
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Neurol. , 02 June 2023
Sec. Neurocritical and Neurohospitalist Care
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1144970
This article is part of the Research Topic Case Reports in Neurocritical and Neurohospitalist Care, volume III - 2023 View all 10 articles
Background: Avermectin Pyridaben (AVP) is an insecticide with extreme neurotoxicity in human, causing critical symptoms such as nausea, vomiting, coma and respiratory failure within a short time after oral ingestion. Neurological sequelae or even death may occur because of delayed treatment or excessive toxic dose.
Case presentation: We report a 15-year-old girl who presented with coma, respiratory failure, limb weakness, ataxia symptoms after ingestion of a toxic dose of AVP. Soon after the poisoning, the patient was treated with life-saving mechanical ventilation and haemodialysis. Subsequently brain Magnetic Resonance Imaging (MRI) and nerve conduction study (NCS) and electromyography (EMG) demonstrated toxic encephalopathy and peripheral nerve injury. Over the next 2 months the patient’s limb function gradually recovered under treatment with hyperbaric oxygen, glucocorticoid pulses and neurotrophic drugs.
Conclusion: This case documents a rare presentation of toxic encephalopathy complicated with peripheral neuropathy following AVP poisoning. Seven other similar cases of poisoning in terms of common symptoms and effective treatment have also been summarised for providing clinicians with experience in diagnosis and therapy.
As a pesticide commonly used in agriculture, Avermectin Pyridaben is a mixed preparation consisting of 0.3% Avermectin and 10.2% Pyridaben. Avermectin which can be extracted from fermenting of Streptomyces avermitilis, presents the chemical structure of a large ring lactone compound, killing insects by the effect of preventing the transmission of electrical impulse which stimulate gamma-aminobutyric acid (GABA) receptors and paralysing nerves (1). Pyridaben is a highly lipohpilic compound with higher bioactivity, lower toxicity, and excellent selectivity compared to benzenoid (2) and can be generated by Intermediate Derivatization Methods (IDM) and terminal group replacement method (TRM) (3), it also can induce neurotoxicity by inhibiting mitochondrial respiration and disrupting oxidative stress and ubiquitin-proteasome system dysfunction (4, 5). The Food and Agriculture Organization of the United Nations (FAO) states that the human intake of Pyridaben should not exceed 0.405 mg/kg in 1 day. With the widespread use of AVP in agriculture, there has been a gradual increase in the incidence of pesticide poisoning, which in severe cases can be life-threatening. This paper reports a case of toxic encephalopathy with peripheral nerve injury after AVP poisoning, which recovered after treatment with hyperbaric oxygen, hormone and neurotrophic therapy. In addition, the symptoms and treatment of seven other similar poisoning cases are discussed in this paper, aiming to increase awareness of the safety of using AVP and to summarise experience in the diagnostic treatment of this type of poisoning.
A female adolescent aged 15 years manifested symptoms of nausea, vomiting, dyspnea and unconsciousness an hour subsequent to the inadvertent ingestion of 50 mL of AVP (comprising 0.3% Avermectin and 10.2% Pyridaben) at home. Two hours later she experienced a state of shock and was admitted to a nearby hospital. She had no previous medical history. Her vital signs showed dilated, non-reactive pupils and no spontaneous breathing. Her blood pressure (BP), pulse rate (PR), respiratory rate (RR), temperature and blood glucose (BS) were 65/30 mmHg, 74 bpm, 18/min with balloon-assisted ventilation, below 35°C, and 26.4 mmol/L, respectively. An arterial blood gas (ABG) analysis showed metabolic acidosis with respiratory alkalosis (pH = 6.77, pO2 = 242 mmHg, pCO2 = 26.2 mmHg, HCO3 = 12.3 mmol/L, BE = -15 mmol/L, Lactate beyond the max range). The 12-lead electrocardiogram was normal. Other laboratory exams are presented in Table 1.
