- 1Department of Otolaryngology Head and Neck Surgery, The Second Affiliated Hospital of Nanchang University, Nanchang, China
- 2Division of Ear, Nose and Throat Section, Department of Clinical Science, Intervention and Technology, Karolinska University Hospital, Karolinska Institutet, Stockholm, Sweden
- 3Department of Otolaryngology Head and Neck & Audiology and Neurotology, Karolinska University Hospital, Karolinska Institutet, Stockholm, Sweden
Objectives: This study aimed to investigate the efficacy and safety of intratympanic or postauricular subperiosteal glucocorticoid injection combined with systemic glucocorticoid in the treatment of sudden sensorineural hearing loss (SSNHL).
Methods: This study is a prospective randomized controlled study. This study included unilateral SSNHL patients who were hospitalized in our department between January 2020 and June 2021. Patients were randomly divided into three groups (groups A, B, and C). Patients in group A were treated with an intratympanic corticosteroid injection combined with systemic corticosteroid treatment, and patients in group B received a postauricular corticosteroid injection combined with systemic corticosteroid treatment. Patients in group C (control group) were treated with systemic corticosteroid alone. The case number of groups A, B, and C was 311, 375, and 369, respectively.
Results: There was no significant difference in gender distribution, the proportion of left and right affected ears, and the average interval from onset to treatment among the three groups (P > 0.05). However, there were significant differences in their average age, distribution of audiogram type, and hearing loss levels among them (P < 0.01). Our study shows that there was no significant difference in average hearing threshold improvement before and after treatment in the three groups (P > 0.05). Regarding the complications, in group A, 33 patients (10.6%) had a transient vertigo attack during tympanic injection, which lasted for ~1–3 min. In group B, 20 patients (6.43%) complained of pain at the injection site, which disappeared after 1–3 days. No other complications occurred in all the other patients.
Conclusion: The addition of intratympanic or postauricular corticosteroid to systemic steroids did not result in a significant effect on hearing recovery in SSNHL. No obvious complications occur in SSNHL patients treated with intratympanic injection or postauricular injection of corticosteroid.
Clinical trial registration: [chictr.org.cn], registration number: ChiCTR2100048762.
Introduction
Sudden sensorineural hearing loss (SSNHL) is an idiopathic emergency disease. The recommended treatments for SSNHL do not target the etiology of SSNHL specifically. As a result, a large number of patients cannot be cured completely despite comprehensive treatments. Therefore, it is an urgent challenge for clinicians to improve the treatment efficacy of SSNHL.
The adopted treatments of SSNHL by clinicians include systemic and local application of corticosteroids, vasodilators, defibrinogenating agents, thrombolytics, neurotrophic drugs, antioxidants, antivirals, and hyperbaric oxygen therapy. Currently, the widely accepted effective treatments are systemic and local use of corticosteroids, which are recommended by the latest Chinese and American SSNHL diagnosis and treatment guidelines (1, 2). The pharmacological mechanism of corticosteroids in the treatment of SSNHL has not been fully clarified, including systemic and local effects. The systemic effect is a systemic immunosuppressive response. Regarding local effects, the glucocorticoid exerts effects by combining receptors in the inner ear. These local effects of glucocorticoid include maintaining ion homeostasis in the inner ear, antioxidation, inhibiting apoptosis, downregulating local pro-inflammatory cytokines, and increasing cochlear blood flow (3).
Intratympanic corticosteroid injection for treating SSNHL was used for the first time in 1996 by Silverstein et al. (4). Since then, many clinicians have used this technology. The corticosteroids of the tympanic cavity can penetrate into the inner ear through the round window membrane. This mechanism has been confirmed in many animal experiments which showed that this technology can produce higher drug concentration in the perilymph than intravenous or oral administration (5, 6). After intratympanic injection, the corticosteroids are mainly distributed in the spiral ligament, basement membrane, Organ of Corti, and spiral ganglion (7). A prospective randomized controlled study conducted by Rauch et al. revealed that the effect of tympanic injection of corticosteroid and systemic medication is equivalent (8). Moreover, narrative and systematic reviews showed that there was a lack of a high-quality study to confirm the effectiveness of intratympanic corticosteroid injection for treating SSNHL (9, 10).
