
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 05 April 2023
Sec. Neurological Biomarkers
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1139598
This article is part of the Research Topic Biomarkers in Neurology, Volume II View all 16 articles
Objective: The purpose of this retrospective study was to establish a numerical model for predicting the risk of pulmonary embolism (PE) in neurology department patients.
Methods: A total of 1,578 subjects with suspected PE at the neurology department from January 2012 to December 2021 were considered for enrollment in our retrospective study. The patients were randomly divided into the training cohort and the validation cohort in the ratio of 7:3. The least absolute shrinkage and selection operator regression were used to select the optimal predictive features. Multivariate logistic regression was used to establish the numerical model, and this model was visualized by a nomogram. The model performance was assessed and validated by discrimination, calibration, and clinical utility.
Results: Our predictive model indicated that eight variables, namely, age, pulse, systolic pressure, hemoglobin, neutrophil count, low-density lipoprotein, D-dimer, and partial pressure of oxygen, were associated with PE. The area under the receiver operating characteristic curve of the model was 0.750 [95% confidence interval (CI): 0.721–0.783] in the training cohort and 0.742 (95% CI: 0.689–0.787) in the validation cohort, indicating that the model showed a good differential performance. A good consistency between the prediction and the real observation was presented in the training and validation cohorts. The decision curve analysis in the training and validation cohorts showed that the numerical model had a good net clinical benefit.
Conclusion: We established a novel numerical model to predict the risk factors for PE in neurology department suspected PE patients. Our findings may help doctors to develop individualized treatment plans and PE prevention strategies.
Pulmonary embolism (PE) is a fatal cardiovascular disorder that remains a challenge to doctors during clinical diagnosis and treatment. Approximately 300,000 people die from PE every year in the United States of America, which ranks PE high among the causes of cardio-cerebrovascular mortality (1). This is especially true in elderly patients as it is hard to distinguish their symptoms of PE from other mild illnesses (2). The proportion of elderly patients in neurology departments cannot be neglected; the characteristics of these neurology department patients include aging, being bedridden for a long term, and presenting many concomitant diseases. These elderly patients are prone to lower extremity deep vein thrombosis and to develop PE (3). The presence of PE is a risk factor for stroke, cerebral infraction, and transient ischemic attack (4–6). Furthermore, PE-related death accounts for 20–25% of early deaths in stroke patients (7). Therefore, timely and accurate diagnosis of PE is crucial for the prognosis of neurology department patients.
Computed tomography pulmonary angiography (CTPA) is recommended for the diagnosis and risk-level assessment of PE (8–10). However, CTPA is time-consuming and expensive and can even cause serious side effects in patients. Therefore, it would be convenient to have a simple and fast risk prediction model to predict the probability of PE occurrence. Many researchers had created a variety of risk assessment models (RAMs) to predict PE, and their usability has been continuously validated. Robert-Ebadi et al. verified the feasibility of the simplified Geneva score in the clinic in 2017 (11). Freund et al. explored the safety of the PE rule-out criteria with a randomized clinical trial in 2018 (12). In addition, in 2019, van der Pol et al. assessed whether a pregnancy-adapted algorithm could help pregnant women avoid the imaging diagnosis for safety reasons (13). Furthermore, Kirsch et al. (14) demonstrated the ability of the Wells score to predict PE, which indicated that a Wells score above 4 was associated with PE; however, the performance of the Wells score was unreliable.
There have been many debates regarding the use of these RAMs; however, there are no consensual methods to diagnose PE. Currently, a RAM specifically for use with neurology department patients has not been developed. In recent years, we published a number of articles related to PE (15–17), and on this basis, we wanted to develop a numerical model that could rapidly determine the risk of PE in neurology department patients.
The numerical model (18, 19) is a graphical description of data, which presents the regression model in an accessible way, thus simplifying the risk assessment, providing a user-friendly interface for medical practitioners to map the probability of events to a single patient, and enhancing the clinical decision-making of doctors and patients. Therefore, the purpose of this study was to develop and validate a numerical model for the prediction of the risk of PE in neurology department suspected PE patients.
Neurology department patients with suspected PE who were admitted at the Affiliated Dongyang Hospital of the Wenzhou Medical University from January 2012 to December 2021 were considered for enrollment in our study. The patients who had undergone CTPA examination were suspected PE. The subjects' data were retrospectively collected from our clinical research data platform. After baseline data clearance and extraction, the medical records of 1,578 subjects were included in the statistical analysis. Subjects were randomly divided into the training cohort and the validation cohort at a ratio of 7:3.
Ethical approval for this retrospective study was obtained from the Medical Ethics Committee of the Affiliated Dongyang Hospital of the Wenzhou Medical University (No.: 2022-YX-160), and the requirement for informed consent was waived as the medical information of all patients was anonymized and de-identified prior to conducting the analysis. Our study was conducted in accordance with the Declaration of Helsinki.
