- 1Department of Otolaryngology, Head and Neck Surgery, The Second Affiliated Hospital of Nanchang University, Nanchang, China
- 2Division of Ear, Nose and Throat Diseases, Department of Clinical Science, Intervention and Technology, Karolinska Institutet, Stockholm, Sweden
- 3Department of Otolaryngology-Head and Neck Surgery, Karolinska University Hospital, Karolinska Institutet, Stockholm, Sweden
- 4Department of Otolaryngology, Head and Neck Surgery, The First Affiliated Hospital of Anhui Medical University, Anhui, Hefei, China
Sudden sensorineural hearing loss (SSNHL) is defined as an abrupt hearing loss of more than 30 dB in three contiguous frequencies within 72 h. It is an emergency disease requiring immediate diagnosis and treatment. The incidence of SSNHL in Western countries' population is estimated between 5 and 20 per 1,00,000 inhabitants. The etiology of SSNHL remains unknown. Due to the uncertainty of the cause of SSNHL, at present, no specific treatment targets the cause of SSNHL, resulting in poor efficacy. Previous studies have reported that some comorbidities are risk factors for SSNHL, and some laboratory results may provide some clues for the etiology of SSNHL. Atherosclerosis, microthrombosis, inflammation, and the immune system may be the main etiological factors for SSNHL. This study confirms that SSNHL is a multifactorial disease. Some comorbidities, such as virus infections, are suggested to be the causes of SSNHL. In summary, by analyzing the etiology of SSNHL, more targeting treatments should be used to achieve a better effect.
1. Introduction
Sudden sensorineural hearing loss (SSNHL) is defined as an abrupt hearing loss of more than 30 dB in three contiguous frequencies within 72 h (1). Associated symptoms, including tinnitus, aural fullness, sound distortion, dizziness, vertigo, and benign paroxysmal positional vertigo (BPPV), may present in some cases (2). Moreover, SSNHL patients with vertigo tend to suffer from more severe hearing loss and worse hearing recovery (3, 4) due to a higher risk of vestibular organ lesions (5).
The incidence of SSNHL in developed countries' populations is an estimated 5–20 per 1,00,000 persons per year (6). There is an overall slight male preponderance, with a male-to-female ratio of 1.07:1 (7). Regarding age distribution, Rauch demonstrated that SSNHL most frequently occurred in 43–53-year-old patients (8). On the contrary, a Japanese survey showed that SSNHL was most prevalent among patients aged 60–69 years old (9). In addition, our study showed that the peak age prevalence was in the group of patients aged 41–50 years (3).
The etiology of SSNHL remains unknown, multiple factors are suggested to be the causes of SSNHL. Some pathophysiological mechanisms, including vascular disease, viral infection, metabolic disease, autoimmunity, and combinations of multiple factors are suggested to be the causes of ISSNHL. Due to the uncertainty of the cause of SSNHL, at present, there is no specific treatment targeting the cause of SSNHL, thus resulting in poor efficacy. This brief review focuses primarily on the etiological comorbidities and laboratory changes of SSNHL. We searched the U.S. National Library of Medicine's PubMed database using the terms “sudden sensorineural hearing loss,” “sudden hearing loss,” “idiopathic sudden sensory neural hearing loss,” and “sudden deafness” as well as the keywords such as “etiology,” “risk factors,” “comorbidity,” and “laboratory results.”
2. Etiological comorbidities
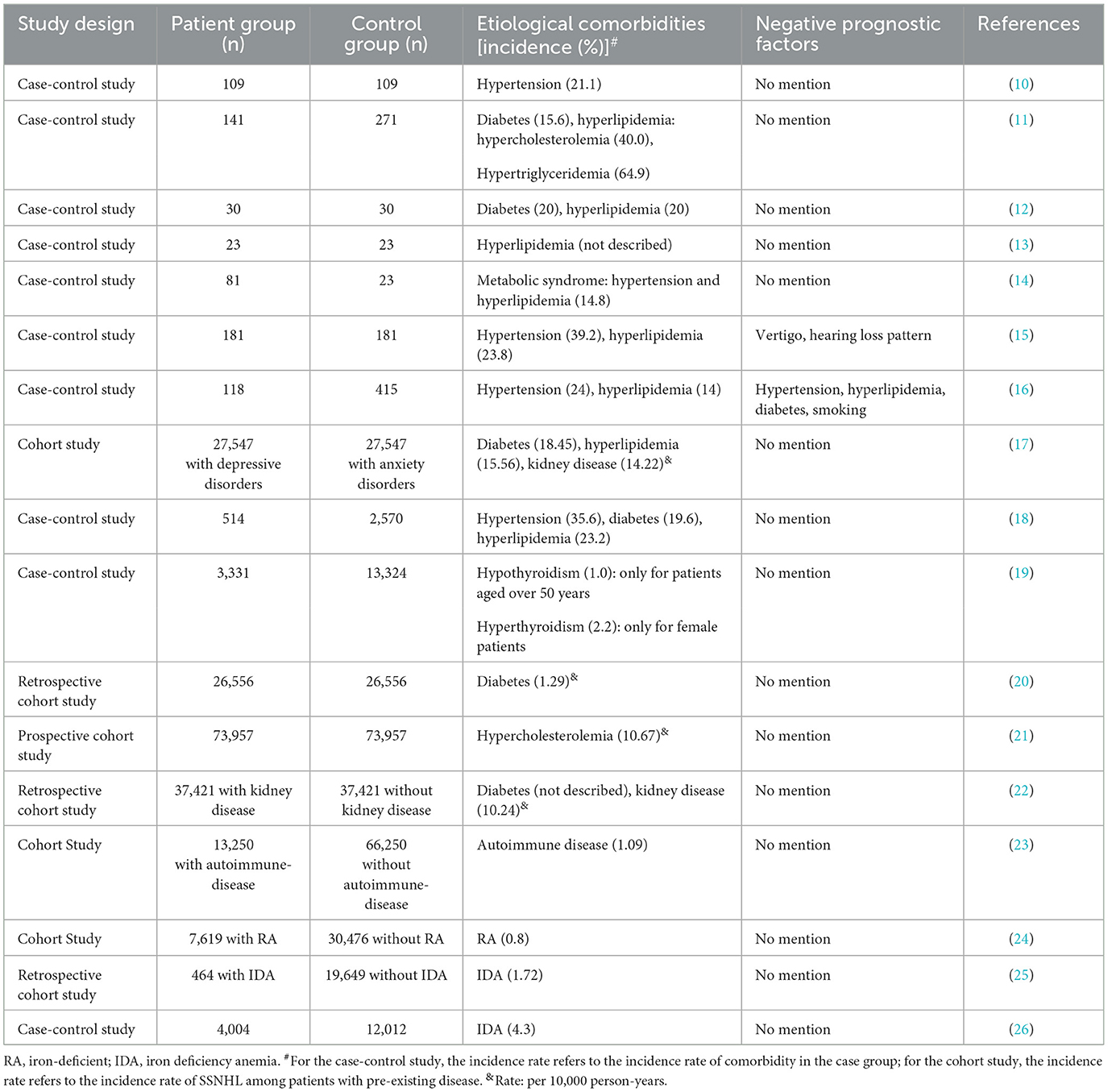
Abundant evidence has proved that several diseases were associated with an increased risk of SSNHL; all these etiological diseases are listed in Table 1.
2.1. Cardiovascular disease
2.1.1. Hypertension
As shown in Table 1, hypertension is considered as one of the most common comorbidities of SSNHL. Animal experiments also showed that the blood flow in different parts of the cochlea was reduced by nearly 80% in hypertensive rats exposed to noise and 50–60% in hypertensive rats fed with an atherogenic diet (27). The cochlea is supplied by the cochlear artery, a terminal artery without any collateral vessels to compensate for any occlusion of the blood vessel. Thrombosis or vasospasm of the internal auditory artery is one of the main hypotheses to explain SSNHL. Hypertension may induce atherosclerotic changes and result in cochlear microcirculation disturbance.