Although the effects of corticosteroid tympanic injection alone for treating SSNHL are controversial, there is some evidence that this technology could be used as a salvage treatment for SSNHL patients whose hearing do not restore after 14-day systematic treatment (11, 12). Therefore, intratympanic corticosteroid therapy is recommended by the SSNHL guidelines both in China and the United States as the salvage treatment for SSNHL. In addition to intratympanic corticosteroid treatment, postauricular subperiosteal corticosteroid injection is recommended by the latest Chinese guideline as a salvage procedure. At present, the mechanism of the drug entering the inner ear using this technology is not entirely clear and the speculated routes include circulation and local penetration routes (13). It is presumed that corticosteroids could be absorbed into the circulation via postauricular capillaries and lymphatic capillaries and transported to the inner ear via its arterial supply, and may also be transported to the inner ear through the bone suture of the auditory vesicle. The drug enters the outer lymph and consequently forms the osmotic gradient between the endolymph and perilymph to exert effects. Currently, the postauricular subperiosteal corticosteroid injection procedure is not mentioned in the American guideline. Therefore, further studies with large sample sizes are needed to evaluate the efficacy of this treatment regimen.
Currently, few reports focus on the treatment effect of local combined with systemic corticosteroid therapy for SSNHL, and most studies have focused on the efficacy of local corticosteroid administration as a salvage treatment for treating SSNHL. However, it is difficult for many patients to return to the hospital several times to receive salvage treatment, and they expect to achieve the best curative effect in the shortest time during hospitalization. In order to evaluate the efficacy of this combined treatment regimen as an initial treatment for SSNHL, we conducted this prospective randomized study. All enrolled SSNHL patients were divided into three groups: intratympanic corticosteroid injection combined with a systemic corticosteroid, postauricular subperiosteal corticosteroid injection combined with a systemic corticosteroid, and systemic corticosteroid treatment alone (control group). Since the prognostic factors of SSNHL include age, the interval from onset to treatment, type of audiometric curve, and degree of hearing loss, we first compared the baseline of clinical characteristics and pre-treatment hearing results of the patients. Then, we compared the hearing efficacy between the patients of the three groups with different audiometric-type SSNHL.
Materials and methods
Patients
In this randomized controlled study, we enrolled SSNHL patients hospitalized in the Second Affiliated Hospital of Nanchang University from January 2020 to June 2021. All patients underwent routine physical examination, general otorhinolaryngological examination, nervous system physical examination, pure tone audiogram and tympanometry, laboratory examination, and brain magnetic resonance imaging (MRI) examination.
The study was performed in accordance with the ethical principles and approved by the Second Affiliated Hospital of Nanchang University Institutional Review Board. Written informed consent was obtained from all patients and/or their guardians.
Inclusion and exclusion criteria
Inclusion criteria
Inclusion criteria were as follows: (1) A diagnosis of unilateral SSNH. The diagnostic criteria were based on the latest guidelines revised by the American Academy of Otolaryngology-Head and Neck Surgery in 2019 (2). (2) The interval from onset to treatment was < 1 month.
Exclusion criteria
Exclusion criteria were as follows: (1) patients with hearing loss due to other causes such as otitis media, Meniere's disease, otosclerosis, congenital deafness, presbycusis, vestibular schwannoma, and inner ear malformation. (2) The interval from onset to treatment was more than 1 month. (3) Patients who did not undergo standard treatment for 14 days and were discharged without restoring to normal hearing. (4) Patients who had previously received other treatment. (5) Patients with bilateral SSNHL. (6) Patients with contraindications of systemic corticosteroids, such as diabetes, gastrointestinal ulcers, mental disorders, and epilepsy. (7) Patients who dropped out of the study or were lost in follow-up. (8) Patients with insufficient medical record data were also excluded.
Patients who met the inclusion criteria were randomly divided into three groups. The patients of group A underwent intratympanic corticosteroid injection combined with systemic corticosteroid treatment; patients of Group B received postauricular subperiosteal corticosteroid injection combined with systemic corticosteroid treatment; and patients of group C were treated only with systemic corticosteroid. Due to the loss of follow-up or lack of complete clinical data, 311, 375, and 369 patients were included in groups A, B, and C, respectively (Figure 1).
Test procedure
All patients underwent a detailed clinical interview. Clinical data, demographic information, past medical history, and personal history were obtained. Routine physical examination, otolaryngology examination, and audiological and laboratory tests were conducted in all subjects. MRI scanning of the ear and the brain was performed in all patients.