Pulmonary embolism (PE) was defined in accordance with the criteria of the European Society of Cardiology Guidelines (20). The diagnosis of PE was based on a filling defect of the pulmonary artery system (including the subsegment pulmonary artery) in computed tomography pulmonary angiography (CTPA). The past medical history, complications, individual clinical features, and clinical biomarker data were collected. The indicators we chose, for example, blood oxygen saturation, systolic blood pressure, and diastolic pressure, were strictly defined from admission to CTPA, and the lowest result was selected; for other indicators, the highest result was selected. Our research flowchart is shown in Figure 1.
Data were statistically analyzed by RStudio software for Windows. Categorical variables were expressed as frequency with percentages and were compared using the chi-square (χ2) test or Fisher's exact test. Continuous variables were expressed as the mean with standard deviation (SD) or median and interquartile range (IQR), and were compared using the Student's t-test or the Mann–Whitney U-test. All subjects contained 58 variables. To guarantee the reliability of the data, five indicators with missing values >20% were deleted. The “mice” package in R software for multiple imputation techniques was used (21) to impute the remaining missing predictor values. The “glmnet” package for the least absolute shrinkage and selection operator regression (LASSO) analysis was used to select the optimal predictive features, and an “rms” package for multivariate logistic regression analysis was used to establish the numerical model (22–24). The “regplot” package in R software was used for the nomogram. The features were presented as the odds ratio (OR) and 95% confidence interval (CI). A two-sided p-value of < 0.05 was considered to statistically significant.
The least absolute shrinkage and selection operator regression analysis was used to select the optimal predictive features, and a multivariable logistic regression analysis was used to establish a numerical model to predict PE in the training cohort. The model performance was assessed and validated for discrimination, calibration, and clinical utility in both cohorts (25). The differentiation in the model was evaluated using the “pROC” package for the area under the receiver operating characteristic (ROC) curve (AUC), the “calibrate” package for calibration curve analysis to evaluate the calibration of the model, and the “rmda” package for decision curve analysis (DCA), which were used to quantify the net benefit under different threshold probabilities to determine the clinical utility of the model.
After excluding five variables with missing information in more than 20% of patients, we involved 53 variables with missing data in < 20% of patients involved in this study (shown in Supplementary Figure 1). The missing data for the 53 variables ranged from 0.00 to 18.69%; thus, the multiple imputation technique was used to impute the missing data. A total of 1,578 subjects with suspected PE were enrolled in this study. The incidence of PE in our study was 24.33%. The baseline characteristics of neurology department patients with suspected PE are displayed in Table 1. We divided the patients into the training cohort (1,113 patients) and the validation cohort (465 patients). Basic characteristics of the patients in the training and validation cohorts are presented in Table 2.
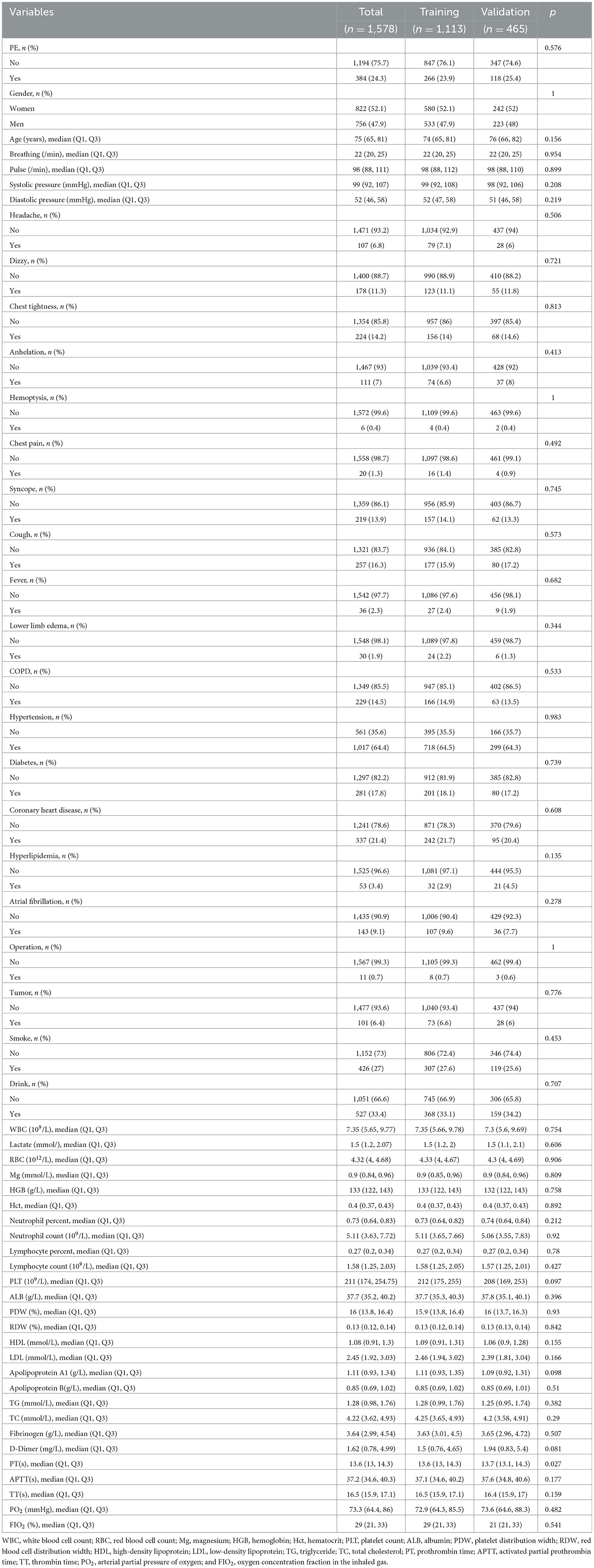
Table 2. The baseline characteristics of the enrolled patients in the training and validation cohorts.