2.1.2. Dysrhythmia
A study elucidated that patients with dysrhythmia showed a significantly higher risk of SSNHL (28). Even after the adjustment of confounders, the incidence of SSNHL in the dysrhythmia group was higher than that in the comparison group. This finding suggests that hemodynamic instability due to dysrhythmia resulting in impaired blood perfusion to the inner ear can lead to SSNHL.
2.2. Metabolic disease
2.2.1. Diabetes
A retrospective cohort study showed that the prevalence of SSNHL was 1.29 per 1,000 person-years among diabetic patients, which was 1.54-fold higher compared with non-diabetic subjects (20). In addition, earlier studies revealed that hearing impairment also occurred in the opposite ear, especially in high frequencies (29). Compared with diabetic patients without SSNHL, the glycated hemoglobin value was significantly higher in diabetes patients with SSNHL, and SSNHL patients with type-2 diabetes had more severe hearing loss (30). Moreover, a cohort study demonstrated that during 14 years of follow-up, a significantly lower percentage of diabetes patients with metformin use developed SSNHL compared with those without metformin intake, indicating that metformin use appeared to reduce the risk of developing SSNHL among diabetes patients (31).
Researchers found that the animal model of type-2 diabetes and obesity exhibited significantly elevated auditory brainstem response (ABR) thresholds. Regarding histological findings, outer hair cell degeneration and spiral ganglion cell loss were present in the middle and basal turns of the cochlear. This study indicates that diabetes and obesity may lead to early sensorineural hearing loss (32).
Microangiopathy may be one of the mechanisms underlying the association between diabetes and SSNHL. Other mechanisms, including upregulation of vascular endothelial growth factor, inducible nitric oxide synthase, and endothelial nitric oxide synthase, may be involved in the pathogenesis of cochlea functional loss (33).
2.2.2. Hyperlipidemia
Previous studies have demonstrated that patients with SSNHL had significantly higher plasma concentrations of cholesterol, triglyceride, lipoprotein A, and low-density lipoprotein cholesterol compared with controls (34, 35).
Animal experiments revealed that after a high-fat diet for 4 months, guinea pigs' inner ears showed impaired hearing sensitivity and pathologic alterations of the cochlear, especially in the basal turn and stria vascularis (36). It has also been reported that cholesterol had different distributions among outer hair cell membranes. Furthermore, after being incubated with water-soluble cholesterol, the outer cell's lateral wall stiffness parameter increased, which impaired the activity of the outer hair cells (37). In addition, Sikora et al. found that after being fed a high-fat diet, chinchillas exposed to noise exhibited more severe hearing loss at high frequency and significantly greater hair cell loss than those in chinchillas fed with a normal diet (38).
Overall, the pathophysiological mechanism of SSNHL caused by hyperlipidemia is through the modification of the microstructure of the stria vascularis and the composition and the electromotility of the outer hair cells by elevated cholesterol, thereby increasing the cochlea's vulnerability to noise. Moreover, hyperlipidemia promotes hyperviscosity, contributes to endothelial function damage, and decreases nitric oxide release. Consequently, it promotes the formation of atheromatous plaque, which might cause occlusion of the cochlear artery, therefore resulting in SSNHL (39, 40).
2.3. Autoimmune diseases
According to a review written by Ralli et al. (41), sensorineural hearing loss was the most common audiovestibular symptom related to systemic autoimmune diseases. Hearing loss may be present in a sudden, slowly, rapidly progressive, or fluctuating form, and is mostly bilateral and asymmetric. SSNHL has been reported as a symptom of some systemic autoimmune diseases, such as autoimmune hepatitis, sympathetic neural hyperalgesia edema syndrome, Cogan's syndrome, systemic lupus erythematosus, multiple sclerosis, rheumatoid arthritis (RA), nodular polyarteritis, and Crohn's disease (42).
Previous studies showed that the risk of SSNHL was significantly higher in patients with antiphospholipid syndrome, multiple sclerosis, RA, and connective-tissue diseases than in patients without autoimmune diseases, and RA was in particular closely related to SSNHL (23, 24). Another retrospective study demonstrated that comorbid systemic lupus erythematosus or RA might negatively affect the prognosis of SSNHL (43). Furthermore, a systematic review reported that SSNHL could be an early manifestation of multiple sclerosis, especially in women. The pathophysiology of SSNHL caused by multiple sclerosis can be explained by the involvement of microglia attacking the central and/or peripheral auditory pathways (44).
Recently, O'Malley et al. (45) reported that the inflammatory cells are distributed in the inner ear. They found the presence of resident cochlear macrophages and the recruitment of inflammatory macrophages to the cochlea in animal models. This result indicates that the innate immune defense system of the human inner ear may involve in many otologic diseases (45).
The pathophysiology of inner ear involvement in systemic autoimmune diseases remains uncertain. The possible pathophysiology may include activated circulating antibodies against inner ear antigens, leading to antibody-dependent cell-mediated cytotoxicity; the activation of the complement system, which directly triggers cytotoxic T cells; or immune complex-mediated damage, which results in vasculitis of the inner ear and causes atrophy of the stria vascularis (46–52).
2.4. Hematological disorders
Hematological disorders such as aplastic anemia, sickle cell anemia, and hyperviscosity syndrome have been described as being associated with inner ear deficits. These hematological diseases may cause inner ear hemorrhage or vasculopathy (53).
2.4.1. Iron deficiency anemia
A retrospective cohort study showed that children with iron deficiency anemia (IDA) demonstrated an increased likelihood of SSNHL (25). Another study also confirmed the link of SSNHL with IDA. In this study, absolute latencies for all ABR waves and interpeak latencies (except I-III interval) were significantly longer in children with IDA than in non-anemic infants (54). A population-based study also showed a significantly higher prevalence of prior IDA among participants with SSNHL compared with the controls, especially in those less than 60 years old. The researchers suggested that patients with IDA, especially those younger than 60 years, should be more aggressively surveyed to reduce hearing-related morbidities (26).
In the animal experiment, an electrophysiological study revealed that the incidence of an auditory threshold elevation of more than 15 dB was 31.85% in the iron-deficient (ID) rats, whereas it was unchanged in all the control animals. The main cochlear histopathological changes were strial atrophy and reduction of spiral ganglion cells in ID rats. So the authors concluded that the observed anomalies may be attributed solely to iron deficiency of the cochlear tissue (55).
The main cochlear pathological changes of SSNHL in ID rats were the synchronous abnormal activity of the iron-containing enzymatic, including succinic dehydrogenase and peroxidase, which in turn would disturb cell respiration and initiate peroxidative damage to the inner ear cells, resulting in a significant reduction of spiral ganglion cells and rapid damage of stereocilia of the outer and inner hair cells (56, 57).
2.4.2. Leukemia
It has been reported that 16–40% of leukemia patients had otolaryngological symptoms, such as SSNHL, vertigo, tinnitus, facial paralysis, and infection (58, 59). Among hematologic malignancies, SSNHL has often been described as the initial presentation in patients with acute lymphocytic leukemia. However, recent studies have indicated that both acute and chronic leukemia were associated with SSNHL (60, 61).
Lin et al. (53) reported that during the 20 years, they had identified 14 cases of SSNHL among patients with hematological disorders, i.e., leukemia or aplastic anemia. Most of these patients presented an abnormal mean hearing level, cervical vestibular-evoked myogenic potential test, ocular vestibular-evoked myogenic potential test, and caloric test results, exhibiting a significant sequential decline in inner ear function.
Chae et al. (62) documented a case of chronic myelogenous leukemia with the first manifestation being SSNHL, and the patient's hearing was restored after leukapheresis and chemotherapy without steroids. The authors presumed that cochlear vessel occlusion as a result of elevated blood viscosity may be responsible for this patient's hearing loss.