Haemodialysis and mechanical ventilation were adopted in the treatment and, in the meantime, norepinephrine (0.2 μg/kg/min) was used to maintain the blood pressure. Having been transferred to ICU, she was treated with normal saline (1 L/d), intravenous pantoprazole (0.1 g q12h) and citicoline (0.25 g qd) which could protect gastic mucosa and the brain. On next day of admission, she regained consciousness, but still be weak, felt powerless and be in mechanical ventilation requirement. Her neurological examination revealed positive Babinski signs, grade IV muscle strength and ataxia in both lower limbs, and no ataxia, grade V muscle strength in both upper limbs, and no abnormalities on sensory examination such as depth sensation, positional sensation, pain and temperature sensation in the limbs. The examination suggested her pyramidal tract involvement, which prompted a request for a brain MRI, but it was not done due to her unpredictable condition. On the day 4 of ICU treatment, the patient was extubated and transferred to the general ward for continued treatment on the day 5. A list of vital signs care during ICU treatment can be found in Table 2. After ten-day treatment at the local hospital, the patient’s BP, PR, RR, BS, O2 saturation, temperature, and ABG indicators returned to the normal range. Then she was transferred to our hospital for lower limb ataxia and positive Babinski signs, which suspected neurological impairment and confirmed by brain MRI NCS and EMG.
Her brain MRI showed abnormal signals in the internal and external capsules of the basal ganglia bilaterally, confirming toxic encephalopathy (Figure 1). Combined with the patient’s respiratory failure after poisoning, the possibility of hypoxia-induced encephalopathy cannot be ruled out. NCS and EMG evoked potential testing suggested peripheral neuropathy of both lower limbs, including axonal damage to motor and sensory nerves (Table 3). Based on her weight of 52 kg the management of her neurological impairment was as follows: Intravenous dexamethasone sodium phosphate injection was given for 11 days (10 mg*5 days, 7.5 mg*3 days, 5 mg*3 days), then switched to oral methylprednisolone tablets with an initial dose of 40 mg/d, and the dose was reduced to one slice a week until the drug was stopped. Glucocorticoid therapy was administered for a duration of 3 months in order to mitigate the effects of inflammation and cerebral oedema. Hyperbaric oxygen (HBO) therapy lasted for 10 days with parameters set at 0.25 MPa and 60 min/d to reduce cerebral hypoxia, but was eventually discontinued due to financial constraints (6). Mecobalamin (0.5 gr qd) and vitamin B1 (0.1 gr qd) injections were used to promote regeneration of peripheral injured nerves and continued for 1 month (7, 8). The patient complied with medical advice and did not have any adverse effects during treatment.
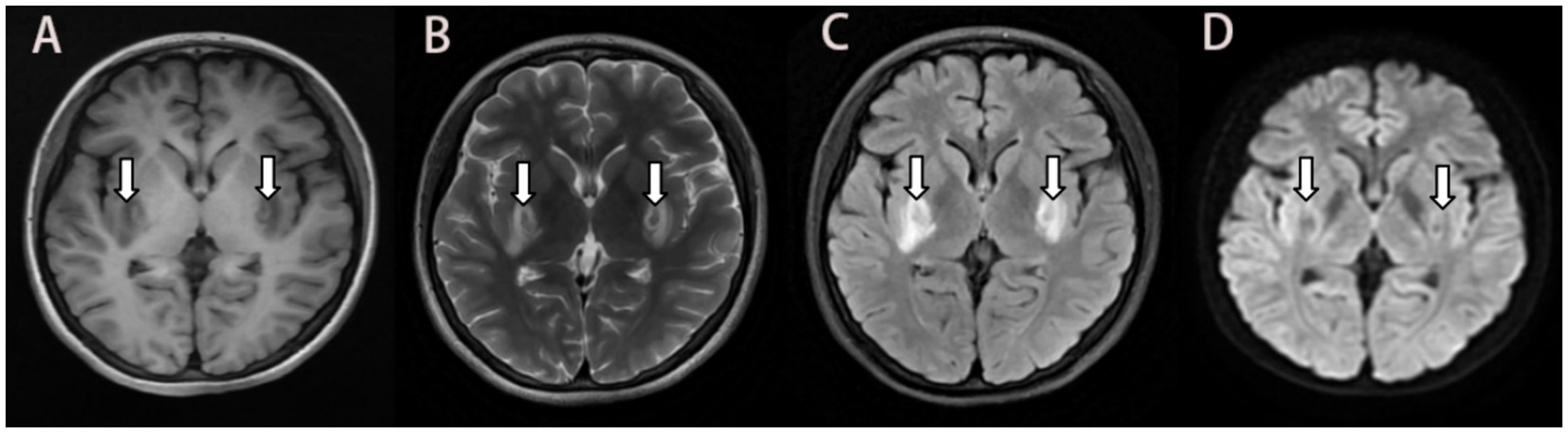
Figure 1. MRI 10 days after ingestion showing bilateral symmetrical lesions in the basal ganglia. Abnormal isointensity on T1-FLAIR (A), and heterogeneous intensity on T2 propeller (B), and hyperintensity on T2-FLAIR (C), and no significant hypersignal on DWI (D). MRI: magnetic resonance imaging; FLAIR: fluid attenuated inversion recovery; DWI: diffusion-weighted imaging.