Of the 53 variables, eight potential predictive features were finally selected based on the LASSO regression analysis (Figures 2A, B). The optimal predictors included age, pulse, systolic pressure, hemoglobin (HGB), neutrophil count (N), low-density lipoprotein (LDL), D-dimer (DD), and partial pressure of oxygen (PO2). The eight potential predictive features screened from the LASSO regression analysis were used to create the final model based on the multivariable logistic regression analysis in the training cohort (Table 3). The prevalence of PE is 23.9% in the training and 25.4% in the validation cohorts (Table 2). The sensitivity of our model is 94.17%, the specificity is 17.56%, the positive predictive value is 77.47%, and the negative predictive value is 50% in the training cohort.
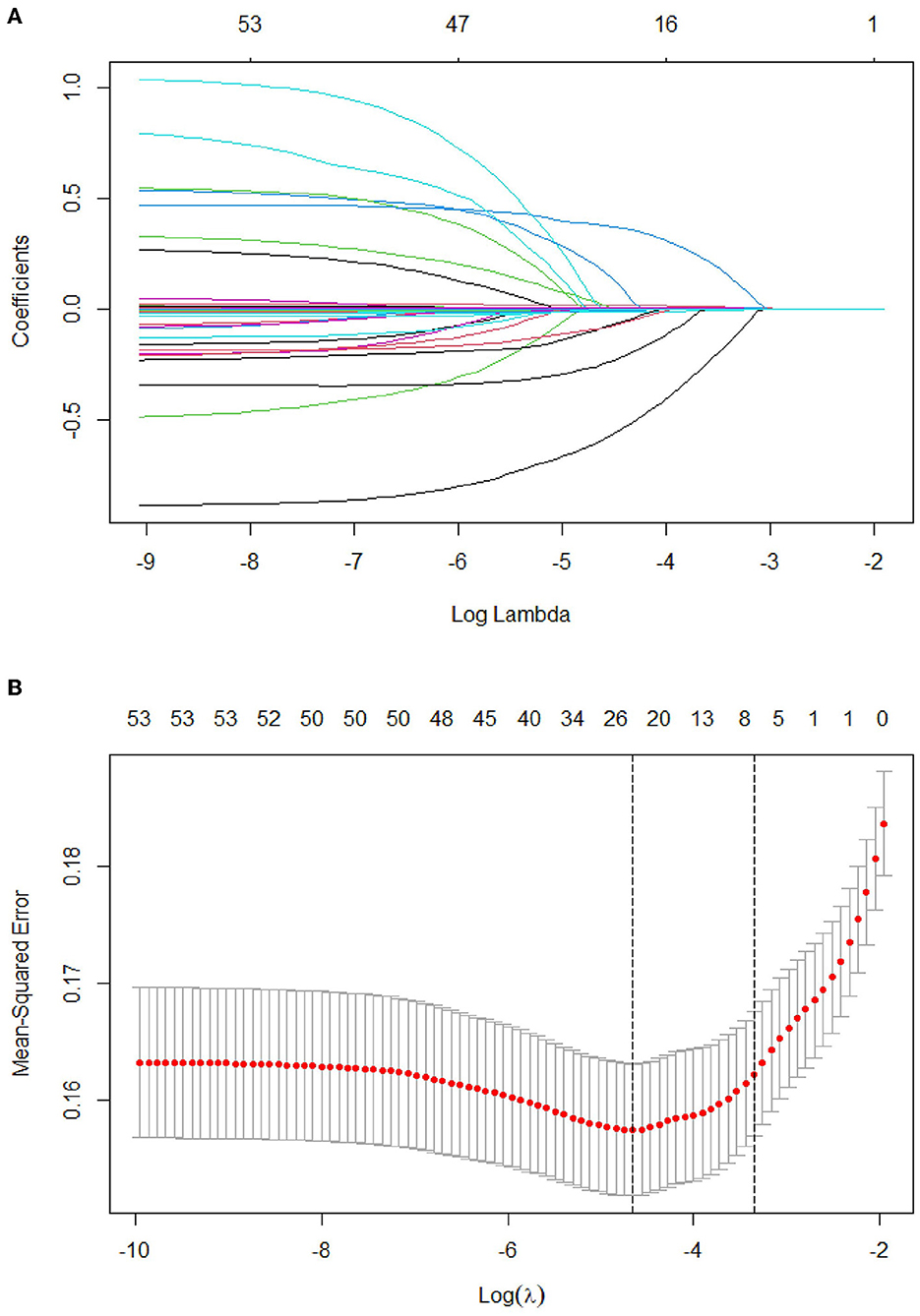
Figure 2. Tuning parameter selection using the LASSO regression in the training cohort. (A) LASSO coefficient profiles of the clinical features. (B) The optimal penalization coefficient lambda was generated in the LASSO via 10-fold cross-validation. The lambda value of the 1-fold mean square error for the training cohort was given.