Treatment procedure
All patients received standard 14-day systemic treatment, which was based on the treatment recommended by the Chinese guidelines for SSNHL diagnosis and treatment revised in 2015 (1). All patients were prescribed 1 mg/kg of prednisone orally per day (maximum dose = 60 mg) for 3 days and were retested audiologically after that. If the treatment was effective, the patients continued to take prednisone for 2 more days; if no effect was seen on the audiogram, the treatment was discontinued on the fourth day. This treatment scheme was in accordance with the latest Chinese guidelines (1), which are revised on the basis of the 2010 German guidelines (14). This guideline recommended that SSNHL patients take prednisone for 3 days, with a total dose of 250 mg. Other medications included antioxidants, neurotrophic, and defibrinogenating agents. The treatment procedure is presented in Table 1.
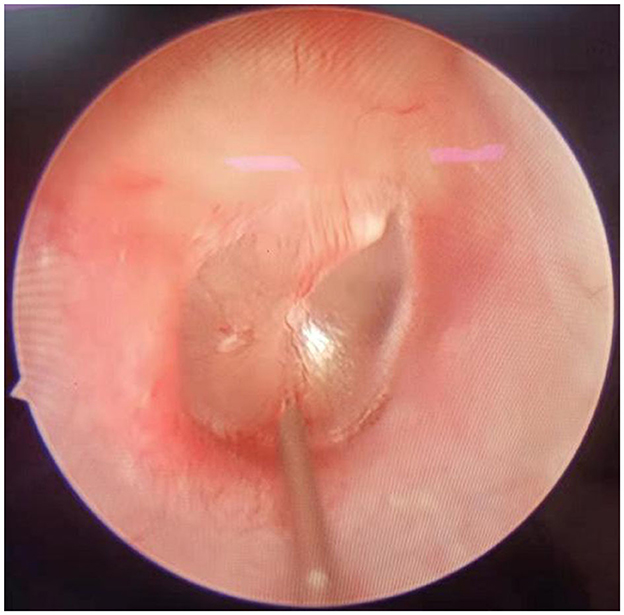
Patients of group A underwent intratympanic dexamethasone injection under otoendoscopy. The procedure was as follows: the patient lay in the lateral position with the ear to be injected upward. After local anesthesia with 1% tetracaine, 0.5 ml of physiological saline and 5 mg of dexamethasone were injected into the middle ear after puncture of the anteroinferior or posteroinferior part of the tympanic membrane (Figure 2). Then, the patient remained still for ~30 min after injection. This procedure was conducted on the first day of treatment and then once every 2 days, a total of four times.
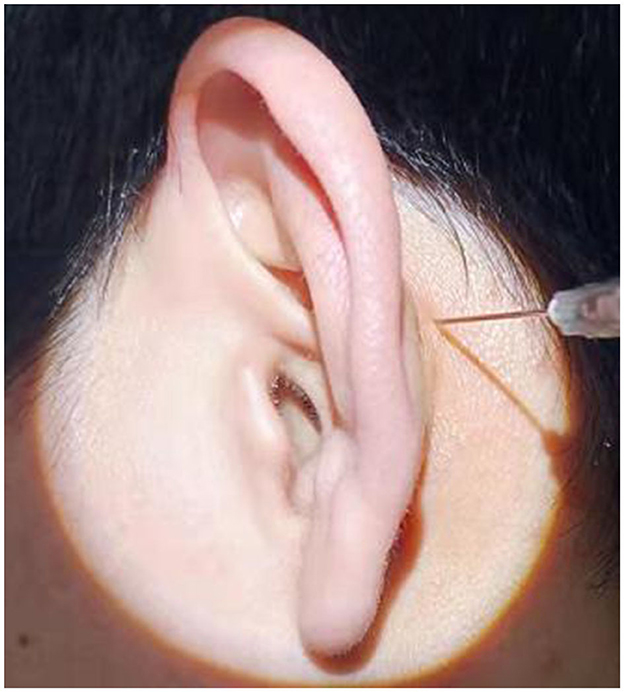
In group B, postauricular subperiosteal methylprednisolone injection was performed in all patients. The procedure was as follows: the patient sat on the chair. After the postauricular skin was disinfected, 40 mg (1 ml) of methylprednisolone was injected at the postauricular site. The injection site was located 0.5 cm behind the posterior sulcus of the affected ear and was level with the posterosuperior part of the external auditory meatus (Figure 3). After the injection, the injection point was compressed for 5 min. This procedure was performed on the first day of treatment and then once every 2 days, a total of four times.
All patients' hearing was evaluated with pure tone audiogram and tympanometry. All hearing tests were carried out by the same audiologist. Air and bone conduction was assessed at frequencies of 250 Hz, 500 Hz, 1 kHz, 2 kHz, 4 kHz, and 8 kHz.
