
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 12 May 2023
Sec. Endovascular and Interventional Neurology
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1146194
Objective: Contrast-induced encephalopathy (CIE) is a rare neurological complication that can occur in the context of various endovascular procedures. Although many potential risk factors for CIE have been reported, it is still unclear whether anesthesia is a risk factor for the occurrence of CIE. The goal of this study was to investigate the incidence of CIE in patients who underwent endovascular treatment under different anesthesia methods and anesthetics administration and to explore whether general anesthesia was a potential risk factor for CIE.
Methods: We retrospectively reviewed available clinical data from 1,043 patients with neurovascular diseases undergoing endovascular treatment between June 2018 and June 2021 in our hospital. A propensity score-based matching strategy and logistic regression were used to analyze the association between anesthesia and the occurrence of CIE.
Results: In this study, we implemented the embolization of intracranial aneurysm in 412 patients, stent implantation of extracranial artery stenosis in 346, stent implantation of intracranial artery stenosis in 187, embolization of cerebral arteriovenous malformation or dural arteriovenous fistula in 54, endovascular thrombectomy in 20, and other endovascular treatments in 24. A total of 370 patients (35.5%) received treatment under local anesthesia, while the remaining 673 (64.5%) underwent treatment under general anesthesia. In total, 14 patients were identified as CIE, resulting in a total incidence rate of 1.34%. After propensity score-based matching of anesthesia methods, the occurrence of CIE was significantly different between the general anesthesia and local anesthesia group (P = 0.007). After propensity score-based matching of CIE, the anesthesia methods were significantly different between the two groups. Pearson contingency coefficients and logistic regression showed a significant correlation between general anesthesia and the risk of CIE.
Conclusion: General anesthesia might be a risk factor for CIE, and propofol might be associated with the increased occurrence of CIE.
Contrast-induced encephalopathy (CIE) is a rare neurological complication following the administration of contrast agents in various angiographic procedures. It has been reported to occur in 1.7–3.6% of neurological endovascular procedures, including cerebral angiography, coil embolization, and endovascular thrombectomy procedure (1–3). The clinical manifestations mainly include encephalopathy, cortical blindness, motor deficit, decreased vigilance, aphasia, headache, and epileptic seizures (4).
The mechanisms of CIE remain unclear, and it is speculated that increased permeability of the blood–brain barrier (BBB), hyperosmolarity, and direct neurotoxicity from the contrast agents may lead to CIE (5). The potential risk factors for CIE may include chronic hypertension, renal dysfunction, history of stroke, high-contrast osmolality, and overdose of contrast medium (3–8). However, it is still unclear whether anesthesia is a risk factor for the occurrence of CIE, considering that anesthetics (e.g., propofol) may affect the permeability of BBB or have direct neurotoxicity. Therefore, our study aimed to investigate the incidence of CIE in patients who underwent endovascular treatment under different anesthesia methods and anesthetics administration and to explore whether general anesthesia was the potential risk factor for CIE.
The clinical data available in our database collected from patients with neurovascular diseases undergoing endovascular treatment were reviewed from June 2018 to June 2021. The participants were fully informed, and signed consent forms were obtained. The study was approved by the medical ethics committee of Ruijin Hospital, affiliated with Shanghai Jiao Tong University School of Medicine.
Clinical data, including age, sex, symptoms, neuroimaging, anesthesia methods, treatment, operation duration, past medical history, and outcomes of patients, were collected and analyzed. The operation duration was defined as the time period from the start of anesthesia to the end of the operation.