Numerous studies have demonstrated histopathological changes in the temporal bones of patients with leukemia. These histopathological changes include leukemic infiltration, inner ear hemorrhage, infection (58, 59, 63), and hyperviscosity syndrome (64, 65).
2.5. Chronic kidney disease
Chronic kidney disease (CKD) can significantly increase the risk of SSNHL (17). A cohort study showed that the incidence of SSNHL was 1.57 times higher in the CKD group compared to the non-CKD group (22).
Another study reported that two patients with kidney failure suffered from profound SSNHL during the course of hemodialysis (66). Moreover, a significant decrease in cochlear microphonic and cochlear nerve action potential has been demonstrated in guinea pigs in a uremic state (67).
One possible explanation of the association between CKD and SSNHL is that the cochlea and kidney have numerous anatomic, physiological, pharmacological, and pathological similarities and have a shared antigenicity, so both are influenced by similar immunologic factors. In addition, many nephrotoxic drugs are also ototoxic. As a result, many patients with CKD may suffer from SSNHL (66, 68).
Dialysis may sometimes result in deteriorated auditory function. Rizviand and Holmes found that the endolymphatic system collapsed in patients on dialysis in a case series (69). They also found edema and atrophy in the majority of the cells of the auditory and vestibular sensory organs. A cohort study reported that hemodialysis patients with SSNHL had higher risks of hemorrhagic stroke, ischemic stroke, acute coronary syndrome, and peripheral arterial occlusive disease than hemodialysis patients without SSNHL (70).
2.6. Thyroid diseases
Some researchers have studied the relationship between thyroid disease and SSNHL. Nakashima et al. explored the SSNHL risk factors in a case–control study including 109 patients, reporting that patients with a history of thyroid disease had a higher odds ratio for SSNHL than those without such history (10). A case–control study with large samples showed that the correlation between hypothyroidism and increased SSNHL risk was significant only for patients aged over 50 years old and that the correlation between hyperthyroidism and SSNHL was remarkable only for female patients (19).
Thyroid autoantibodies can result in peripheral or central hearing organ dysfunction, increasing patients' susceptibility to SSNHL (71). In addition, thyroid dysfunction may lead to hypercoagulability and venous thrombosis, which may impair cochlear circulation, thus causing SSNHL (72, 73).
Overall, all these comorbidities which may affect the blood supply to the inner ear or alter the metabolism of the inner ear can cause SSNHL. Another evidence of circulatory disorder may be the main pathophysiology of SSNHL is that hyperbaric oxygen is effective for treating SSNHL. This treatment contributes to supply the oxygen needs to the peripheral neuronal structures of the inner ear (74).
3. Laboratory test results
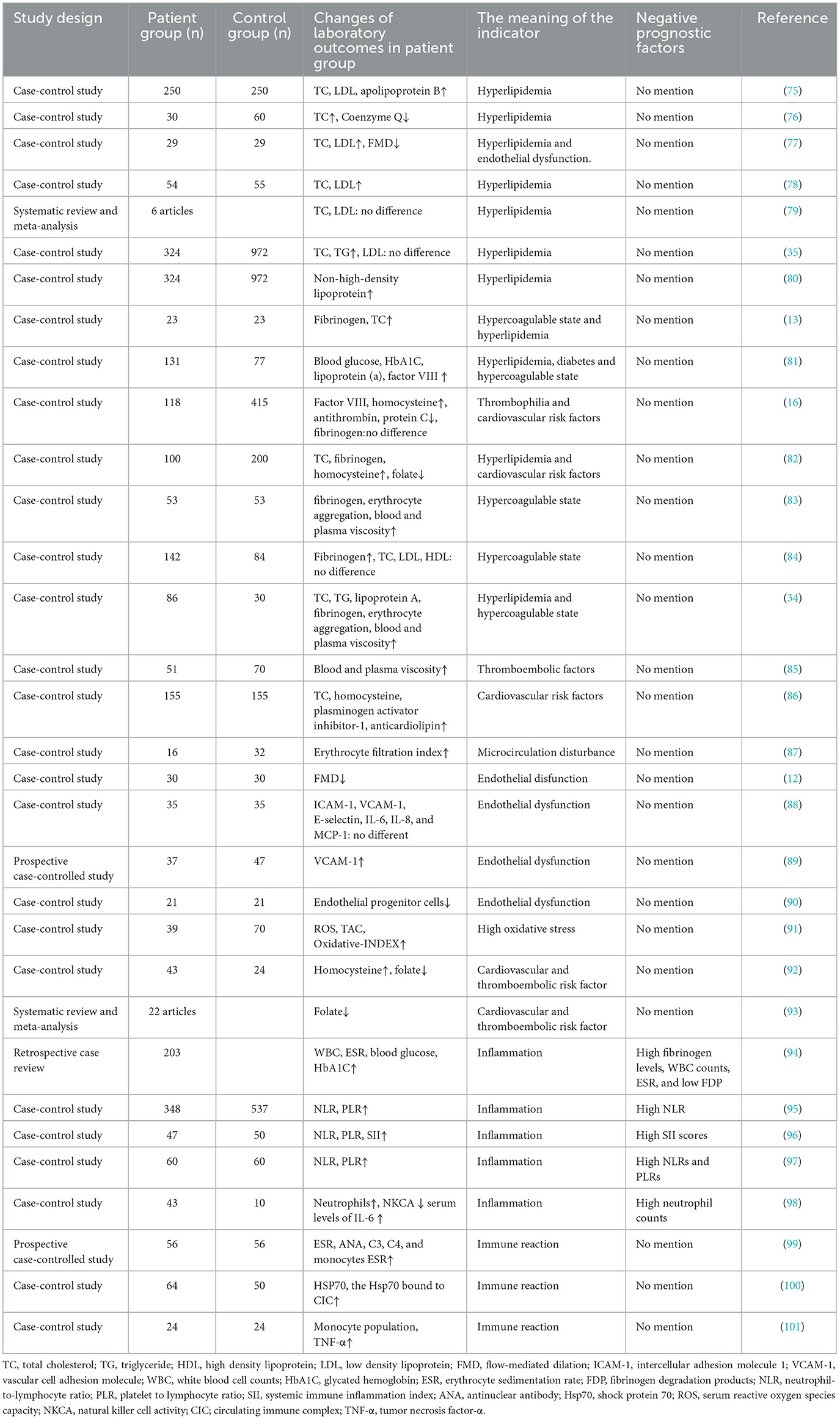
In addition to hyperglycemia and hyperlipidemia, several laboratory abnormalities were reported in SSNHL patients (Table 2). The alterations of several major hematological parameters are reviewed and listed as follows.
3.1. Blood coagulation systems
Table 2 shows that laboratory abnormalities, such as hyperfibrinogenemia, antithrombin, protein C or protein S deficiency, and high factor VIII plasma levels, were associated with SSNHL. All these changes contribute to hypercoagulability and microthrombosis, which may cause cochlear ischemia and result in SSNHL.
Animal models showed increased levels of fibrinogen, accompanied by decreased cochlear blood flow as well as increased hearing thresholds. Moreover, hearing thresholds correlated negatively with cochlear blood flow (102).
Additional evidence for the role of hyperfibrinogenemia as one etiological factor of SSNHL is that acute and drastic removal of plasma fibrinogen and low-density lipoproteins can be used to effectively treat SSNHL. This treatment approach had a rapidly beneficial effect on endothelial dysfunction in SSNHL patients (103, 104).
3.2. Hemorheology
The changes in hemorheology observed in SSNHL patients included increased blood and plasma viscosity, erythrocyte aggregation index, and erythrocyte filtration index (Table 2). These changes can lead to impaired blood perfusion in the inner ear either by thrombosis or impaired regional blood flow.
