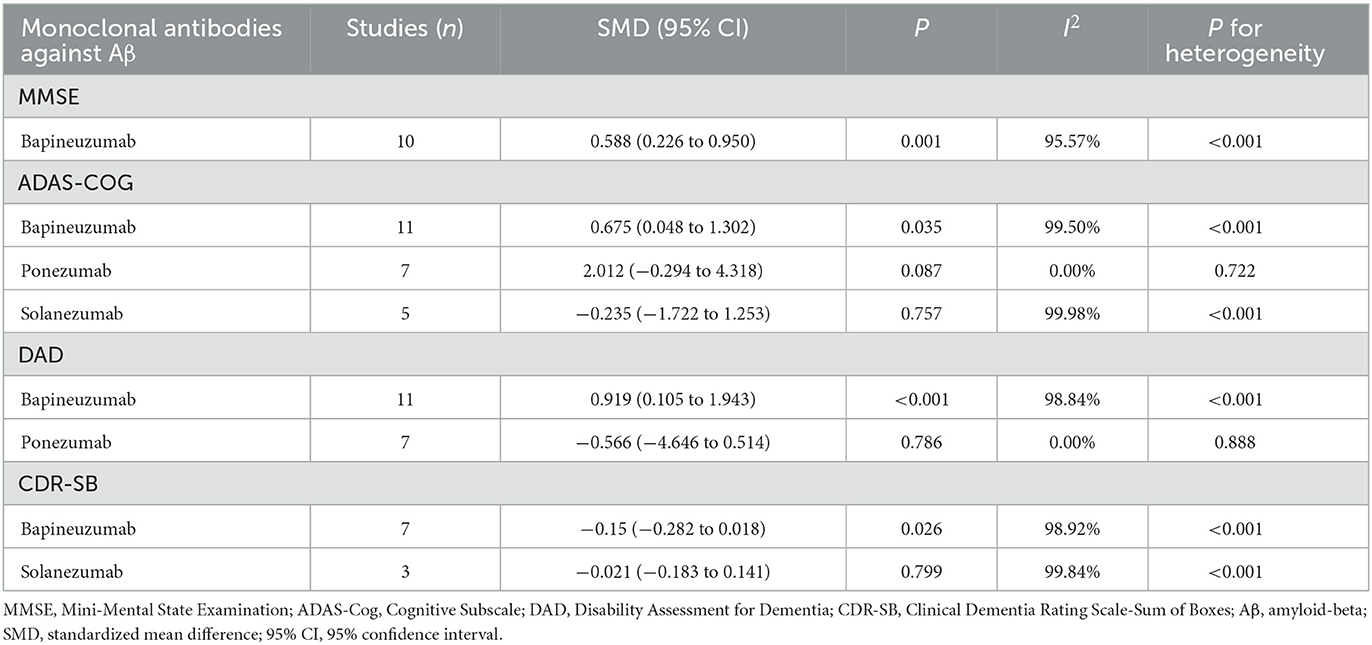
As different monoclonal antibodies against Aβ might exert a diverse impact on assessment scales, drug-specific subsidiary analyses were done accordingly (Table 3). To control potential bias from small-scale estimation, only subgroups involving three or more trials are displayed. Specifically, bapineuzumab was associated with a significant increase in MMSE (SMD: 0.588; 95% CI: 0.226–0.95) and DAD (SMD: 0.919; 95% CI: 0.105–1.943), while a significant decrease in CDR-SB (SMD: −0.15; 95% CI: −0.282–0.018), indicating that bapineuzumab can not only improve cognitive outcomes and functional abilities but also instrumental activities of daily life.
In addition, it is surprising to note that bapineuzumab can significantly increase ADAS-Cog (SMD: 0.675; 95% CI: 0.048–1.302). Changes in the four scores were not significant for the other types of monoclonal antibodies against Aβ.