The patients were arranged to receive local anesthesia or general anesthesia, depending on the type and location of the lesion and also by the request of the patient. Generally, the patients with intracranial aneurysm, cerebral arteriovenous malformation, intracranial artery stenosis, dural arteriovenous fistula, or acute ischemic stroke were recommended to undergo general anesthesia. Otherwise, the patients with extracranial artery stenosis (i.e., stenosis of the initial segment of the internal carotid artery) or extracranial arterial dissection, or the patients who would undergo embolization of feeding arteries of convex meningioma, were recommended to receive local anesthesia. In patients with local anesthesia, only 2% lidocaine was injected into the inguinal region locally, while in patients with general anesthesia, anesthetic drugs were given intravenously by experienced anesthetists. Generally, the anesthetic drugs included sedatives (propofol and dexmedetomidine), muscular relaxants (rocuronium and cisatracurium), and analgesics (fentanyl, sufentanil, and remifentanil). The choice of these abovementioned anesthetic drugs depended on the need for anesthesia. The dose of each drug administrated was based on the body weight of the patients. Additionally, if the patients stayed in prolonged intubation after endovascular treatment, dexmedetomidine would be used for sedation, and the dosage of dexmedetomidine would also be documented (Supplementary Table 1).
A non-ionic contrast medium was administrated intravenously in all cases during endovascular treatment. The main contrast medium used was iopamidol or iodixanol. In addition, the contrast dose of a CT angiography (CTA), in which a non-ionic contrast medium was used, would also be included in the total contrast dose if the CTA was performed within 24 h prior to endovascular treatment.
The patient was diagnosed with CIE if both the clinical and radiological criteria were fulfilled within 24 h following endovascular treatment. The clinical criteria were unequivocal clinical deterioration (i.e., a decrease of ≥2 in the Glasgow Coma Scale score and a decrease in muscle strength) and/or onset of new neurological symptoms (i.e., disorientation and cortical blindness) that could not be explained by recurrent ischemic stroke, hemorrhage, and metabolic abnormalities. The radiological criterion was regional cerebral edema accompanied by contrast staining, which was defined as the presence of a hyperdense lesion in the brain parenchyma or subarachnoid space that persisted on follow-up neuroimaging (3). The patients receiving general anesthesia were neurologically evaluated immediately after awakening from anesthesia and at the onset of the new event. Neuroimaging was arranged in all of them at an appropriate time for ruling out stroke or hemorrhage (Figures 1, 2).
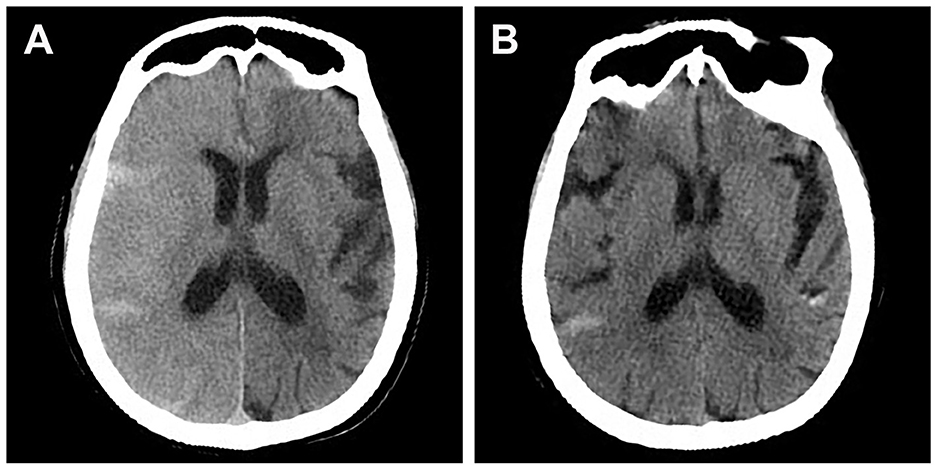
Figure 1. Brain CT scan of a patient who presented with lethargy, aphasia, and contralateral side limb weakness after stent-assisted embolization of posterior communicating aneurysm. (A) CT scan performed 2 h after symptom onset showing diffuse right cerebral edema and contrast staining. (B) CT scan performed after clinical resolution (36 h after symptom onset) showing resolution of cerebral edema.