She was reexamined by brain MRI, NCS, and EMG 45 days after glucocorticoid treatment and 4 months after poisoning, respectively. The brain MRI showed a remarkable reduction in the size of the lesions and the FLAIR hyperintensities in both basal ganglia regions, together with the observation of gliosis in the adjacent affected areas (Figures 2, 3), but NCS and EMG showed persistence of motor and sensory axonal damage in the lower limbs (Table 3). Her neurological examination showed a positive Babinski sign, no ataxia and normal muscle strength of the limbs.
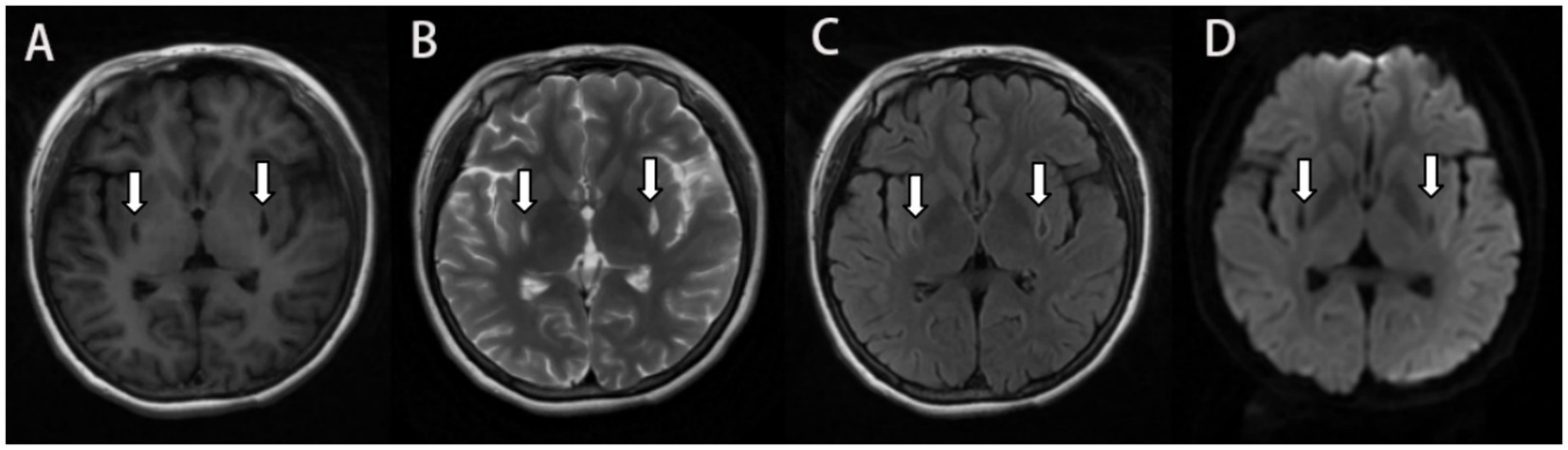
Figure 2. MRI 2 months after ingestion showing encephalomalacia in the bilateral basal ganglia with surrounding gliosis and the lesion size was smaller than before. Symmetrical small patchy with long T1 long T2 signal on T1 Flair (A) and T2 propeller (B), and hyperintense in the edge of the lesion on T2-FLAIR (C), and no significant hypersignal on DWI (D).