Pure tone audiogram (PTA) was calculated by averaging air conduction thresholds at 0.5, 1, 2, and 4 kHz (15). The hearing loss levels were categorized into four grades: mild (26–40 dB HL), moderate (41–55 dB HL), moderate to severe (56–70 dB HL), severe (71–90 dB HL), and profound (>90 dB HL) (15). Audiogram patterns were classified into five types: ascending (the average threshold of 0.25–0.50 kHz was 20 dB higher than that of 4–8 kHz), descending (the average threshold of 4–8 kHz was 20 dB higher than that of 0.25–0.50 kHz), flat (all frequencies present similar thresholds and the hearing threshold was below 80 dB HL), profound (all frequencies show similar threshold and the hearing threshold was over 80 dB HL), and concave or convex type (average hearing degree of the mid-tone frequency was 20 dB higher than low and high frequencies) (1).
In addition to calculating patients' PTA, we evaluated patients' hearing by calculating the average air conduction hearing threshold at hearing impairment frequency. The patients' hearing of the affected ear was assessed by referring to the unaffected ears' hearing or their affected ears' initial hearing. Referring to the 2015 Chinese guidelines (1), the average air conduction hearing threshold at hearing impairment frequency was calculated as follows: among patients with flat-type or profound hearing loss, the hearing threshold at hearing impairment frequency was equivalent to the average hearing threshold of all frequencies. For the patients with low-frequency or high-frequency hearing loss, it was calculated as the average hearing threshold at hearing impairment low or high frequencies.
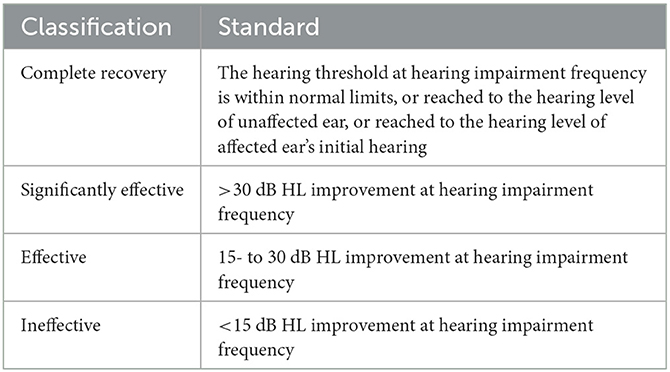
With reference to the Chinese guidelines for the diagnosis and treatment of SSNHL revised in 2015 (1), by comparing the hearing results before and 6 months after treatment, the hearing recovery of all patients was categorized into four grades: complete recovery, effective, significantly effective, and ineffective, as shown in Table 2.
Follow-up procedure
We tested the patients' hearing on the seventh day and 1 day before discharge after treatment, and retest their hearing immediately as long as they reported hearing improvement during hospitalization. If their hearing recovered, the treatment was terminated. If their hearing did not recover to normal, they would be instructed to continue taking 7.5 mg of Ginkgo biloba extract, 10 mg of vitamin B1, and 0.5 mg of Mecobalamin orally three times a day for 30 days. The second time of hearing examination was 30 days after discharge. If their hearing completely reached normal, drugs would be discontinued. If their hearing did not recover completely, the same cure would be given for 2 months. Three months after discharge, all treatments were terminated and patients revisited our hospital. The last follow-up time was 6 months after treatment, and their hearing was reevaluated and was taken as the final hearing result to evaluate the hearing effect. During the follow-up period, in addition to evaluating the patient's hearing, the general otorhinolaryngological examination and otoendoscopy were performed at each follow-up visit.
Statistical analysis
Quantitative data were presented as mean ± standard deviation for comparison, while frequency data were presented as cases and the ratio for comparison. An analysis of variance (ANOVA) was performed for the data conforming to normal distribution and homogeneity, while a Kruskal–Wallis test was conducted for those not conforming to normal distribution or homogeneity. Categorical data were shown as percentages and compared using the chi-square test. The Fisher exact test was used when expected counts in the chi-square test were insufficient. All analyses were conducted using SPSS version 25 for Windows. All statistical tests were two-sided, and statistically significant levels were set at a P-value of 0.05.