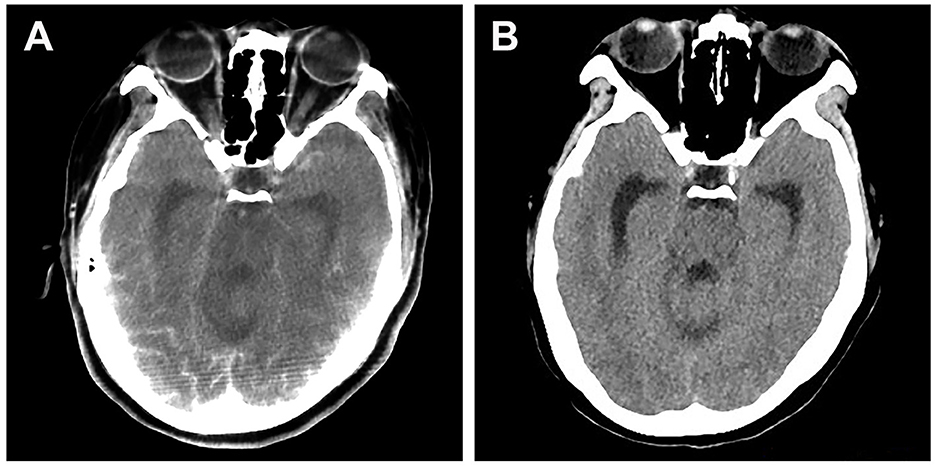
Figure 2. Brain CT scan of a patient who presented with cortical blindness after stent-assisted embolization of vertebral aneurysm. (A) CT scan performed 2 h after symptom onset showing significant contrast staining at the occipital lobes. (B) CT scan performed after clinical resolution (24 h after symptom onset) showing effacement of contrast staining.
A propensity score-based matching strategy was used to select a control group. For patients who underwent treatment with general anesthesia, controls with local anesthesia were matched by age, sex, hypertension, diabetes, renal dysfunction, history of stroke, and dose of contrast agent to the corresponding case with a ratio of 1:1 with the nearest propensity score (caliper width of 0.10). The differences between general and local anesthesia after matching were shown by standardized mean difference (SMD). All continuous data were presented as mean ± standard deviation (SD) and compared by t-test. All categorical data were presented as N (%) and compared by chi-square. The correlation between the anesthesia methods and the event of CIE was analyzed by Pearson's contingency coefficients.
Considering the low prevalence of CIE in the real world and the limited events of CIE in our data, the controls were matched by age, sex, hypertension, diabetes, renal dysfunction, history of stroke, and dose of contrast agent to the patients with CIE to further study the association between the anesthesia methods and risk of CIE. Each patient with CIE was matched to four control subjects with the nearest propensity score (caliper width of 0.10). All continuous data were presented as mean ± standard deviation (SD) and compared by t-test. All categorical data were presented as N (%) and compared by chi-square. Logistic regression was used to estimate the odds ratio (OR) of CIE and its 95% confidence interval (95% CI). The additional sensitivity analysis comprised the risk of CIE associated with propofol dosage in unit weight. We calculated the median propofol dosage in unit weight in patients with general anesthesia first. The patients were grouped into three groups: never used, used less than median propofol dosage in unit weight, and used more than median propofol dosage in unit weight. The test for trend was based on variables containing a median value for each quintile.
A p-value of < 0.05 (two-tailed) was considered statistically significant. R (version 4.1.1) and R Studio (version 1.1.442) were used to perform the statistical analysis.
During the study period, 1,043 patients underwent endovascular treatment, among whom 14 patients were diagnosed with CIE, resulting in a total incidence rate of 1.34% (Table 1). Before endovascular treatment, a CT angiography was given to 453 patients, among whom 26 patients had the CT angiography within 24 h prior to endovascular treatment. In these 1,043 patients, we implemented embolization of intracranial aneurysm in 412, stent implantation of extracranial artery stenosis in 346, stent implantation of intracranial artery stenosis in 187, embolization of cerebral arteriovenous malformation or dural arteriovenous fistula in 54, endovascular thrombectomy in 20, and other endovascular treatments in 24. Out of the 1,043 patients, 370 received treatment under local anesthesia, while the remaining 673 patients underwent treatment under general anesthesia. In total, 18 patients stayed in prolonged intubation after endovascular treatment under general anesthesia and received an intravenous injection of dexmedetomidine by micropump for sedation. The duration of sedation in these 18 patients varied from 8 h to 36 h.