3.3. Endothelial function
The biomarkers of endothelial function include flow-mediated dilation (FMD) of the brachial artery, endothelial progenitor cells (EPCs), and the expression of circulating adhesion molecules, such as soluble intercellular adhesion molecule 1 (ICAM-1) and soluble vascular cell adhesion molecule 1 (VCAM-1). Other factors, including oxidative stress, homocysteine, and folate also take part in the endothelial function.
3.3.1. FMD
FMD is a simple, non-invasive, and highly repeatable method to assess endothelial function. The mechanism of FMD is that after compression of the brachial artery for some minutes, the increased blood flow can induce shear stress, which can activate the endothelium to release nitric oxide with the consequence of vasodilation. This phenomenon can be monitored by ultrasonography. Diminished FMD is an early sign of subclinical atherosclerosis and is associated with coronary atherosclerosis (105–107). Recently, researchers also found reduced FMD among SSNHL patients (12, 77).
3.3.2. EPCs
EPCs are circulating cells, and their properties are similar to embryonal angioblasts. They can differentiate into mature endothelial cells. Increased EPCs have been found in case of acute vascular damage such as limb ischemia, acute myocardial infarction, or vascular trauma. By contrast, decreased EPCs were linked to a higher incidence of cardiovascular events (108, 109).
By analyzing peripheral blood CD34+KDR+CD133+ cells, researchers found that the circulating levels of EPCs were much lower in SSNHL patients compared with controls (90). The results of this study confirm the existence of endothelial dysfunction in SSNHL patients.
3.3.3. Circulating adhesion molecules
Increased expression of some molecules is the early evidence of endothelial dysfunction. The activated endothelial cells can increase the expression of soluble ICAM-1 and soluble VCAM-1, and these molecules can mediate leukocyte adhesion to the endothelium and activate atherosclerosis formation (89, 110).
One prospective case–control study showed higher ICAM-1 and VCAM-1 in SSNHL patients (89). However, inconsistent results have been documented by another study, indicating that there was no difference between ICAM-1 and VCAM-1 between SSNHL patients and the controls. The authors considered that the role of soluble adhesion molecules in the pathogenesis of SSNHL remained unclear and needed further investigation (88).
3.3.4. Oxidative stress
The balanced reactive oxygen species (ROS) and antioxidant system can maintain the normal physiological oxidative status in living organisms. On the contrary, the imbalance between ROS and total antioxidant capacity is thought to be a potential pathogenetic mechanism leading to endothelial dysfunction. If excessive ROS are not buffered by the cellular antioxidants, they can react with cellular macromolecules and promote lipid peroxidation, which may cause DNA damage and induce protein and nucleic acid modifications (111).
Recent studies have reported a significantly higher ROS in SSNHL patients, as well as oxidative stress status, supporting the vascular impairment involvement in ISSNHL etiopathogenesis (91, 112). The microcirculation disturbance due to an ischemic event may relate to increased oxidative stress, which may synergistically account for endothelial damage, especially in terminal microvascular systems (91, 113).
Other findings also reflect the involvement of oxidative stress in SSNHL. In a successive pioneering study, Cadoni et al. described an association between SSNHL and low serum levels of the antioxidant Co-enzyme Q (CoQ) (76).
3.3.5. Homocysteine and folate
Hyperhomocysteinemia is considered to be a cardiovascular and thromboembolic risk factor for atheromatous and vascular events (114). Homocysteine can promote platelet aggregation, hypercoagulability, oxidative stress response, endothelial impairment, and smooth muscle cell proliferation (115).
As an important regulator of homocysteine, folate is a coenzyme necessary for one-carbon metabolism. Low levels of folate may contribute to increased plasma levels of homocysteine (92). Lower serum folate and higher homocysteine levels have been found among SSNHL patients than among controls (92).
In general, it is known that endothelial dysfunction has a primary role in regulating vascular tone by modifying lipoproteins, thrombogenesis, and transformation of circulating monocytes into foam cells (82). Moreover, it can counterbalance pro-aggregation and anti-aggregation properties or even regulate coagulation conditions by mediums such as heparin. If endothelial dysfunction exists, the blood supply to the inner ear will be disturbed because of the sudden and transient thrombotic event, which could explain the nature of SSNHL (77, 92).
4. Inflammation
Chronic inflammation may lead to microvascular damage and atherogenesis, which increases ischemic risk in a direct way (116). Several studies revealed that some biomarkers of inflammation, including white blood cell (WBC) counts, neutrophil count, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic immune inflammation index (SII) values, tumor necrosis factor-α level, and monocyte population were higher in SSNHL patients compared to the control groups. By contrast, lymphocyte count was significantly higher in the control group (Table 2). The lower NLR level might be taken into account as a novel potential marker to predict a better prognosis. A meta-analysis including 12 retrospective cohort studies also confirmed that NLR might be a useful biomarker to determine the onset and prognosis of SSNHL (95).
The high WBC counts among SSNHL may reflect an immune response to inner ear damage induced by ischemic changes or infections (94). In addition, the interrelation between neutrophils and endothelium may contribute to increased damage to the endothelium and was reported to explain platelet adhesion in patients with unstable angina (117). An elevated platelet count leading to an increased PLR might therefore lead to an increase in vascular endpoints. The SII, which is defined as platelets × neutrophils/lymphocytes, can serve as a prognostic marker for malignancies and inflammatory conditions. According to Ulu et al., as a novel index, the SII can be an indicator of SSNHL and it can predict the prognosis of SSNHL (96).
Masuda et al. (98) recruited 43 patients with SSNHL and found that, in SSNHL patients, neutrophils were above the reference range, natural killer cell activity (NKCA) was low and serum levels of interleukin-6 (IL-6) were higher compared to controls. Moreover, neutrophil count level was correlated with more severe hearing loss and a worse prognosis. The authors hypothesized that high neutrophils together with low NKCA and high IL-6 may activate nuclear factor-κB in the cochlea and lead to SSNHL (98).
5. Immune system
As shown previously, immune factors are involved in the onset of SSNHL. Studies have found elevated levels of Circulating Immune Complexes and Heat Shock Proteins 70 in SSNHL patients, as well as IgG antibodies against the inner ear-specific proteins cochlin and β-tectorin (100, 118). These findings have provided compelling evidence that antibody-mediated tissue damage and Type III immunocomplex-mediated immune reaction in the inner ear are the pathogenetic mechanisms of the development of SSNHL. In addition, Baradaranfar M. reported that mean erythrocyte sedimentation rate, antinuclear antibody, C3, C4, and monocytes were higher in the case group (99).
In addition, no matter what kind of administration method, the use of steroids greatly improved the recovery of hearing in patients with SSNHL (119). The beneficial effect of corticosteroids in SSNHL could be due to an immunosuppressive and anti-inflammatory effect.
6. Conclusion
SSNHL is a multifactorial disease and its underlying mechanism remains uncertain. Some etiological comorbidities involving multiple systems may play a role in its pathogenesis. Atherosclerosis, microthrombosis, inflammation, and immune system may be the main etiological factors of SSNHL. In summary, by analyzing the etiology of SSNHL, more targeting treatments should be directed at the underlying cause to achieve a better effect.
Author contributions
Based on discussions with all authors, WX drafted the manuscript, which all authors revised. All authors contributed to the study design. All authors approved the final version submitted for publication.