The predictive model for PE was visualized by a nomogram in the training cohort, which is shown in Figure 3. Model discrimination, as quantified by the AUC, was 0.750 (95% CI: 0.721–0.783) in the training cohort and 0.742 (95% CI: 0.689–0.787) in the validation cohort, indicating that this numerical model can successfully distinguish PE from non-PE (Figures 4A, B). The calibration plots in the training and validation cohorts are shown in Figures 5A, B, which demonstrate a good consistency between the prediction and the real observation. The DCA in the training and validation cohorts indicated that the numerical model had a good net clinical benefit (Figures 6A, B).
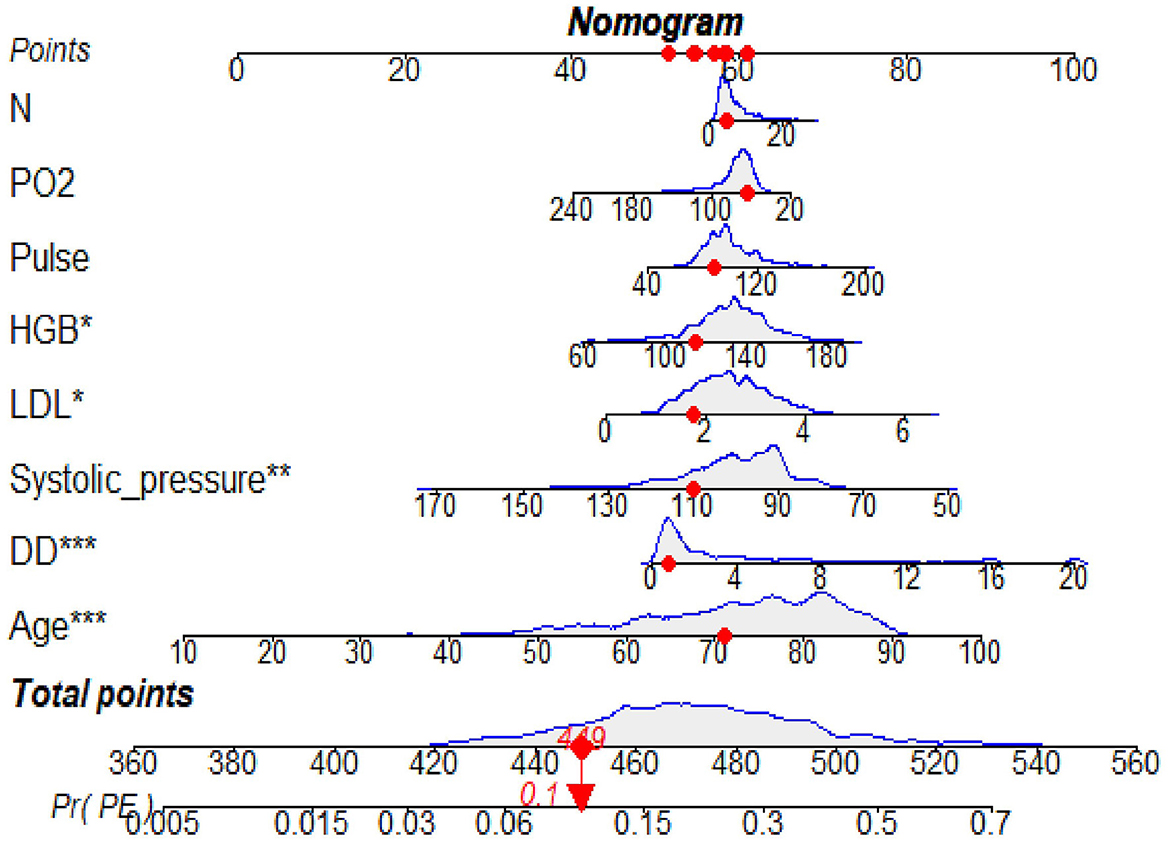
Figure 3. A nomogram based on the combination of eight indicators was developed using logistic regression analysis. If the neurology department patient had a total score of 449 points, this corresponded to an ~10% risk of PE and then the probability of the PE was 0.1 (red numbers). HGB, hemoglobin; N, neutrophil count; LDL, low-density lipoprotein; DD, D-dimer; PO2, oxygen partial pressure (PO2). *p < 0.05, **p < 0.01, ***p < 0.001.