The incidence of CIE was 0.27% (1/370) and 1.93% (13/673) in the local anesthesia and general anesthesia groups, respectively. Also, a higher dose of the contrast medium was used in the general anesthesia group than in the local anesthesia group. The baseline characteristics of the two groups of patients are presented in Table 2.
After propensity score-based matching of anesthesia methods, 290 patients with general anesthesia were matched to 290 patients with local anesthesia. The variables of age, sex, hypertension, diabetes mellitus, nephropathy, history of stroke, and dose of contrast medium were more balanced between the two groups after matching (Table 3). The occurrence of CIE was significantly different between the general anesthesia and local anesthesia group (P = 0.007, Table 3). Pearson's contingency coefficients showed a significant correlation between general anesthesia and the event of CIE (r = 0.117, P = 0.004).
To further study the association between the anesthesia methods and the risk of CIE, a propensity score-based matching of CIE was performed, and 14 patients with CIE were matched to 54 patients without CIE. The variables of age, sex, hypertension, diabetes mellitus, nephropathy, history of stroke, and dose of contrast agent were more balanced between the two groups after matching (Table 4). The anesthesia methods were significantly different between the two groups (P = 0.027, Table 4).
Further logistic analysis showed that the risk of CIE after general anesthesia was 7.273 times higher than that after local anesthesia (OR = 8.273, P = 0.049). Moreover, we analyzed the association between propofol dosage in unit weight and CIE and demonstrated a dose–response manner. An increased risk was seen for the lower dosage ( ≤ 11.1 mg/kg) in trend (OR = 7.412, P = 0.076) than the higher dosage (>11.1 mg/kg) (OR = 9.188, P = 0.048), indicating that the risk of CIE increased in a statistically significant dose-dependent pattern with increased propofol dosage in unit weight (P trend = 0.035).
In the present study, the overall incidence of CIE in the 1,043 patients who underwent endovascular treatment was 1.34%, which was similar to the incidence reported in previous literature (1–3). The incidences of CIE were 0.27% and 1.93% in the local anesthesia and general anesthesia groups, respectively, and the latter incidence was significantly higher than the former. After propensity score-based matching of anesthesia methods, the occurrence of CIE was still significantly different between the general anesthesia and local anesthesia groups (P = 0.007). This difference in incidence between these two groups indicated that anesthesia methods and/or anesthetics might be associated with the occurrence of CIE.
In order to further study the association between anesthesia methods and the risk of CIE, we performed a propensity score-based matching of CIE. Similarly, the anesthesia methods were significantly different between the CIE and control groups after balancing the variables of risk factors. Further logistic regression analysis also showed a positive association between general anesthesia and the risk of CIE, indicating that general anesthesia might be a risk factor for CIE. To the best of our knowledge, this was the first comprehensive analysis of the relationship between anesthesia and CIE in patients with neurovascular diseases undergoing endovascular treatment. The propensity score-based matching technique performed in this study for patient cohort selection has provided assurance for the better control of confounders and therefore may add more strength to the validation of our results.