Funding
This study was funded by the Natural Science Foundation of Jiangxi Province (Project No.: 20224BAB206051).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Stachler RJ, Chandrasekhar SS, Archer SM, Rosenfeld RM, Schwartz SR, Barrs DM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngology. (2012) 146:S1–35. doi: 10.1177/0194599812436449
2. Hong SM, Yeo SG. Clinical analysis of patients with idiopathic sudden sensorineural hearing loss and benign paroxysmal positional vertigo. Acta Otolaryngol. (2013) 133:439–42. doi: 10.3109/00016489.2012.754996
3. Xie W, Dai Q, Liu J, Liu Y, Hellström S, Duan M. Analysis of clinical and laboratory findings of idiopathic sudden sensorineural hearing loss. Sci Rep. (2020) 10:6057. doi: 10.1038/s41598-020-63046-z
4. Yu H, Li H. Association of vertigo with hearing outcomes in patients with sudden sensorineural hearing loss: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. (2018) 144:677–83. doi: 10.1001/jamaoto.2018.0648
5. Yu H, Li H. Vestibular dysfunctions in sudden sensorineural hearing loss: a systematic review and meta-analysis. Front Neurol. (2018) 9:45. doi: 10.3389/fneur.2018.00045
6. Byl FM. Sudden hearing loss: eight years' experience and suggested prognostic table. Laryngoscope. (1984) 94:647–61. doi: 10.1288/00005537-198405000-00014
7. Alexander TH, Harris JP. Incidence of sudden sensorineural hearing loss. Otol Neurotol. (2013) 34:1586–9. doi: 10.1097/MAO.0000000000000222
8. Rauch SD. Clinical practice. Idiopathic sudden sensorineural hearing loss. N Engl J Med. (2008) 359:833–40. doi: 10.1056/NEJMcp0802129
9. Kitoh R, Nishio SY, Ogawa K, Kanzaki S, Hato N, Sone M, et al. Nationwide epidemiological survey of idiopathic sudden sensorineural hearing loss in Japan. Acta Otolaryngol. (2017) 137:S8–S16. doi: 10.1080/00016489.2017.1297537
10. Nakashima T, Tanabe T, Yanagita N, Wakai K, Ohno Y. Risk factors for sudden deafness: a case-control study. Auris Nasus Larynx. (1997) 24:265–70. doi: 10.1016/S0385-8146(96)00024-7
11. Aimoni C, Bianchini C, Borin M, Ciorba A, Fellin R, Martini A, et al. Diabetes, cardiovascular risk factors and idiopathic sudden sensorineural hearing loss: a case-control study. Audiol Neurootol. (2010) 15:111–5. doi: 10.1159/000231636
12. Berjis N, Moeinimehr M, Hashemi SM, Hashemi SM, Bakhtiari EK, Nasiri S. Endothelial dysfunction in patients with sudden sensorineural hearing loss. Adv Biomed Res. (2016) 5:5. doi: 10.4103/2277-9175.174978
13. Suckfull M, Thiery J, Wimmer C, Mees K, Schorn K. Hypercholesteremia and hyperfibrinogenemia in sudden deafness. Laryngorhinootologie. (1997) 76:453–7. doi: 10.1055/s-2007-997460
14. Jalali MM, Nasimidoust Azgomi M. Metabolic syndrome components and sudden sensorineural hearing loss: a case-control study. Eur Arch Otorhinolaryngol. (2020) 277:1023–9. doi: 10.1007/s00405-020-05808-z
15. Chien CY, Tai SY, Wang LF, Hsi E, Chang NC, Wu MT, et al. Metabolic syndrome increases the risk of sudden sensorineural hearing loss in Taiwan: a case-control study. Otolaryngol Head Neck Surg. (2015) 153:105–11. doi: 10.1177/0194599815575713
16. Passamonti SM, Di Berardino F, Bucciarelli P, Berto V, Artoni A, Gianniello F, et al. Risk factors for idiopathic sudden sensorineural hearing loss and their association with clinical outcome. Thromb Res. (2015) 135:508–12. doi: 10.1016/j.thromres.2015.01.001
17. Lin CS, Lin YS, Liu CF, Weng SF, Lin C, Lin BS. Increased risk of sudden sensorineural hearing loss in patients with depressive disorders: population-based cohort study. J Laryngol Otol. (2016) 130:42–9. doi: 10.1017/S0022215115002960
18. Koo M, Hwang JH. Risk of sudden sensorineural hearing loss in patients with common preexisting sensorineural hearing impairment: a population-based study in Taiwan. PLoS ONE. (2015) 10:e0121190. doi: 10.1371/journal.pone.0121190
19. Tsai YT, Chang IJ, Hsu CM, Yang YH, Liu CY, Tsai MS, et al. Association between sudden sensorineural hearing loss and preexisting thyroid diseases: a nationwide case-control study in Taiwan. Int J Environ Res Public Health. (2020) 17:834. doi: 10.3390/ijerph17030834
20. Lin SW, Lin YS, Weng SF, Chou CW. Risk of developing sudden sensorineural hearing loss in diabetic patients: a population-based cohort study. Otol Neurotol. (2012) 33:1482–8. doi: 10.1097/MAO.0b013e318271397a
21. Chang SL, Hsieh CC, Tseng KS, Weng SF, Lin YS. Hypercholesterolemia is correlated with an increased risk of idiopathic sudden sensorineural hearing loss: a historical prospective cohort study. Ear Hear. (2014) 35:256–61. doi: 10.1097/AUD.0b013e3182a76637
22. Lin C, Hsu HT, Lin YS, Weng SF. Increased risk of getting sudden sensorineural hearing loss in patients with chronic kidney disease: a population-based cohort study. Laryngoscope. (2013) 123:767–73. doi: 10.1002/lary.23669
23. Jeong J, Lim H, Lee K, Hong CE, Choi HS. High risk of sudden sensorineural hearing loss in several autoimmune diseases according to a population-based national sample cohort study. Audiol Neurootol. (2019) 24:224–30. doi: 10.1159/000502677
24. Lee SY, Kong IG, Oh DJ, Choi HG. Increased risk of sudden sensory neural hearing loss in patients with rheumatoid arthritis: a longitudinal follow-up study using a national sample cohort. Clin Rheumatol. (2019) 38:683–9. doi: 10.1007/s10067-018-4333-6
25. Schieffer KM, Connor JR, Pawelczyk JA, Sekhar DL. The relationship between iron deficiency anemia and sensorineural hearing loss in the pediatric and adolescent population. Am J Audiol. (2017) 26:155–62. doi: 10.1044/2017_AJA-16-0093
26. Chung SD, Chen PY, Lin HC, Hung SH. Sudden sensorineural hearing loss associated with iron-deficiency anemia: a population-based study. JAMA Otolaryngol Head Neck Surg. (2014) 140:417–22. doi: 10.1001/jamaoto.2014.75
27. Sidman JD, Prazma J, Pulver SH, Pillsbury HC. Cochlea and heart as end-organs in small vessel disease. Ann Otolaryngol. (1988) 97:9–13. doi: 10.1177/000348948809700102
28. Luan CW, Chang JJ, Hsu CM, Tsai MS, Chang GH, Huang EI, et al. Risk of sudden sensorineural hearing loss in patients with dysrhythmia: A nationwide population-based cohort study. PLoS ONE. (2019) 14:e0218964. doi: 10.1371/journal.pone.0218964
29. Weng SF, Chen YS, Hsu CJ, Tseng FY. Clinical features of sudden sensorineural hearing loss in diabetic patients. Laryngoscope. (2005) 115:1676–80. doi: 10.1097/01.mlg.0000184790.91675.e3