Adverse events
Table 4 summarizes the common adverse events associated with monoclonal antibodies against Aβ vis-à-vis placebo in the treatment of AD. Relative to the other types of monoclonal antibodies against Aβ, bapineuzumab can increase the significant risk of serious adverse events (OR: 1.281; 95% CI: 1.075–1.525) during the treatment of patients with mild or moderate AD. As for donanemab, there was a significantly increased risk of urinary tract infection (OR: 2.452; 95% CI: 1.107–5.428), nervous system disorders (OR: 3.368; 95% CI: 1.49–7.612), intracranial hemorrhage (OR: 4.966; 95% CI: 1.68–10.674), and amyloid-related imaging abnormalities (OR: 3.063; 95% CI: 3.525–23.3).
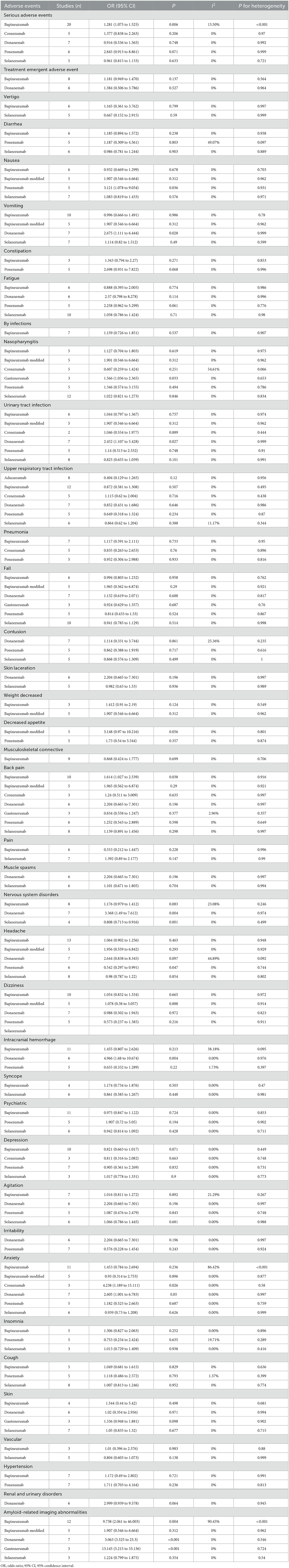
Table 4. Common adverse events associated with monoclonal antibodies against Aβ in the treatment of mild or moderate AD.
Regarding solanezumab, there was a significantly reduced risk for nervous system disorders (OR: 0.808; 95% CI: 0.713–0.916). For ponezumab, the risk of headache was reduced significantly (OR: 0.542; 95% CI: 0.297–0.991). In contrast, gantenerumab was associated with a significantly increased risk of amyloid-related imaging abnormalities (OR: 13.145; 95% CI: 5.215–33.136).
Rare adverse events associated with monoclonal antibodies against Aβ vis-à-vis placebo in the treatment of mild or moderate AD are presented in Supplementary Table 2.
Discussion
The aim of this meta-analysis was to summarize data on the effectiveness and safety of monoclonal antibodies against Aβ vis-à-vis placebo in the treatment of mild or moderate AD. It is noteworthy that monoclonal antibodies against Aβ as a whole can effectively improve instrumental activities of daily life based on CDR-SB scores. Moreover, analysis of individual antibodies revealed that bapineuzumab can improve cognition and function, as well as activities of daily life, yet it also triggers the occurrence of serious adverse events. To the best of our knowledge, this is the largest meta-analysis thus far that has synthesized data on monoclonal antibodies against Aβ compared with placebo for mild or moderate AD.