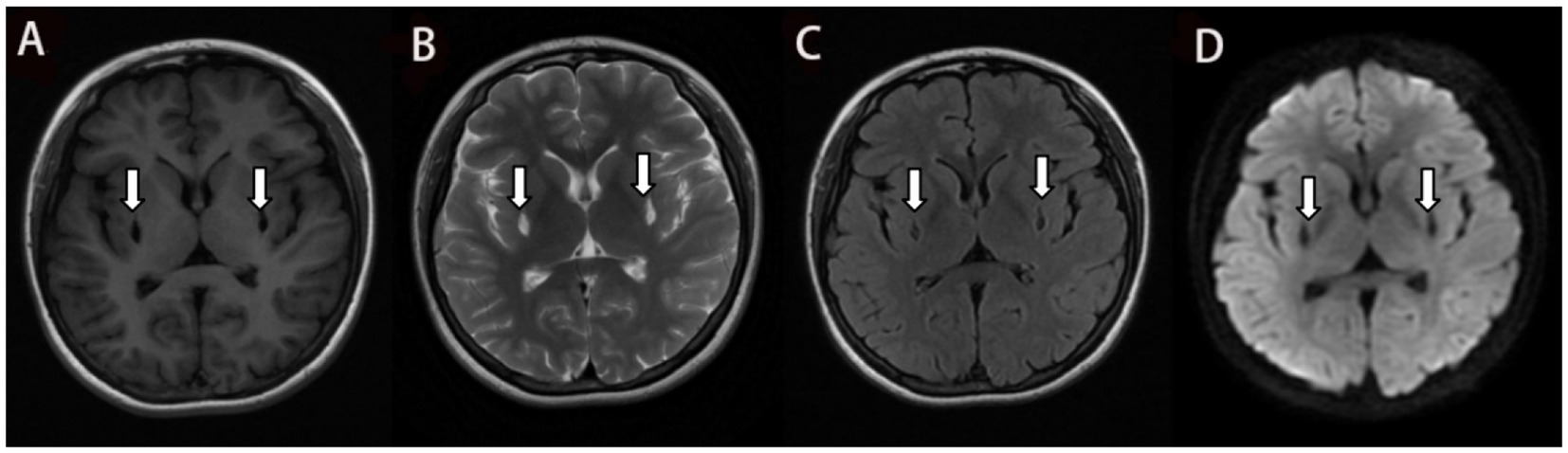
Figure 3. The T1 Flair (A), T2 Propeller (B) and DWI (D) in the MRI 4 months after ingestion show similar to Figure 2. and the gliosis around the lesion is less than before which is shown in the T2 Flair (C).
Avermectin can stimulate gamma-aminobutyric acid (GABA) receptors in the central nervous system to produce inhibitory neurotransmitters (9) and reduce cell viability and adenosine triphosphate (ATP) production by affecting mitochondrial function, thereby impairing the function of other organelles, leading to an imbalance in intracellular calcium homeostasis and eventually leading to cell necrosis (10). Pyridaben, an inhibitor of mitochondrial oxidation complex I, can significantly increase the production of reactive oxygen species (ROS) during part of the action period (11), which can induce apoptosis by affecting mitochondrial function, inducing ROS, inducing calcium imbalance, increasing the endoplasmic reticulum stress response, and promoting apoptosis (12). The patient reported taking 50 mL of a mixture of Avermitin 150 mg and Pyridine 15,100 mg and was found to have symptoms of toxic encephalopathy, and Peripheral neuropathy was found the next day after rescue. This is similar to the case of Avermectin poisoning with neurological injury-related sequelae reported by Song, which suggests that simultaneous central and peripheral neuropathy may have a poor prognosis (13). The poison reaches the brain tissue through the blood, inhibits mitochondrial function, and leads to tissue hypoxia and necrosis. Gray matter is more vulnerable to anoxic or ischemic insults due to its higher metabolic demands for oxygen and glucose, and deep gray nuclei are often involved in acute severe brain tissue hypoxia, which is associated with poor prognosis (14–16).