30. Fukui M, Kitagawa Y, Nakamura N, Kadono M, Mogami S, Ohnishi M, et al. Idiopathic sudden hearing loss in patients with type 2 diabetes. Diabetes Res Clin Pract. (2004) 63:205–11. doi: 10.1016/j.diabres.2003.09.013
31. Chen HC, Chung CH, Lu CH, Chien WC. Metformin decreases the risk of sudden sensorineural hearing loss in patients with diabetes mellitus: A 14-year follow-up study. Diab Vasc Dis Res. (2019) 16:324–7. doi: 10.1177/1479164119826292
32. Lee HS, Kim KR, Chung WH, Cho YS, Hong SH. Early sensorineural hearing loss in ob/ob mouse, an animal model of type 2 diabetes. Clin Exp Otorhinolaryngol. (2008) 1:211–6. doi: 10.3342/ceo.2008.1.4.211
33. Liu F, Xia M, Xu A. Expression of VEGF, iNOS, and eNOS is increased in cochlea of diabetic rat. Acta Otolaryngol. (2008) 128:1178–86. doi: 10.1080/00016480801901774
34. Lu YY, Jin Z, Tong BS, Yang JM, Liu YH, Duan M, et al. clinical study of microcirculatory disturbance in Chinese patients with sudden deafness. Acta Otolaryngol. (2008) 128:1168–72. doi: 10.1080/00016480801901626
35. Lee JS, Kim DH, Lee HJ, Kim HJ, Koo JW, Choi HG, et al. Lipid profiles and obesity as potential risk factors of sudden sensorineural hearing loss. PLoS ONE. (2015) 10:e0122496. doi: 10.1371/journal.pone.0122496
36. Satar B, Ozkaptan Y, Surucu HS, Ozturk H. Ultrastructural effects of hypercholesterolemia on the cochlea. Otol Neurotol. (2001) 22:786–9. doi: 10.1097/00129492-200111000-00012
37. Nguyen TV, Brownell WE. Contribution of membrane cholesterol to outer hair cell lateral wall stiffness. Otolaryngol Head Neck Surg. (1998) 119:14–20. doi: 10.1016/S0194-5998(98)70167-6
38. Sikora MA, Morizono T, Ward WD, Paparella MM, Leslie K. Diet-induced hyperlipidemia and auditory dysfunction. Acta Otolaryngol. (1986) 102:372–81. doi: 10.3109/00016488609119420
39. Suzuki K, Kaneko M, Murai K. Influence of serum lipids on auditory function. Laryngoscope. (2000) 110:1736–8. doi: 10.1097/00005537-200010000-00033
40. Suckfull M. Fibrinogen and LDL apheresis in treatment of sudden hearing loss: a randomised multicentre trial. Lance. (2002) 360:1811–7. doi: 10.1016/S0140-6736(02)11768-5
41. Ralli M, D'Aguanno V, Di Stadio A, De Virgilio A, Croce A, Longo L, et al. Audiovestibular symptoms in systemic autoimmune diseases. J Immunol Res. (2018) 2018:5798103. doi: 10.1155/2018/5798103
42. Li G, You D, Ma J, Li W, Li H, Sun S. The role of autoimmunity in the pathogenesis of sudden sensorineural hearing loss. Neural Plast. (2018) 2018:7691473. doi: 10.1155/2018/7691473
43. Xie S, Ning H, She Y, Jing Q, Jiang Q, Zhang Y, et al. Effect of systemic lupus erythematosus and rheumatoid arthritis on sudden sensorineural hearing loss. Laryngoscope. (2020) 130:2475–80. doi: 10.1002/lary.28455
44. Di Stadio A, Dipietro L, Ralli M, Meneghello F, Minni A, Greco A, et al. Sudden hearing loss as an early detector of multiple sclerosis: a systematic review. Eur Rev Med Pharmacol Sci. (2018) 22:4611–24. doi: 10.26355/eurrev_201807_15520
45. O'Malley JT, Nadol JB, McKenna MJ. Anti CD163+, Iba1+, and CD68+ Cells in the adult human inner ear: normal distribution of an unappreciated class of macrophages/microglia and implications for inflammatory otopathology in humans. Otol Neurotol. (2016) 37:99–108. doi: 10.1097/MAO.0000000000000879
46. Rossini BAA, Penido NO, Munhoz MSL, Bogaz EA, Curi RS. Sudden sensorioneural hearing loss and autoimmune systemic diseases. Int Arch Otorhinolaryngol. (2017) 21:213–23. doi: 10.1055/s-0036-1586162
47. Malik MU, Pandian V, Masood H, Diaz DA, Varela V, Dávalos-Balderas AJ, et al. Spectrum of immune-mediated inner ear disease and cochlear implant results. Laryngoscope. (2012) 122:2557–62. doi: 10.1002/lary.23604
48. Hervier B, Bordure P, Masseau A, Calais C, Agard C, Hamidou M. Auto-immune sensorineural deafness: physiopathology and therapeutic approach. Rev Med Interne. (2010) 31:222–8. doi: 10.1016/j.revmed.2008.12.017
49. Greco A, De Virgilio A, Gallo A, Fusconi M, Ruoppolo G, Turchetta R, et al. Idiopathic bilateral vestibulopathy: an autoimmune disease? Autoimmun Rev. (2014) 13:1042–7. doi: 10.1016/j.autrev.2014.08.035
50. Jeffries MA, Sawalha AH. Autoimmune disease in the epigenetic era: how has epigenetics changed our understanding of disease and how can we expect the field to evolve? Expert Rev Clin Immunol. (2015) 11:45–58. doi: 10.1586/1744666X.2015.994507
51. Kanzaki J. Immune-mediated sensorineural hearing loss. Acta Otolaryngol Suppl. (1994) 514:70–2. doi: 10.3109/00016489409127564
52. Di Stadio A, Ralli M. Systemic lupus erythematosus and hearing disorders: literature review and meta-analysis of clinical and temporal bone findings. Int J Appl Basic Med Res. (2017) 45:1470–80. doi: 10.1177/0300060516688600
53. Lin CT, Chiang CW, Young YH. Acute hearing loss in patients with hematological disorders. Acta Otolaryngol. (2015) 135:673–80. doi: 10.3109/00016489.2015.1015608
54. Algarin C, Peirano P, Garrido M, Pizarro F, Lozoff B. Iron deficiency anemia in infancy: long-lasting effects on auditory and visual system functioning. Pediatr Res. (2003) 53:217–23. doi: 10.1203/01.PDR.0000047657.23156.55
55. Sun AH, Xiao SZ Li BS, Li ZJ, Wang TY, Zhang YS. Iron deficiency and hearing loss: Experimental study in growing rats. ORL J Otorhinolaryngol Relat Spec. (1987) 49:118–22. doi: 10.1159/000275920
56. Sun AH, Wang ZM, Xiao SZ Li ZJ, Zheng Z, Li JY. Sudden sensorineural hearing loss induced by experimental iron deficiency in rats. ORL J Otorhinolaryngol Relat Spec. (1992) 54:246–50. doi: 10.1159/000276307
57. Sun AH Li JY, Xiao SZ Li ZJ, Wang TY. Changes in the cochlear iron enzymes and adenosine triphosphatase in experimental iron deficiency. Ann Otol Rhinol Laryngol. (1990) 99:988–92. doi: 10.1177/000348949009901211
58. Veling MC, Windmill I, Bumpous JM. Sudden hearing loss as a presenting manifestation of leukemia. Otolaryngol Head Neck Surg. (1999) 120:954–6. doi: 10.1016/S0194-5998(99)70349-9
59. Baer MR, Stein RS, Dessypris EN. Chronic lymphocytic leukemia with hyperleukocytosis. The hyperviscosity syndrome. Cancer. (1985) 56:2865–9. doi: 10.1002/1097-0142(19851215)56:12<2865::AID-CNCR2820561225>3.0.CO;2-6
60. Genden EM, Bahadori RS. Bilateral sensorineural hearing loss as a first symptom of chronic myelogenous leukemia. Otolaryngol Head Neck Surg. (1995) 113:499–501. doi: 10.1016/S0194-59989570095-1
61. Chim CS, Woo JK. Deafness in chronic myeloid leukemia. Leuk Lymphoma. (1997) 26:209–10. doi: 10.3109/10428199709109179