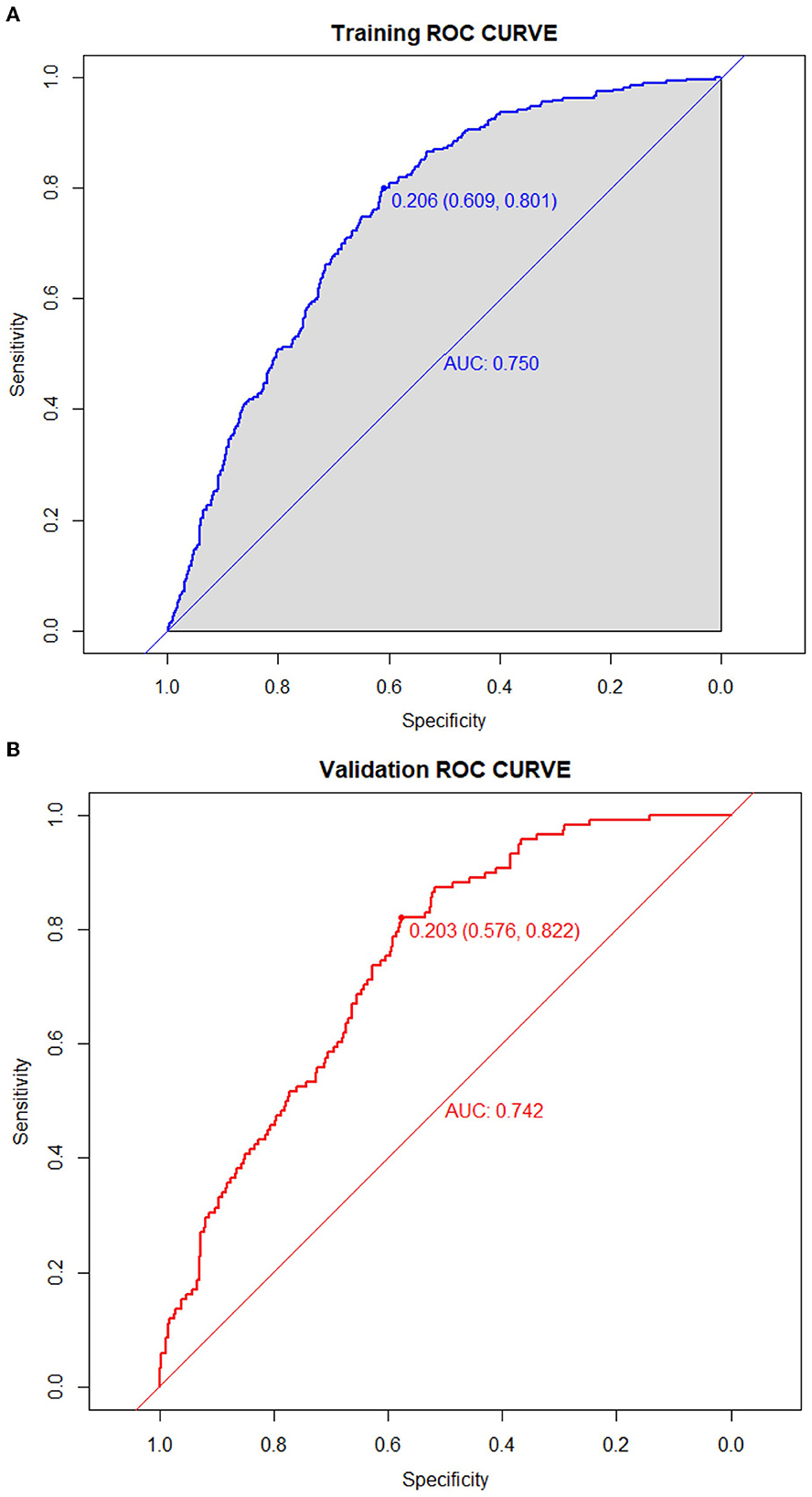
Figure 4. Receiver operating characteristic curves of the model distinguishing PE from non-PE in the training (A) and validation (B) cohorts.

Figure 6. The decision curve of the model in the training (A) and validation (B) cohort. If the risk threshold is < 50%, the nomogram model will obtain more benefit than all treatments (assuming that all neurology department patients were PE) or no treatment (assuming all neurology department patients were non-PE).