This paper presents a discussion regarding a total of eight patients who suffered from poisoning, each exhibiting varying degrees of symptoms related to neurological damage include ataxia, muscle weakness, coma, blepharoptosis, hypoesthesia and myoclonus et al. (Table 4) (13, 17–22). In addition to nervous system symptoms, gastrointestinal irritation symptoms were common, and more than half of the patients had respiratory system involvement. One patient had allergic symptoms, and another one had cerebral haemorrhage, multiple organ failure and eventually died. The dose of poisoning, the rescue time and the presence or absence of major organ damage were the main factors influencing prognosis.This paper presents an overview of treatment methods for AVP poisoning, as no singular antidote for its mitigation is currently available.
Toxins should be removed from the body as soon as possible after poisoning. Activated charcoal gastric lavage, intestinal perfusion, hemodialysis and other methods could be used to avoid the occurrence of MOF. At the same time, vasoactive drugs were used to maintain blood pressure in the normal range, and mechanical ventilation support was given to patients with dyspnea. Patients with toxic encephalopathy can use glucocorticoid to control the inflammatory response of cells, reduce brain edema, and prevent compression of the surrounding normal brain area. Hyperbaric oxygen therapy (HBO) inhibits neutrophil adherence to the walls of ischemic vessels, which decreases free radical production, vasoconstriction and tissue destruction (14). Patients with Peripheral neuropathy should be given timely nutritional nerve treatment to prevent irreversible sequelae. Liver damage, pulmonary infection, gastrointestinal ulcer, and allergy are all possible complications, and doctors need to predict the possible situation to reduce the incidence of complications.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Research ethics committee of the second hospital of shandong university. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
JD collected the clinical information, and examination data and wrote the manuscript. QZ provides thesis guidance and financial support. All authors contributed to the article and approved the submitted version.
Supported by Natural Science Foundation of Shandong Province of China (ZR2021MH098).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. El-Saber Batiha, G, Alqahtani, A, Ilesanmi, OB, Saati, AA, El-Mleeh, A, Hetta, HF, et al. Avermectin derivatives, pharmacokinetics, therapeutic and toxic dosages, mechanism of action, and their biological effects. Pharmaceuticals (Basel). (2020) 13:196. doi: 10.3390/ph13080196
2. Liu, C. L. Proceedings of Research and Development of New Agrochemicals ; Chemical Industry Press Publishers: Beijing, (2002), pp. 93–100.
3. Guan, AY, Liu, CL, Sun, XF, Xie, Y, and Wang, MA. Discovery of pyridine-based agrochemicals by using intermediate derivatization methods. Bioorg Med Chem. (2016) 24:342–53. doi: 10.1016/j.bmc.2015.09.031
4. Hoglinger, GU, Carrard, G, Michel, PP, Medja, F, Lombes, A, Ruberg, M, et al. Dysfunction of mitochondrial complex I and the proteasome: interactions between two biochemical deficits in a cellular model of Parkinson’s disease. J Neurochem. (2003) 86:1297–307. doi: 10.1046/j.1471-4159.2003.01952.x
5. Ham, J, You, S, Lim, W, and Song, G. Pyridaben induces mitochondrial dysfunction and leads to latent male reproductive abnormalities. Pestic Biochem Physiol. (2021) 171:104731. doi: 10.1016/j.pestbp.2020.104731
6. Ermolov, AS, Epifanova, NM, Romasenko, MV, Luzhnikov, EA, Ishmukhametov, AI, Golikov, PP, et al. Role of hyperbaric oxygenation in the treatment of posthypoxic encephalopathy of toxic etiology. Anesteziol Reanimatol. (1998) 6:20–5.