Result
Clinical characteristics and pre- and post-treatment hearing results of the patients in the three groups
Table 3 shows the clinical characteristics and post-treatment hearing results of the patients in the three groups. The case number of groups A, B, and C was 311, 375, and 369, respectively. The mean age of the patients in the three groups was 46.35 ± 15.104, 44.79 ± 13.893, and 41.85 ± 16.185 years. The number of male patients in the three groups was 151, 211, and 272, and the number of female patients in the three groups was 160, 164, and 197, respectively. There was a significant difference in the average ages of the three groups (P < 0.01). However, no significant difference existed in patients' gender distribution, the proportion of affected left or right ears, and the average interval from onset to treatment between the three groups (P > 0.05).
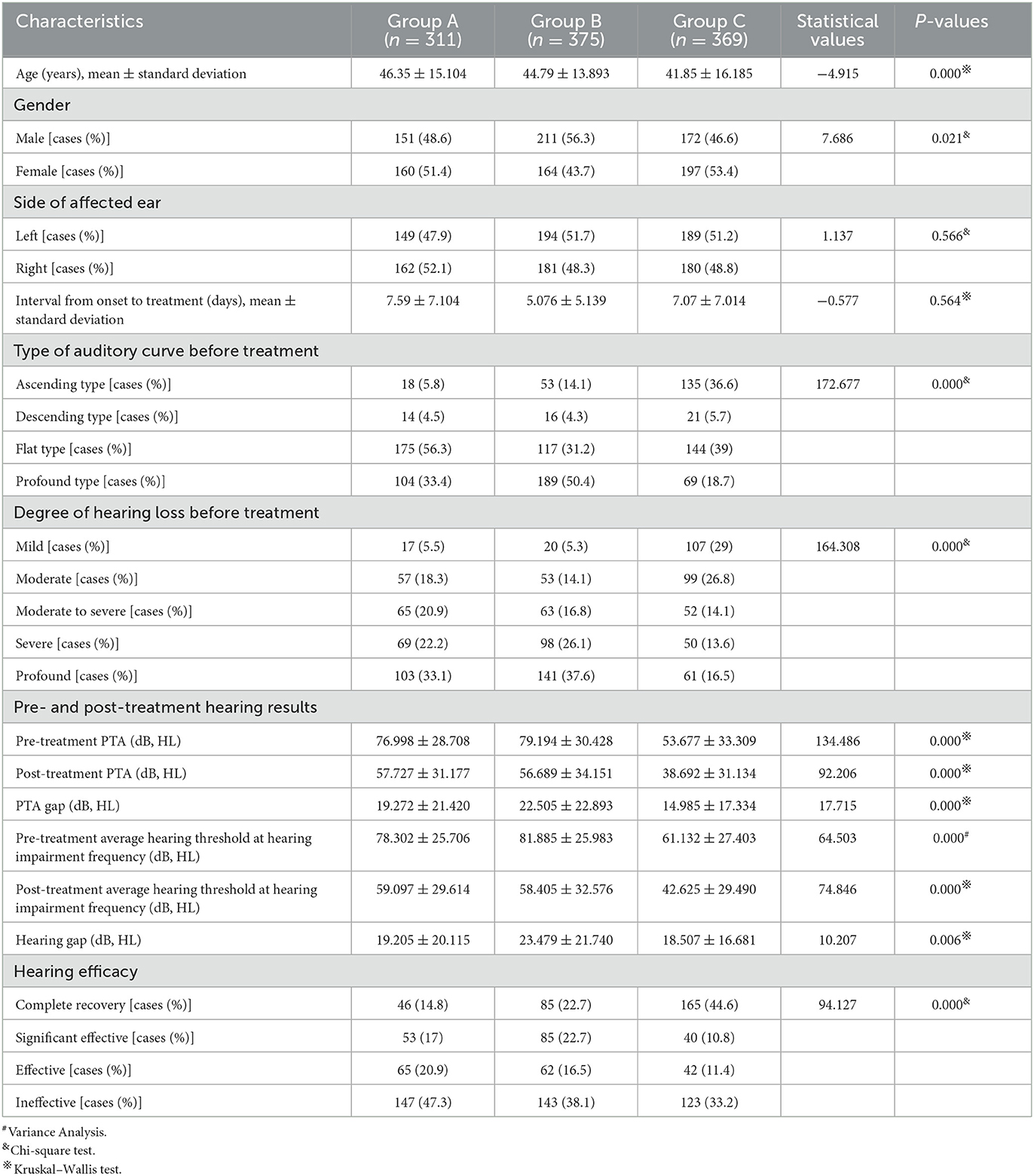
Table 3. Clinical characteristics and pre- and post-treatment hearing of patients of the three groups.