In this study, we developed and validated a simple model to determine the possible risk factors for PE based on a 10-year retrospective study in a comprehensive hospital in China. Our novel numerical model incorporated eight parameters, namely, age, pulse, systolic pressure, HGB, N, LDL, DD, and PO2. All parameters are readily available clinical features and biomarkers in routine health examinations. Notably, the ROC analysis showed that the AUC was 0.750 (95% CI: 0.721–0.783), indicating that our model displayed good discrimination and calibration. Furthermore, the DCA in the training and validation cohorts indicated that our model had a good net clinical benefit.
Our research found that DD (OR: 1.10; 95% CI: 1.07–1.13) is an independent predictive factor for the increased risk of PE. This result is in accordance with other observations (26, 27), which found that a high DD level was attributable to the possibility of developing PE. In terms of biomarkers, DD is the only biomarker currently used in routine practice for predicting PE; however, it is unlikely to have adequate specificity in neurology department patients for positivity. A large sample study from 2000 to 2015 showed increased hospitalization rates and the highest inpatient mortality due to PE in elderly patients (28). In addition, a retrospective study demonstrated an association between age and the severity of submassive PE stadium (29). Our model also showed that age (OR: 1.04; 95% CI: 1.02–1.06) is a high-risk factor for PE, which is similar to previous studies. In our model, most of the factors were positively associated with the risk of PE, whereas systolic blood pressure and PO2 were negatively associated. A previous study showed that low systolic pressure was connected with an increased risk of PE-related mortality (30, 31). In hemodynamically stable patients, a lower PO2 (< 8 kPa) was still associated with an elevated risk of mortality (32). These conclusions were consistent with those of our study. Our data also revealed that two clinical symptoms, including pulse and systolic pressure, were incorporated into the model to predict PE. Consistent with our result, a previous study (33) showed that pulse and systolic pressure were good predictors in a model for the prognosis of PE. Relevant studies (34–37) have also shown that inflammation and dyslipidemia are factors affecting PE, which is similar to the presence of inflammation and blood lipid indicators in our model. However, there are still some differences between the indicators included in our model and those included in the previous models (38). Three possible explanations for the discrepant results are as follows: (1) there are no such indicators in our clinical research data platform; (2) indicators with missing values >20% were deleted; and (3) indicators were not included in the model after the analysis.
This retrospective study suggested that a nomogram developed with clinical features and biomarkers to generate a personalized evaluation of PE risk in neurology department patients may distinguish patients at high risk of PE. For example, if the neurology department patient had a total score of 449 points, this corresponded to an ~10% risk of PE, and the probability of the PE was 0.1. Clinicians can use this simple numerical model to categorize the neurology department patients as PE-likely or PE-unlikely, thus reducing unnecessary CTPA examinations. This model may also be helpful to identify high-risk patients early, evaluate thrombosis, and implement active and individualized anticoagulation therapy.
This study is subject to certain limitations. In this retrospective study, five indicators (blood oxygen saturation, BMI, ejection fraction, troponin T, and brain natriuretic peptide precursor) with missing values > 20% were deleted. Moreover, the additional disadvantages of this study were the limited sample of participants and a lack of information on sufficient variables. Additionally, the data were collected as a single-center retrospective study.
In conclusion, we developed a novel numerical model for selecting the risk factors for PE in suspected-PE patients in a neurology department. Our findings may help decision-makers weigh the risk of PE and appropriately select PE prevention strategies. In the future, a large-scale prospective multicenter cohort study would help to form an improved and updated clinical decision-making system.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of Affiliated Dongyang Hospital of Wenzhou Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
WM conceived and designed the research strategy. QJ, JL, and WM wrote the manuscript text. QL, FL, MX, and LW collected the clinical data and participated in the writing of the manuscript. WM, MX, and LW contributed to the analysis and interpretation of the data. All authors contributed to this manuscript and approved the submitted version of the manuscript.