62. Chae SW, Cho JH, Lee JH, Kang HJ, Hwang SJ. Sudden hearing loss in chronic myelogenous leukaemia implicating the hyperviscosity syndrome. J Laryngol Otol. (2002) 116:291–3. doi: 10.1258/0022215021910564
63. Paparella MM, Berlinger NT, Oda M, el-Fiky F. Otological manifestations of leukemia. Laryngoscope. (1973) 83:1510–26. doi: 10.1288/00005537-197309000-00010
64. Preston FE, Sokol RJ, Lilleyman JS, Winfield DA, Blackburn EK. Cellular hyperviscosity as a cause of neurological symptoms in leukaemia. Br Med J. (1978) 1:476–8. doi: 10.1136/bmj.1.6111.476
65. Harada T, Namiki S, Kawabata I. Acute profound sensorineural hearing loss as the initial manifestation of acute leukemia–report of a case. Auris Nasus Larynx. (2000) 27:359–62. doi: 10.1016/S0385-8146(99)00074-7
66. Lasisi OA, Salako BL, Kadiri S, Arije A, Oko-Jaja R, Ipadeola A, et al. Sudden sensorineural hearing loss and hemodialysis. Ear Nose Throat J. (2006) 85:819–21. doi: 10.1177/014556130608501212
67. Ikeda K, Kusakari J, Arakawa E, Ohyama K, Inamura N, Kawamoto K. Cochlear potentials of guinea pigs with experimentally induced renal failure. Acta Otolaryngol Suppl. (1987) 435:40–5. doi: 10.3109/00016488709107349
68. Wang IK, Wang CY, Muo CH, Yen TH, Sung FC. Risk of sudden sensorineural hearing loss in patients with end-stage renal disease undergoing dialysis. Nephrology (Carlton). (2017) 22:397–402. doi: 10.1111/nep.12800
69. Rizvi SS, Holmes RA. Hearing loss from hemodialysis. Arch Otolaryngol. (1980) 106:751–6. doi: 10.1001/archotol.1980.00790360029009
70. Chou CL, Hsieh TC, Chen JS, Fang TC. Sudden sensorineural hearing loss in hemodialysis patients could be a marker of pathogenic progression in the mortality and atherosclerotic events: a national cohort study. Otol Neurotol. (2018) 39:1241–9. doi: 10.1097/MAO.0000000000001967
71. Chiarella G, Russo D, Monzani F, Petrolo C, Fattori B, Pasqualetti G, et al. Hashimoto thyroiditis and vestibular dysfunction. Endocr Pract. (2017) 23:863–8. doi: 10.4158/EP161635.RA
72. Hostiuc M, Curca GC, Dermengiu D, Sinescu C, Hostiuc S. Can subclinical hypothyroidism explain some sudden deaths due to pulmonary embolism without evident risk factors? Med hypotheses. (2011) 76:855–7. doi: 10.1016/j.mehy.2011.02.035
73. Segna D, Méan M, Limacher A, Baumgartner C, Blum MR, Beer JH, et al. Association between thyroid dysfunction and venous thromboembolism in the elderly: a prospective cohort study. J Thromb Haemost. (2016) 14:685–94. doi: 10.1111/jth.13276
74. Tong B, Niu K, Ku W, Xie W, Dai Q, Hellström S, et al. Comparison of therapeutic results with/without additional hyperbaric oxygen therapy in idiopathic sudden sensorineural hearing loss: a randomized prospective study. Audiol Neurooto. (2020) 26:1–6. doi: 10.1159/000507911
75. Weng T, Devine EE, Xu H, Yan Z, Dong P, A. clinical study of serum lipid disturbance in Chinese patients with sudden deafness. Lipids Health Dis. (2013) 12:95. doi: 10.1186/1476-511X-12-95
76. Cadoni G, Scipione S, Agostino S, Addolorato G, Cianfrone F, Leggio L, et al. Coenzyme Q 10 and cardiovascular risk factors in idiopathic sudden sensorineural hearing loss patients. Otol Neurotol. (2007) 28:878–83. doi: 10.1097/MAO.0b013e3180686e4a
77. Ciccone MM, Cortese F, Pinto M, Di Teo C, Fornarelli F, Gesualdo M, et al. Endothelial function and cardiovascular risk in patients with idiopathic sudden sensorineural hearing loss. Atherosclerosis. (2012) 225:511–6. doi: 10.1016/j.atherosclerosis.2012.10.024
78. Oreskovic Z, Shejbal D, Bicanic G, Kekic B. Influence of lipoproteins and fibrinogen on pathogenesis of sudden sensorineural hearing loss. J Laryngol Otol. (2011) 125:258–61. doi: 10.1017/S0022215110002252
79. Chang IJ, Kang CJ, Yueh CY, Fang KH, Yeh RM, Tsai YT. The relationship between serum lipids and sudden sensorineural hearing loss: a systematic review and meta-analysis. PLoS ONE. (2015) 10:e0121025. doi: 10.1371/journal.pone.0121025
80. Wang S, Ye Q, Pan Y. Serum non-high-density lipoprotein cholesterol is associated with the risk of sudden sensorineural hearing loss. Medicine (Baltimore). (2020) 99:e19175. doi: 10.1097/MD.0000000000019175
81. Fasano T, Pertinhez TA, Tribi L, Lasagni D, Pilia A, Vecchia L, et al. Laboratory assessment of sudden sensorineural hearing loss: A case-control study. Laryngoscope. (2017) 127:2375–81. doi: 10.1002/lary.26514
82. Capaccio P, Ottaviani F, Cuccarini V, Bottero A, Schindler A, Cesana BM, et al. Genetic and acquired prothrombotic risk factors and sudden hearing loss. Laryngoscope. (2007) 117:547–51. doi: 10.1097/MLG.0b013e31802f3c6a
83. Suckfull M, Wimmer C, Reichel O, Mees K, Schorn K. Hyperfibrinogenemia as a risk factor for sudden hearing loss. Otol Neurotol. (2002) 23:309–11. doi: 10.1097/00129492-200205000-00013
84. Rudack C, Langer C, Stoll W, Rust S, Walter M. Vascular risk factors in sudden hearing loss. Thromb Haemost. (2006) 95:454–61. doi: 10.1160/TH05-08-0554
85. Ohinata Y, Makimoto K, Kawakami M, Haginomori S, Araki M, Takahashi H. Blood viscosity and plasma viscosity in patients with sudden deafness. Acta Otolaryngol. (1994) 114:601–7. doi: 10.3109/00016489409126112
86. Marcucci R, Alessandrello Liotta A, Cellai AP, Rogolino A, Berloco P, Leprini E, et al. Cardiovascular and thrombophilic risk factors for idiopathic sudden sensorineural hearing loss. J Thromb Haemost. (2005) 3:929–34. doi: 10.1111/j.1538-7836.2005.01310.x
87. Ciuffetti G, Scardazza A, Serafini G, Lombardini R, Mannarino E, Simoncelli C. Whole-blood filterability in sudden deafness. Laryngoscope. (1991) 101:65–7. doi: 10.1288/00005537-199101000-00011
88. Haubner F, Martin L, Steffens T, Strutz J, Kleinjung T. The role of soluble adhesion molecules and cytokines in sudden sensorineural hearing loss. Otolaryngol Head Neck Surg. (2011) 144:575–80. doi: 10.1177/0194599810394324
89. Quaranta N, Ramunni A, Brescia P, D'Elia A, Vacca A, Ria R. Soluble intercellular adhesion molecule 1 and soluble vascular cell adhesion molecule 1 in sudden hearing loss. Otol Neurotol. (2008) 29:470–4. doi: 10.1097/MAO.0b013e318170b650
90. Quaranta N, Ramunni A, De Luca C, Brescia P, Dambra P, De Tullio G, et al. Endothelial progenitor cells in sudden sensorineural hearing loss. Acta Otolaryngol. (2011) 131:347–50. doi: 10.3109/00016489.2010.536990
91. Capaccio P, Pignataro L, Gaini LM, Sigismund PE, Novembrino C, De Giuseppe R, et al. Unbalanced oxidative status in idiopathic sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol. (2012) 269:449–53. doi: 10.1007/s00405-011-1671-2