7. Sawangjit, R, Thongphui, S, Chaichompu, W, and Phumart, P. Efficacy and safety of Mecobalamin on peripheral neuropathy: a systematic review and Meta-analysis of randomized controlled trials. J Altern Complement Med. (2020) 26:1117–29. doi: 10.1089/acm.2020.0068
8. Calderon-Ospina, CA, Nava-Mesa, MO, and Paez-Hurtado, AM. Update on safety profiles of vitamins B1, B6, and B12: a narrative review. Ther Clin Risk Manag. (2020) 22:1275–88. doi: 10.2147/TCRM.S274122
9. Njoo, FL, Beek, WM, Keukens, HJ, van Wilgenburg, H, Oosting, J, Stilma, JS, et al. Ivermectin detection in serum of onchocerciasis patients: relationship to adverse reactions. Am J Trop Med Hyg. (1995) 52:94–7. doi: 10.4269/ajtmh.1995.52.94
10. Maioli, MA, de Medeiros, HC, Guelfi, M, Trinca, V, Pereira, FTV, and Mingatto, FE. The role of mitochondria and biotransformation in abamectin-induced cytotoxicity in isolated rat hepatocytes. Toxicol In Vitro. (2013) 27:570–9. doi: 10.1016/j.tiv.2012.10.017
11. Chen, Q, Vazquez, EJ, Moghaddas, S, Hoppel, CL, and Lesnefsky, EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem. (2003) 278:36027–31. doi: 10.1074/jbc.M304854200
12. Bae, H, You, S, Lim, W, and Song, G. Pyridaben leads to inhibition of cell growth and induction of cell death through intracellular mechanisms in early pregnancy. Pestic Biochem Physiol. (2021) 171:104733. doi: 10.1016/j.pestbp.2020.104733
13. Sung, YF, Huang, CT, Fan, CK, Lin, CH, and Lin, SP. Avermectin intoxication with coma, myoclonus, and polyneuropathy. Clin Toxicol (Phila). (2009) 47:686–8. doi: 10.1080/15563650903070901
14. Hantson, P, and Duprez, T. The value of morphological neuroimaging after acute exposure to toxic substances. Toxicol Rev. (2006) 25:87–98. doi: 10.2165/00139709-200625020-00003
15. Luigetti, M, Goldsberry, GT, and Cianfoni, A. Brain MRI in global hypoxia-ischemia: a map of selective vulnerability. Acta Neurol Belg. (2012) 112:105–7. doi: 10.1007/s13760-012-0007-3
16. Chao, CP, Zaleski, CG, and Patton, AC. Neonatal hypoxic-ischemic encephalopathy: multimodality imaging findings. Radiographics. (2006) 26:S159–72. doi: 10.1148/rg.26si065504
17. Karunatilake, H, Amarasinghe, S, Dassanayake, S, Saparamadu, T, and Weerasinghe, S. Partial ptosis, dilated pupils and ataxia following abamectin poisoning. Ceylon Med J. (2012) 57:125–6. doi: 10.4038/cmj.v57i3.4706
18. Wenjie, W, Houqing, L, Ping, Z, and Gengyun, S. Nystagmus following acute avermectins poisoning. Toxicol Ind Health. (2016) 32:751–2. doi: 10.1177/0748233713503377
19. Aminiahidashti, H, Jamali, SR, and Heidari Gorji, AM. Conservative care in successful treatment of abamectin poisoning. Toxicol Int. (2014) 21:322–4. doi: 10.4103/0971-6580.155386
20. Soyuncu, S, Oktay, C, Berk, Y, and Eken, C. Abamectin intoxication with coma and hypotension. Clin Toxicol (Phila). (2007) 45:299–300. doi: 10.1080/15563650601072225
21. Jiezhen, D, Linshan, Q, Yanzhen, M, Chunyan, W, and Xuan, J. Poisoning by a compound preparation of abamectin and pyridine. Chin J Ind Hyg Occup Dis. (2015) 33:57.
Keywords: Avermectin and Pyridine, toxic encephalopathy, peripheral neuropathy, therapy, case report
Citation: Diao J and Zhou Q (2023) Toxic encephalopathy and peripheral neuropathy of poisoning by Avermectin Pyridine: a case report and a review of the literature. Front. Neurol. 14:1144970. doi: 10.3389/fneur.2023.1144970
Received: 28 January 2023; Accepted: 17 May 2023;
Published: 02 June 2023.
Edited by:
Silke Walter, Saarland University Hospital, GermanyReviewed by:
Nakul Katyal, Stanford Healthcare, United StatesCopyright © 2023 Diao and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingbo Zhou, bGlhbnhpYW5nMTIxMEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.