This study received funding from the Zhejiang Provincial Natural Science and Public Welfare Foundation of China (Grant No. LTGY23H200002) and the Jinhua Science and Technology Foundation, Zhejiang Province, China (Grant No. 2022-3-012).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1139598/full#supplementary-material
1. Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ Res. (2016) 118:1340–7. doi: 10.1161/CIRCRESAHA.115.306841
2. Toplis E, Mortimore G. The diagnosis and management of pulmonary embolism. Br J Nurs. (2020) 29:22–6. doi: 10.12968/bjon.2020.29.1.22
3. Kim KA, Choi SY, Kim R. Endovascular treatment for lower extremity deep vein thrombosis: an overview. Korean J Radiol. (2021) 22:931–43. doi: 10.3348/kjr.2020.0675
4. Cherng SC, Wang YF, Fan YM, Yang SP, Huang WS. Iliofemoral vein thrombosis and pulmonary embolism associated with a transient ischemic attack in a patient with antiphospholipid syndrome. Clin Nucl Med. (2001) 26:84–5. doi: 10.1097/00003072-200101000-00029
5. Tassi R, Guideri F, Acampa M, Domenichelli C, Martini G. Acute ischemic stroke and concomitant massive pulmonary embolism: a challenge. Neurol Sci. (2021) 42:4777–80. doi: 10.1007/s10072-021-05494-7
6. Vindis D, Hutyra M, Sanak D, Kral M, Cechakova E, Littnerova S, et al. Patent foramen ovale and the risk of cerebral infarcts in acute pulmonary embolism-a prospective observational study. J Stroke Cerebrovasc Dis. (2018) 27:357–64. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.004
7. Sherman DG, Albers GW, Bladin C, Fieschi C, Gabbai AA, Kase CS, et al. The efficacy and safety of enoxaparin versus unfractionated heparin for the prevention of venous thromboembolism after acute ischaemic stroke (prevail study): an open-label randomised comparison. Lancet. (2007) 369:1347–55. doi: 10.1016/S0140-6736(07)60633-3
8. Konstantinides SV, Meyer G. The 2019 Esc guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. (2019) 40:3453–5. doi: 10.1093/eurheartj/ehz726
9. Pang W, Zhang Z, Wang Z, Zhen K, Zhang M, Zhang Y, et al. Higher incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism in Asians than in Europeans: a meta-analysis. Front Med. (2021) 8:721294. doi: 10.3389/fmed.2021.721294
10. Wang X, Zhao H, Cui N. The role of electrical impedance tomography for management of high-risk pulmonary embolism in a postoperative patient. Front Med. (2021) 8:773471. doi: 10.3389/fmed.2021.773471
11. Robert-Ebadi H, Mostaguir K, Hovens MM, Kare M, Verschuren F, Girard P, et al. Assessing clinical probability of pulmonary embolism: prospective validation of the simplified geneva score. J Thromb Haemost. (2017) 15:1764–9. doi: 10.1111/jth.13770
12. Freund Y, Cachanado M, Aubry A, Orsini C, Raynal PA, Feral-Pierssens AL, et al. Effect of the pulmonary embolism rule-out criteria on subsequent thromboembolic events among low-risk emergency department patients: the proper randomized clinical trial. JAMA. (2018) 319:559–66. doi: 10.1001/jama.2017.21904
13. van der Pol LM, Tromeur C, Bistervels IM, Ni Ainle F, van Bemmel T, Bertoletti L, et al. Pregnancy-adapted years algorithm for diagnosis of suspected pulmonary embolism. N Engl J Med. (2019) 380:1139–49. doi: 10.1056/NEJMoa1813865
14. Kirsch B, Aziz M, Kumar S, Burke M, Webster T, Immadi A, et al. Wells score to predict pulmonary embolism in patients with coronavirus disease 2019. Am J Med. (2021) 134:688–90. doi: 10.1016/j.amjmed.2020.10.044
15. Wang MF, Li FX, Feng LF, Zhu CN, Fang SY, Su CM, et al. Development and validation of a novel risk assessment model to estimate the probability of pulmonary embolism in postoperative patients. Sci Rep. (2021) 11:18087. doi: 10.1038/s41598-021-97638-0
16. Ji QY, Wang MF, Su CM, Yang QF, Feng LF, Zhao LY, et al. Clinical symptoms and related risk factors in pulmonary embolism patients and cluster analysis based on these symptoms. Sci Rep. (2017) 7:14887. doi: 10.1038/s41598-017-14888-7
17. Wang M, Zhang J, Ji Q, Yang Q, Zhao F, Li W, et al. Evaluation of platelet distribution width in chronic obstructive pulmonary disease patients with pulmonary embolism. Biomark Med. (2016) 10:587–96. doi: 10.2217/bmm.15.112
18. Grimes DA. The nomogram epidemic: resurgence of a medical relic. Ann Intern Med. (2008) 149:273–5. doi: 10.7326/0003-4819-149-4-200808190-00010
19. Levy DA Li H, Sterba KR, Hughes-Halbert C, Warren GW, Nussenbaum B, et al. Development and validation of nomograms for predicting delayed postoperative radiotherapy initiation in head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. (2020) 146:455–64. doi: 10.1001/jamaoto.2020.0222
20. Nolan MT, Creati L, Koczwara B, Kritharides L, Lynam J, Lyon AR, et al. First European Society of Cardiology Cardio-Oncology Guidelines: a big leap forward for an emerging specialty. Heart Lung Circ. (2022) 31:1563–7. doi: 10.1016/j.hlc.2022.11.003