92. Cadoni G, Agostino S, Scipione S, Galli J. Low serum folate levels: a risk factor for sudden sensorineural hearing loss? Acta Otolaryngol. (2004) 124:608–11. doi: 10.1080/00016480410016216
93. Lin RJ, Krall R, Westerberg BD, Chadha NK, Chau JK. Systematic review and meta-analysis of the risk factors for sudden sensorineural hearing loss in adults. Laryngoscope. (2012) 122:624–35. doi: 10.1002/lary.22480
94. Kanzaki S, Sakagami M, Hosoi H, Murakami S, Ogawa K. High fibrinogen in peripheral blood correlates with poorer hearing recovery in idiopathic sudden sensorineural hearing loss. PLoS ONE. (2014) 9:e104680. doi: 10.1371/journal.pone.0104680
95. Seo YJ, Jeong JH, Choi JY, Moon IS. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio: novel markers for diagnosis and prognosis in patients with idiopathic sudden sensorineural hearing loss. Dis Markers. (2014) 2014:702807. doi: 10.1155/2014/702807
96. Ulu S, Kinar A, Bucak A, Özdemir M. Systemic immune inflammatory index of patients with idiopathic sudden sensorineural hearing loss: comparison of NLR and PRL values. Ear Nose Throat J. (2020) 100:726–30. doi: 10.1177/0145561320924312
97. Qiao XF Li X, Wang GP, Bai YH, Zheng W, Li TL. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with sudden sensorineural hearing loss. Med Princ Pract. (2019) 28:23–7. doi: 10.1159/000494556
98. Masuda M, Kanzaki S, Minami S, Kikuchi J, Kanzaki J, Sato H, et al. Correlations of inflammatory biomarkers with the onset and prognosis of idiopathic sudden sensorineural hearing loss. Otol Neurotol. (2012) 33:1142–50. doi: 10.1097/MAO.0b013e3182635417
99. Baradaranfar M, Dadgarnia M, Zand V, Vaziribozorg S, Mirzade FS, Mirzade M. The role of immunological factors on sudden sensoryneural hearing loss. Iran J Otorhinolaryngol. (2018) 30:219–23.
100. Pawlak-Osinska K, Golda R, Osinski S, Kazmierczak H, Krumrych W, Marzec M, et al. Circulating immune complexes and heat shock protein 70 in the sera of patients with sudden sensorineural hearing loss. J Int Adv Otol. (2018) 14:426–31. doi: 10.5152/iao.2018.5694
101. Yoon SH, Kim ME, Kim HY, Lee JS, Jang CH. Inflammatory cytokines and mononuclear cells in sudden sensorineural hearing loss. J Laryngol Otol. (2019) 133:95–101. doi: 10.1017/S0022215119000100
102. Ihler F, Strieth S, Pieri N, Gohring P, Canis M. Acute hyperfibrinogenemia impairs cochlear blood flow and hearing function in guinea pigs in vivo. Int J Audiol. (2012) 51:210–5. doi: 10.3109/14992027.2011.622302
103. Valbonesi M, Mora F, Mora R, Carlier P. Rheopheresis for sudden hearing loss (SHL). Int J Artif Organs. (2004) 27:806–9. doi: 10.1177/039139880402700911
104. Mosges R, Koberlein J, Heibges A, Erdtracht B, Klingel R, Lehmacher W. Rheopheresis for idiopathic sudden hearing loss: results from a large prospective, multicenter, randomized, controlled clinical trial. Eur Arch Otorhinolaryngol. (2009) 266:943–53. doi: 10.1007/s00405-008-0823-5
105. Ciccone MM, Scicchitano P, Zito A, Agati L, Gesualdo M, Mandolesi S, et al. Correlation between coronary artery disease severity, left ventricular mass index and carotid intima media thickness, assessed by radio-frequency. Cardiovasc Ultrasound. (2011) 9:32. doi: 10.1186/1476-7120-9-32
106. Ciccone MM, De Pergola G, Porcelli MT, Scicchitano P, Caldarola P, Iacoviello M, et al. Increased carotid IMT in overweight and obese women affected by Hashimoto's thyroiditis: an adiposity and autoimmune linkage? BMC Cardiovasc Disord. (2010) 10:22. doi: 10.1186/1471-2261-10-22
107. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. (2002) 39:257–65. doi: 10.1016/S0735-1097(01)01746-6
108. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. (1997) 275:964–7. doi: 10.1126/science.275.5302.964
109. Shantsila E, Watson T, Lip GY. Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol. (2007) 49:741–52. doi: 10.1016/j.jacc.2006.09.050
110. Krieglstein CF, Granger DN. Adhesion molecules and their role in vascular disease. Am J Hypertens. (2001) 14:44s–54s. doi: 10.1016/S0895-7061(01)02069-6
111. Halliwell B, Gutteridge JM. Lipid peroxidation, oxygen radicals, cell damage, and antioxidant therapy. Lancet. (1984) 1:1396–7. doi: 10.1016/S0140-6736(84)91886-5
112. Vassalle C, Pratali L, Boni C, Mercuri A, Ndreu R. An oxidative stress score as a combined measure of the pro-oxidant and anti-oxidant counterparts in patients with coronary artery disease. Clin Biochem. (2008) 41:1162–7. doi: 10.1016/j.clinbiochem.2008.07.005
113. Joachims HZ, Segal J, Golz A, Netzer A, Goldenberg D. Antioxidants in treatment of idiopathic sudden hearing loss. Otol Neurotol. (2003) 24:572–5. doi: 10.1097/00129492-200307000-00007
114. Sainani GS, Sainani R. Homocysteine and its role in the pathogenesis of atherosclerotic vascular disease. J Assoc Physicians India. (2002) 50 Suppl:16–23.
115. Outinen PA, Sood SK, Liaw PC, Sarge KD, Maeda N, Hirsh J, et al. Characterization of the stress-inducing effects of homocysteine. Biochem J. (1998) 332:213–21. doi: 10.1042/bj3320213
116. Hoffman M, Blum A, Baruch R, Kaplan E, Benjamin M. Leukocytes and coronary heart disease. Atherosclerosis. (2004) 172:1–6. doi: 10.1016/S0021-9150(03)00164-3
117. Ott I, Neumann FJ, Gawaz M, Schmitt M, Schömig A. Increased neutrophil-platelet adhesion in patients with unstable angina. Circulation. (1996) 94:1239–46. doi: 10.1161/01.CIR.94.6.1239
118. Naumann A, Hempel JM, Schorn K. Detection of humoral immune response to inner ear proteins in patients with sensorineural hearing loss. Laryngorhinootologie. (2001) 80:237–44. doi: 10.1055/s-2001-13883
Keywords: sudden sensorineural hearing loss, hearing loss, etiology, laboratory results, comorbidities
Citation: Xie W, Karpeta N, Tong B, Liu Y, Zhang Z and Duan M (2023) Comorbidities and laboratory changes of sudden sensorineural hearing loss: a review. Front. Neurol. 14:1142459. doi: 10.3389/fneur.2023.1142459
Received: 11 January 2023; Accepted: 29 March 2023;
Published: 18 April 2023.
Edited by:
Arianna Di Stadio, University of Catania, ItalyCopyright © 2023 Xie, Karpeta, Tong, Liu, Zhang and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maoli Duan, bWFvbGkuZHVhbkBraS5zZQ==
 Wen Xie
Wen Xie Niki Karpeta
Niki Karpeta Busheng Tong4
Busheng Tong4 Yuehui Liu
Yuehui Liu Zhilin Zhang
Zhilin Zhang Maoli Duan
Maoli Duan