The deposit of extracellular Aβ plaques is a key feature of AD, and mounting evidence indicates that aberrant Aβ production or clearance is a potential harbinger in the pathogenesis of AD (48). Immunotherapy with monoclonal antibodies is increasingly identified as an effective therapeutic regime against AD, and dozens of clinical trials have been undertaken to explore the effectiveness and safety of monoclonal antibodies against Aβ in patients with AD (11, 49, 50). However, the results of these trials are not often reproducible. For example, Doody et al. in a multicenter, randomized, placebo-controlled trial demonstrated a marginally significant increase in MMSE scores in favor of donepezil (51), and contrastingly, Rinne et al. found that bapineuzumab exerted an unfavorable effect on MMSE scores (21). The reasons for these inconsistencies are likely several-fold. One reason might be related to sample sizes, because the magnitude of changes in instrumental scores between interventions is small in most cases. Another reason is probably due to the diverse types of monoclonal antibodies against Aβ, in view of the different targeted Aβ epitopes (31, 36, 52–56). A third reason rests with the differences in demographic and clinical characteristics, as well as genetic undergrounds across trials. Fortunately, meta-analysis offers a rational and helpful approach to dealing with inconsistencies from many studies of the same research topic. With the help of this approach and based on 29 articles and 21,383 participants, we interestingly found that monoclonal antibodies against Aβ as a whole can effectively improve instrumental activities of daily life based on CDR-SB scores in patients with mild or moderate AD, in line with the observations of many clinical trials (26, 36–38).
In addition, we explored the effectiveness and safety of individual monoclonal antibodies against Aβ in patients with AD. Because of the limited number of eligible trials, statistical significance was merely identified for bapineuzumab, an antibody targeted against the N-terminus of Aβ as reflected by MMSE and DAD scores, which can not only improve cognition and function but also enhance activities of daily life, as reflected by CDR-SB scores in terms of effectiveness. Simultaneously, the administration of bapineuzumab was associated with the development of serious adverse events. We agree that the safety profile is paramount, and the long-term benefits and risks of bapineuzumab treatment for mild or moderate AD are not yet known (25, 41). However, we here express concerns that such warnings may discourage patients and their families from choosing bapineuzumab in practice. From another aspect, Aβ might not be the best treatment target in patients with mild or moderate AD, or monoclonal antibodies against Aβ cannot remove an important species of Aβ that plays a contributing role in the pathogenesis of AD (37). Nevertheless, we agree that more large-scale clinical trials with long-term extended follow-ups are warranted to unveil the full potential of monoclonal antibodies against Aβ in AD.
In addition to the clear strengths of this meta-analysis, including the largest sample size, comprehensive analyses, and solid observations, several limitations should be acknowledged. First, only clinical trials published in English were retrieved, which leaves selection bias an open question, as some excellent trials may be published in other languages. However, explorations on publication bias revealed a low probability. Second, the power to detect significance in some subgroups was limited, and between-trial heterogeneity cannot be totally accounted for. Third, only the effectiveness and safety of monoclonal antibodies against Aβ vis-à-vis placebo were examined in the current meta-analysis, and comparison between other classes of drugs targeting AD will be addressed in the future. Fourth, definitions of adverse effects evaluated in this meta-analysis differed across trials, and caution is needed when interpreting the safety profiles of monoclonal antibodies.
Taken together, our findings indicate that monoclonal antibodies against Aβ as a whole can effectively improve instrumental activities of daily life based on CDR-SB scores in mild or moderate AD. Individually, bapineuzumab can improve cognition and function, as well as activities of daily life, yet it also triggers the occurrence of serious adverse events. Further functional investigations on the molecular mechanisms of monoclonal antibodies against Aβ, in particular, bapineuzumab, in the pathophysiology of AD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committees of all institutes or hospitals involved in this meta-analysis. The patients/participants provided their written informed consent to participate in this study.