21. Peyre H, Leplege A, Coste J. Missing data methods for dealing with missing items in quality of life questionnaires. a comparison by simulation of personal mean score, full information maximum likelihood, multiple imputation, and hot deck techniques applied to the Sf-36 in the French 2003 Decennial Health Survey. Qual Life Res. (2011) 20:287–300. doi: 10.1007/s11136-010-9740-3
22. Hu X, Shen F, Zhao Z, Qu X, Ye J. An individualized gait pattern prediction model based on the least absolute shrinkage and selection operator regression. J Biomech. (2020) 112:110052. doi: 10.1016/j.jbiomech.2020.110052
23. Narala S, Li SQ, Klimas NK, Patel AB. Application of least absolute shrinkage and selection operator logistic regression for the histopathological comparison of chondrodermatitis nodularis helicis and hyperplastic actinic keratosis. J Cutan Pathol. (2021) 48:739–44. doi: 10.1111/cup.13931
24. Yang S, Wu H. A novel Pm25 concentrations probability density prediction model combines the least absolute shrinkage and selection operator with quantile regression. Environ Sci Pollut Res Int. (2022) 29:78265–91. doi: 10.1007/s11356-022-21318-3
25. Wu J, Zhang H, Li L, Hu M, Chen L, Xu B, et al. A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: a population-based analysis. Cancer Commun. (2020) 40:301–12. doi: 10.1002/cac2.12067
26. Damodaram M, Kaladindi M, Luckit J, Yoong W. D-dimers as a screening test for venous thromboembolism in pregnancy: is it of any use? J Obstet Gynaecol. (2009) 29:101–3. doi: 10.1080/01443610802649045
27. Hassanin IM, Shahin AY, Badawy MS, Karam K. D-dimer testing versus multislice computed tomography in the diagnosis of postpartum pulmonary embolism in symptomatic high-risk women. Int J Gynaecol Obstet. (2011) 115:200–1. doi: 10.1016/j.ijgo.2011.05.024
28. Pauley E, Orgel R, Rossi JS, Strassle PD. Age-stratified national trends in pulmonary embolism admissions. Chest. (2019) 156:733–42. doi: 10.1016/j.chest.2019.05.021
29. Keller K, Beule J, Coldewey M, Dippold W, Balzer JO. Impact of advanced age on the severity of normotensive pulmonary embolism. Heart Vessels. (2015) 30:647–56. doi: 10.1007/s00380-014-0533-4
30. Keller K, Beule J, Balzer JO, Dippold W. Blood pressure for outcome prediction and risk stratification in acute pulmonary embolism. Am J Emerg Med. (2015) 33:1617–21. doi: 10.1016/j.ajem.2015.07.009
31. Quezada A, Jimenez D, Bikdeli B, Moores L, Porres-Aguilar M, Aramberri M, et al. Systolic blood pressure and mortality in acute symptomatic pulmonary embolism. Int J Cardiol. (2020) 302:157–63. doi: 10.1016/j.ijcard.2019.11.102
32. Ye W, Chen X, Li X, Guo X, Gu W. Arterial partial pressure of oxygen and diffusion function as prognostic biomarkers for acute pulmonary embolism. Respir Med. (2022) 195:106794. doi: 10.1016/j.rmed.2022.106794
33. Lei M, Liu C, Luo Z, Xu Z, Jiang Y, Lin J, et al. Diagnostic management of inpatients with a positive D-dimer test: developing a new clinical decision-making rule for pulmonary embolism. Pulm Circ. (2021) 11:2045894020943378. doi: 10.1177/2045894020943378
34. Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. (2012) 379:1835–46. doi: 10.1016/S0140-6736(11)61904-1
35. Shao C, Wang J, Tian J, Tang YD. Coronary artery disease: from mechanism to clinical practice. Adv Exp Med Biol. (2020) 1177:1–36. doi: 10.1007/978-981-15-2517-9_1
36. Jara-Palomares L, Otero-Candelera R, Elias-Hernandez T, Cayuela-Dominguez A, Ferrer-Galvan M, Alfaro MJ, et al. dyslipidemia as a long-term marker for survival in pulmonary embolism. Rev Port Pneumol. (2011) 17:205–10. doi: 10.1016/j.rppneu.2011.03.006
37. Rodríguez-Núñez N, Ruano-Raviña A, Lama A, Ferreiro L, Ricoy J, Álvarez-Dobaño JM, et al. Impact of cardiovascular risk factors on the clinical presentation and survival of pulmonary embolism without identifiable risk factor. J Thorac Dis. (2020) 12:5411–9. doi: 10.21037/jtd-20-1634
Keywords: pulmonary embolism, neurology department, retrospective analysis, risk assessment models, nomogram
Citation: Jianling Q, Lulu J, Liuyi Q, Lanfang F, Xu M, Wenchen L and Maofeng W (2023) A nomogram for predicting the risk of pulmonary embolism in neurology department suspected PE patients: A 10-year retrospective analysis. Front. Neurol. 14:1139598. doi: 10.3389/fneur.2023.1139598
Received: 07 January 2023; Accepted: 06 March 2023;
Published: 05 April 2023.
Edited by:
Wael M. Y. Mohamed, International Islamic University Malaysia, MalaysiaReviewed by:
Dorota Zyśko, Wroclaw Medical University, PolandCopyright © 2023 Jianling, Lulu, Liuyi, Lanfang, Xu, Wenchen and Maofeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wang Maofeng, d3ptY3dtZkAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.