Increased permeability of the BBB, hyperosmolarity, and direct neurotoxicity from the contrast agents are considered to be involved in the occurrence of CIE (5). During the general anesthesia procedure in our study, the sedatives, muscular relaxants, and analgesics were intravenously administrated to the patients, and propofol was used in all these patients. The lipophilic property of propofol allows it to cross the BBB rapidly and distribute into the central nervous system (9). Remsen et al. reported that propofol was observed to provide better disruption of the BBB in tumor-bearing rats than isoflurane did, and thus, drug delivery to tumors and the brain tissue around the tumors was significantly improved with propofol anesthesia (10). Fortin et al. also observed that the neurotoxicity of chemotherapy delivery increased significantly in BBB-disruption rats with propofol anesthesia (11). Thus, we further explored whether the dosage of propofol in unit weight was associated with CIE in our studies and demonstrated a dose-dependent pattern with increased propofol dosage in unit weight. Combining these findings with our observation, we hypothesized that propofol might be associated with the increased occurrence of CIE in the general anesthesia group, although the administration of propofol was in the normal dose range in our study. The possible reason might involve the transiently increased permeability or disruption of BBB caused by propofol, which increased the amount of contrast distributed in the brain tissue. However, the sample in our study was small, especially in the case of CIE, and thus, results from studies with larger samples are needed to support our findings.
Fentanyl was another common anesthetic agent used in most of our patients with general anesthesia. However, there is little evidence of changes in the permeability of the in vitro BBB caused by fentanyl (12). Similarly, rare evidence showed that other sedatives, muscular relaxants, and analgesics were related to the disruption or increased permeability of BBB. However, whether these anesthetic drugs will contribute to the occurrence of CIE needs to be investigated in further studies with larger sample sizes.
There is still no guideline recommendation for the treatment of CIE. Considering the potential risk factors for CIE, including chronic hypertension, renal dysfunction, history of stroke, high-contrast osmolality, overdose of contrast medium, and anesthetics, the patients at a high risk of CIE should receive a minimized dose of contrast and sedatives (13). CIE usually alleviates after several days to weeks of supportive management (3). The therapy of vigorous hydration, steroids, and mannitol is controversial. They have been reported to be used to treat severe CIE (14–17). However, Quintas-Neves et al. found no relation between the institution of treatments and complete clinical recovery in a systematic review (4).
This study had several limitations. First, the present study was retrospective as opposed to the well-designed, prospective, and randomized comparative clinical trials. Methodological bias could influence the reliability of these results. However, the use of propensity score matching might control for biases in our study. Second, it still lacks unified diagnostic criteria of CIE. We could not exclude the possibility of underdiagnosis of CIE in our cohort. Third, the low incidence of CIE and the small population of cases may limit the statistical power. Therefore, the effects of anesthetic drugs on CIE need further confirmation.
The incidence of CIE was significantly higher in the general anesthesia group than in the local anesthesia group. Logistic regression analysis showed a positive association between general anesthesia and the risk of CIE, indicating that general anesthesia might be a risk factor for CIE.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. Written informed consent to participate in this study was provided by the patient/participants or patient/participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
ZZ and HN analyzed the data and were the major contributors in writing the manuscript. JZ and HJ provided assistance for the data acquisition and literature search. JH, DL, and LB carried out the manuscript preparation and editing. DL performed a manuscript review. All authors read and approved the final manuscript.
The authors would like to thank all the patients who participated in this study and Shishuang Cui for providing help with statistical analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1146194/full#supplementary-material
1. Niimi Y, Kupersmith MJ, Ahmad S, Song J, Berenstein A. Cortical blindness, transient and otherwise, associated with detachable coil embolization of intracranial aneurysms. AJNR Am J Neuroradiol. (2008) 29:603–7. doi: 10.3174/ajnr.A0858
2. Wishart DL. Complications in vertebral angiography as compared to non-vertebral cerebral angiography in 447 studies. Am J Roentgenol Radium Ther Nucl Med. (1971) 113:527–37. doi: 10.2214/ajr.113.3.527
3. Chu YT, Lee KP, Chen CH, Sung PS, Lin YH, Lee CW, et al. Contrast-induced encephalopathy after endovascular thrombectomy for acute ischemic stroke. Stroke. (2020) 51:3756–9. doi: 10.1161/STROKEAHA.120.031518
4. Quintas-Neves M, Araújo JM, Xavier SA, Amorim JM, Cruz E., Silva V, et al. Contrast-induced neurotoxicity related to neurological endovascular procedures: a systematic review. Acta Neurol Belg. (2020) 120:1419–24. doi: 10.1007/s13760-020-01508-x
5. Yu J, George Dangas MD. Commentary: New insights into the risk factors of contrast-induced encephalopathy. J Endovasc Ther. (2011) 18:545–6. doi: 10.1583/11-3476C.1