Author contributions
WN and XD planned and designed the study. WN directed its implementation. YH and MD contributed to data acquisition and conducted statistical analyses. YH, MD, YS, and XD had access to all raw data. YH and WN wrote the manuscript. All authors have read and approved the final manuscript prior to submission.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1147757/full#supplementary-material
References
1. World Health Organization. Dementia. (2020). Available online at: https://www.who.int/news-room/fact942sheets/detail/dementia
2. Scheltens P, Strooper BD, Kivipelto M, Holstege H, Chetelat G, Teunissen CE, et al. Alzheimer's disease. Lancet. (2021) 397:1577–90. doi: 10.1016/S0140-6736(20)32205-4
3. Cui L, Hou NN, Wu HM, Zuo X, Lian YZ, Zhang CN, et al. Prevalence of Alzheimer's disease and Parkinson's disease in China: An updated systematical analysis. Front Aging Neurosci. (2020) 12:603854. doi: 10.3389/fnagi.2020.603854
4. Mathuranath PS, George A, Ranjith N, Justus S, Kumar MS, Menon R, et al. Incidence of Alzheimer's disease in India: A 10 years follow-up study. Neurol India. (2012) 60:625–30. doi: 10.4103/0028-3886.105198
5. Tokuchi R, Hishikawa N, Sato K, Hatanaka N, Fukui Y, Takemoto M, et al. Differences between the behavioral and psychological symptoms of Alzheimer's disease and Parkinson's disease. J Neurol Sci. (2016) 369:278–82. doi: 10.1016/j.jns.2016.08.053
6. Behl T, Kaur I, Sehgal A, Singh S, Albarrati A, Albratty M, et al. The road to precision medicine: Eliminating the “One Size Fits All” approach in Alzheimer's disease. Biomed Pharmacother. (2022) 153:113337. doi: 10.1016/j.biopha.2022.113337
7. Barker WW, Luis CA, Kashuba A, Luis M, Harwood DG, Loewenstein D, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. (2002) 16:203–12. doi: 10.1097/00002093-200210000-00001
8. Ohm DT, Fought AJ, Martersteck A, Coventry C, Sridhar J, Gefen T, et al. Accumulation of neurofibrillary tangles and activated microglia is associated with lower neuron densities in the aphasic variant of Alzheimer's disease. Brain Pathol. (2021) 31:189–204. doi: 10.1111/bpa.12902
9. Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer's disease: The IWG-2 criteria. Lancet Neurol. (2014) 13:614–29. doi: 10.1016/S1474-4422(14)70090-0
10. Behl T, Kaur I, Fratila O, Brata R, Bungau S. Exploring the potential of therapeutic agents targeted towards mitigating the events associated with amyloid-beta cascade in Alzheimer's disease. Int J Mol Sci. (2020) 21:7443. doi: 10.3390/ijms21207443
11. van Dyck CH. Anti-amyloid-beta monoclonal antibodies for Alzheimer's disease: Pitfalls and promise. Biol Psychiatry. (2018) 83:311–9. doi: 10.1016/j.biopsych.2017.08.010
12. Sperling RA, Jack CR Jr, Aisen PS. Testing the right target and right drug at the right stage. Sci Transl Med. (2011) 3:111cm.33. doi: 10.1126/scitranslmed.3002609
13. Ricciarelli R, Fedele E. The amyloid cascade hypothesis in Alzheimer's disease: It's time to change our mind. Curr Neuropharmacol. (2017) 15:926–35. doi: 10.2174/1570159X15666170116143743
14. Behl T, Kaur I, Sehgal A, Singh S, Sharma N, Makeen HA, et al. “Aducanumab” making a comeback in Alzheimer's disease: An old wine in a new bottle. Biomed Pharmacother. (2022) 148:112746. doi: 10.1016/j.biopha.2022.112746
15. Panza F, Lozupone M, Logroscino G, Imbimbo BP. A critical appraisal of amyloid-beta-targeting therapies for Alzheimer disease. Nat Rev Neurol. (2019) 15:73–88. doi: 10.1038/s41582-018-0116-6
16. Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
17. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2, a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366:l4898. doi: 10.1136/bmj.l4898
18. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. (2010) 1:97–111. doi: 10.1002/jrsm.12
19. Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. (2009) 73:2061–70. doi: 10.1212/WNL.0b013e3181c67808
20. Black RS, Sperling RA, Safirstein B, Motter RN, Pallay A, Nichols A, et al. A single ascending dose study of bapineuzumab in patients with Alzheimer disease. Alzheimer Dis Assoc Disord. (2010) 24:198–203. doi: 10.1097/WAD.0b013e3181c53b00
21. Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer's disease treated with bapineuzumab: A phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. (2010) 9:363–72. doi: 10.1016/S1474-4422(10)70043-0
22. Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med. (2014) 370:322–33. doi: 10.1056/NEJMoa1304839
23. Arai H, Umemura K, Ichimiya Y, Iseki E, Eto K, Miyakawa K, et al. Safety and pharmacokinetics of bapineuzumab in a single ascending-dose study in Japanese patients with mild to moderate Alzheimer's disease. Geriatr Gerontol Int. (2016) 16:644–50. doi: 10.1111/ggi.12516
24. Brody M, Liu E, Di J, Lu M, Margolin RA, Werth JL, et al. A phase II, randomized, double-blind, placebo-controlled study of safety, pharmacokinetics, and biomarker results of subcutaneous bapineuzumab in patients with mild to moderate Alzheimer's disease. J Alzheimers Dis. (2016) 54:1509–19. doi: 10.3233/JAD-160369
25. Ivanoiu A, Pariente J, Booth K, Lobello K, Luscan G, Hua L, et al. Long-term safety and tolerability of bapineuzumab in patients with Alzheimer's disease in two phase 3 extension studies. Alzheimers Res Ther. (2016) 8:24. doi: 10.1186/s13195-016-0193-y
26. Vandenberghe R, Rinne JO, Boada M, Katayama S, Scheltens P, Vellas B, et al. Bapineuzumab for mild to moderate Alzheimer's disease in two global, randomized, phase 3 trials. Alzheimers Res Ther. (2016) 8:18. doi: 10.1186/s13195-016-0189-7
27. Brashear HR, Ketter N, Bogert J, Di J, Salloway SP, Sperling R, et al. Clinical evaluation of amyloid-related imaging abnormalities in bapineuzumab phase III studies. J Alzheimers Dis. (2018) 66:1409–24. doi: 10.3233/JAD-180675
28. Lu M, Brashear HR. Pharmacokinetics, pharmacodynamics, and safety of subcutaneous bapineuzumab: A single-ascending-dose study in patients with mild to moderate Alzheimer's disease. Clin Pharmacol Drug Dev. (2019) 8:326–35. doi: 10.1002/cpdd.584
29. Delnomdedieu M, Duvvuri S, Li DJ, Atassi N, Lu M, Brashear HR, et al. First-In-Human safety and long-term exposure data for AAB-003 (PF-05236812) and biomarkers after intravenous infusions of escalating doses in patients with mild to moderate Alzheimer's disease. Alzheimers Res Ther. (2016) 8:12. doi: 10.1186/s13195-016-0177-y
30. Ferrero J, Williams L, Stella H, Leitermann K, Mikulskis A, O'Gorman J, et al. First-in-human, double-blind, placebo-controlled, single-dose escalation study of aducanumab (BIIB037) in mild-to-moderate Alzheimer's disease. Alzheimers Dement. (2016) 2:169–76. doi: 10.1016/j.trci.2016.06.002
31. Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M, et al. The antibody aducanumab reduces Abeta plaques in Alzheimer's disease. Nature. (2016) 537:50–6. doi: 10.1038/nature19323