6. Junck L, Marshall WH. Fatal brain edema after contrast-agent overdose. AJNR Am J Neuroradiol. (1986) 7:522–5.
7. Leong S, Fanning NF. Persistent neurological deficit from iodinated contrast encephalopathy following intracranial aneurysm coiling. A case report and review of the literature. Interv Neuroradiol. (2012) 18:33–41. doi: 10.1177/159101991201800105
8. Potsi S, Chourmouzi D, Moumtzouoglou A, Nikiforaki A, Gkouvas K, Drevelegas A. Transient contrast encephalopathy after carotid angiography mimicking diffuse subarachnoid haemorrhage. Neurol Sci. (2012) 33:445–8. doi: 10.1007/s10072-011-0765-3
9. Kanto J, Gepts E. Pharmacokinetic implications for the clinical use of propofol. Clin Pharmacokinet. (1989) 17:308–26. doi: 10.2165/00003088-198917050-00002
10. Remsen LG, Pagel MA, McCormick CI, Fiamengo SA, Sexton G, Neuwelt EA. The influence of anesthetic choice, PaCO2, and other factors on osmotic blood-brain barrier disruption in rats with brain tumor xenografts. Anesth Analg. (1999) 88:559–67. doi: 10.1213/00000539-199903000-00018
11. Fortin D, McCormick CI, Remsen LG, Nixon R, Neuwelt EA. Unexpected neurotoxicity of etoposide phosphate administered in combination with other chemotherapeutic agents after blood-brain barrier modification to enhance delivery, using propofol for general anesthesia, in a rat model. Neurosurgery. (2000) 47:199–207. doi: 10.1227/00006123-200007000-00041
12. Fischer S, Renz D, Schaper W, Karliczek GF. In vitro effects of fentanyl, methohexital, and thiopental on brain endothelial permeability. Anesthesiology. (1995) 82:451–8. doi: 10.1097/00000542-199502000-00015
13. Neilan P, Urbine D. A case of contrast-induced encephalopathy. BMJ Case Rep. (2019) 12:9717. doi: 10.1136/bcr-2019-229717
14. Kamimura T, Nakamori M, Imamura E, Hayashi Y, Matsushima H, Mizoue T, et al. Low-dose contrast-induced encephalopathy during diagnostic cerebral angiography. Intern Med. (2021) 60:629–33. doi: 10.2169/internalmedicine.5139-20
15. Bender M, Bogdan G, Radančević D, Pejanović-Škobić N. Contrast-induced encephalopathy following cerebral angiography in a hemodialysis patient. Case Rep Neurol Med. (2020) 2020:3985231. doi: 10.1155/2020/3985231
16. Liu MR, Jiang H, Li XL, Yang P. Case report and literature review on low-osmolar, non-ionic iodine-based contrast-induced encephalopathy. Clin Interv Aging. (2020) 15:2277–89. doi: 10.2147/CIA.S280931
Keywords: contrast-induced encephalopathy, anesthesia, endovascular treatment, risk factor, propofol
Citation: Zhong Z, Ni H, Zhu J, Jiang H, Hu J, Lin D and Bian L (2023) Association between general anesthesia and contrast-induced encephalopathy after endovascular treatment on neurovascular diseases. Front. Neurol. 14:1146194. doi: 10.3389/fneur.2023.1146194
Received: 17 January 2023; Accepted: 12 April 2023;
Published: 12 May 2023.
Edited by:
Osama O. Zaidat, Northeast Ohio Medical University, United StatesReviewed by:
Konstantinos Dimitriadis, LMU Munich University Hospital, GermanyCopyright © 2023 Zhong, Ni, Zhu, Jiang, Hu, Lin and Bian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Lin, bGQxMDUwMUByamguY29tLmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.