32. Siemers ER, Friedrich S, Dean RA, Gonzales CR, Farlow MR, Paul SM, et al. Safety and changes in plasma and cerebrospinal fluid amyloid beta after a single administration of an amyloid beta monoclonal antibody in subjects with Alzheimer disease. Clin Neuropharmacol. (2010) 33:67–73. doi: 10.1097/WNF.0b013e3181cb577a
33. Farlow M, Arnold SE, van Dyck CH, Aisen PS, Snider BJ, Porsteinsson AP, et al. Safety and biomarker effects of solanezumab in patients with Alzheimer's disease. Alzheimers Dement. (2012) 8:261–71. doi: 10.1016/j.jalz.2011.09.224
34. Uenaka K, Nakano M, Willis BA, Friedrich S, Ferguson-Sells L, Dean RA, et al. Comparison of pharmacokinetics, pharmacodynamics, safety, and tolerability of the amyloid β monoclonal antibody solanezumab in Japanese and white patients with mild to moderate alzheimer disease. Clin Neuropharmacol. (2012) 35:25–9. doi: 10.1097/WNF.0b013e31823a13d3
35. Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med. (2014) 370:311–21. doi: 10.1056/NEJMoa1312889
36. Siemers ER, Sundell KL, Carlson C, Case M, Sethuraman G, Liu-Seifert H, et al. Phase 3 solanezumab trials: Secondary outcomes in mild Alzheimer's disease patients. Alzheimers Dement. (2016) 12:110–20. doi: 10.1016/j.jalz.2015.06.1893
37. Honig LS, Vellas B, Woodward M, Boada M, Bullock R, Borrie M, et al. Trial of solanezumab for mild dementia due to Alzheimer's disease. N Engl J Med. (2018) 378:321–30. doi: 10.1056/NEJMoa1705971
38. Salloway S, Farlow M, McDade E, Clifford DB, Wang G, Llibre-Guerra JJ, et al. A trial of gantenerumab or solanezumab in dominantly inherited Alzheimer's disease. Nat Med. (2021) 27:1187–96. doi: 10.1038/s41591-021-01369-8
39. Ostrowitzki S, Lasser RA, Dorflinger E, Scheltens P, Barkhof F, Nikolcheva T, et al. A phase III randomized trial of gantenerumab in prodromal Alzheimer's disease. Alzheimers Res Ther. (2017) 9:95. doi: 10.1186/s13195-017-0318-y
40. Cummings JL, Cohen S, van Dyck CH, Brody M, Curtis C, Cho W, et al. ABBY: A phase 2 randomized trial of crenezumab in mild to moderate Alzheimer disease. Neurology. (2018) 90:e1889–97. doi: 10.1212/WNL.0000000000005550
41. Salloway S, Honigberg LA, Cho W, Ward M, Friesenhahn M, Brunstein F, et al. Amyloid positron emission tomography and cerebrospinal fluid results from a crenezumab anti-amyloid-beta antibody double-blind, placebo-controlled, randomized phase II study in mild-to-moderate Alzheimer's disease (BLAZE). Alzheimers Res Ther. (2018) 10:96. doi: 10.1186/s13195-018-0424-5
42. Guthrie H, Honig LS, Lin H, Sink KM, Blondeau K, Quartino A, et al. Safety, tolerability, and pharmacokinetics of crenezumab in patients with mild-to-moderate Alzheimer's disease treated with escalating doses for up to 133 weeks. J Alzheimers Dis. (2020) 76:967–79. doi: 10.3233/JAD-200134
43. Landen JW, Zhao Q, Cohen S, Borrie M, Woodward M, Billing CB, et al. Safety and pharmacology of a single intravenous dose of ponezumab in subjects with mild-to-moderate Alzheimer disease: A phase I, randomized, placebo-controlled, double-blind, dose-escalation study. Clin Neuropharmacol. (2013) 36:14–23. doi: 10.1097/WNF.0b013e31827db49b
44. Landen JW, Andreasen N, Cronenberger CL, Schwartz PF, Börjesson-Hanson A, Östlund H, et al. Ponezumab in mild-to-moderate Alzheimer's disease: Randomized phase II PET-PIB study. Alzheimers Dement. (2017) 3:393–401. doi: 10.1016/j.trci.2017.05.003
45. Landen JW, Cohen S, Billing CB Jr, Cronenberger C, Styren S, Burstein AH, et al. Multiple-dose ponezumab for mild-to-moderate Alzheimer's disease: Safety and efficacy. Alzheimers Dement. (2017) 3:339–47. doi: 10.1016/j.trci.2017.04.003
46. Lowe SL, Duggan Evans C, Shcherbinin S, Cheng YJ, Willis BA, Gueorguieva I, et al. Donanemab (LY3002813) phase 1b study in Alzheimer's disease: Rapid and sustained reduction of brain amyloid measured by florbetapir F18 imaging. J Prev Alzheimers Dis. (2021) 8:414–24. doi: 10.14283/jpad.2021.56
47. Mintun MA, Lo AC, Duggan Evans C, Wessels AM, Ardayfio PA, Andersen SW, et al. Donanemab in early Alzheimer's disease. N Engl J Med. (2021) 384:1691–704. doi: 10.1056/NEJMoa2100708
48. Khan S, Barve KH, Kumar MS. Recent advancements in pathogenesis, diagnostics and treatment of Alzheimer's disease. Curr Neuropharmacol. (2020) 18:1106–25. doi: 10.2174/1570159X18666200528142429
49. Shi M, Chu F, Zhu F, Zhu J. Impact of anti-amyloid-beta monoclonal antibodies on the pathology and clinical profile of Alzheimer's disease: A focus on aducanumab and lecanemab. Front Aging Neurosci. (2022) 14:870517. doi: 10.3389/fnagi.2022.870517
50. Lacorte E, Ancidoni A, Zaccaria V, Remoli G, Tariciotti L, Bellomo G, et al. Safety and efficacy of monoclonal antibodies for Alzheimer's disease: A systematic review and meta-analysis of published and unpublished clinical trials. J Alzheimers Dis. (2022) 87:101–29. doi: 10.3233/JAD-220046
51. Doody RS, Ferris SH, Salloway S, Sun Y, Goldman R, Watkins WE, et al. Donepezil treatment of patients with MCI: A 48-week randomized, placebo-controlled trial. Neurology. (2009) 72:1555–61. doi: 10.1212/01.wnl.0000344650.95823.03
52. Doggrell SA. Still grasping at straws: Donanemab in Alzheimer's disease. Expert Opin Investig Drugs. (2021) 30:797–801. doi: 10.1080/13543784.2021.1948010
53. Kerchner GA, Boxer AL. Bapineuzumab. Expert Opin Biol Ther. (2010) 10:1121–30. doi: 10.1517/14712598.2010.493872
54. Ultsch M, Li B, Maurer T, Mathieu M, Adolfsson O, Muhs A, et al. Structure of crenezumab complex with abeta shows loss of beta-hairpin. Sci Rep. (2016) 6:39374. doi: 10.1038/srep39374
55. La Porte SL, Bollini SS, Lanz TA, Abdiche YN, Rusnak AS, Ho WH, et al. Structural basis of C-terminal beta-amyloid peptide binding by the antibody ponezumab for the treatment of Alzheimer's disease. J Mol Biol. (2012) 421:525–36. doi: 10.1016/j.jmb.2011.11.047
Keywords: Alzheimer's disease, monoclonal antibodies against amyloid-beta, effectiveness, adverse events, meta-analysis, MMSE, ADAS-Cog
Citation: Hao Y, Dong M, Sun Y, Duan X and Niu W (2023) Effectiveness and safety of monoclonal antibodies against amyloid-beta vis-à-vis placebo in mild or moderate Alzheimer's disease. Front. Neurol. 14:1147757. doi: 10.3389/fneur.2023.1147757
Received: 19 January 2023; Accepted: 16 February 2023;
Published: 15 March 2023.
Edited by:
Jennifer S. Yokoyama, University of San Francisco, United StatesReviewed by:
Simona Gabriela Bungau, University of Oradea, RomaniaCristina Monteiro, University of Beira Interior, Portugal
Copyright © 2023 Hao, Dong, Sun, Duan and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohui Duan, eXVmZWVkdWFuQHNpbmEuY29t; Wenquan Niu, bml1d2VucXVhbl9zaGNuQDE2My5jb20=
 Ying Hao1
Ying Hao1 Xiaohui Duan
Xiaohui Duan Wenquan Niu
Wenquan Niu