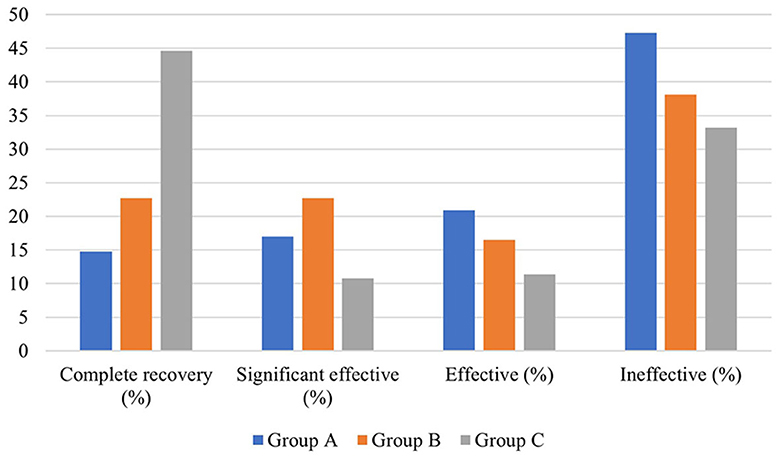
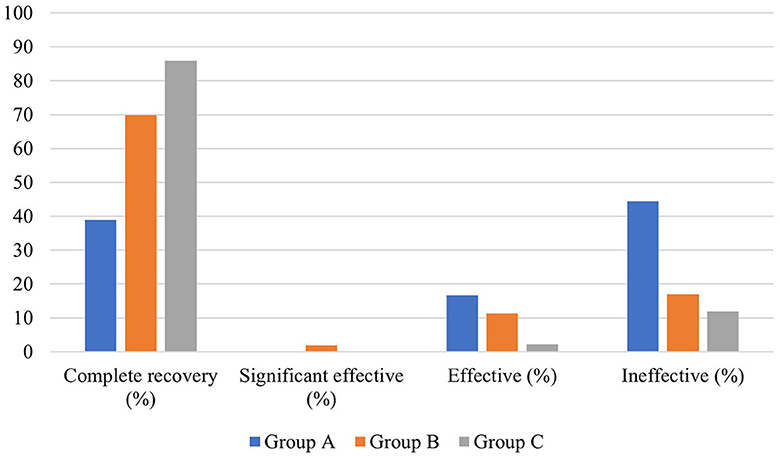
In terms of hearing results, a significant difference existed in the distribution of pre-treatment auditory curve type and degree of hearing loss among the three groups (P < 0.01). The pre- and post-treatment PTA and the PTA gap of the patients in the three groups were also significantly different (P < 0.05). The biggest pre- and post-treatment PTA gap existed in group B, followed by group A and group C. In addition, the pre- and post-treatment average hearing thresholds at the impairment frequency in patients of the three groups were significantly different (P < 0.05). As for the pre- and post-treatment gap of hearing thresholds at hearing impairment frequency, the largest one existed in group B (23.479 ± 21.740 dB HL), followed by group A (19.205 ± 20.115 dB, HL) and group C (18.507 ± 16.681 dB, HL). This gap was statistically significant (P < 0.05). In terms of grading of hearing efficacy, the highest one was in patients of group C (66.8%), followed by group B (61.9%) and group A (52.7%) (Figure 4).
Efficacy of different topical corticosteroid administration methods in patients with different auditory types
The baseline of patients in the three groups including the average age, gender distribution, and the distribution of auditory types and hearing degrees was inequivalent, which may affect the post-treatment hearing effect. Therefore, we analyzed the efficacy of SSNHL patients with different audiogram-type SSNHL.
Efficacy of different local corticosteroids combined with systemic corticosteroid administration in patients with ascending-type SSNHL
There was no significant difference in average age, gender distribution, and average interval from onset to treatment among patients in the three groups (P > 0.05). In terms of hearing results, a significant difference existed in the pre- and post-treatment average hearing threshold at the hearing impairment frequency among the patients in the three groups (P < 0.05), but no significant difference was observed in the gap between pre- and post-treatment average hearing threshold at the hearing impairment frequency (P > 0.05), which were 17.454 ± 15.166 dB, 22.296 ± 14.959 dB, and 18.91 ± 13.142 dB, respectively. However, there was a significant difference in the proportion of patients with different hearing efficacy grading in the three groups (P < 0.05). Furthermore, the hearing recovery rate of patients in group C was higher than those in the other two groups (Table 4, Figure 5).
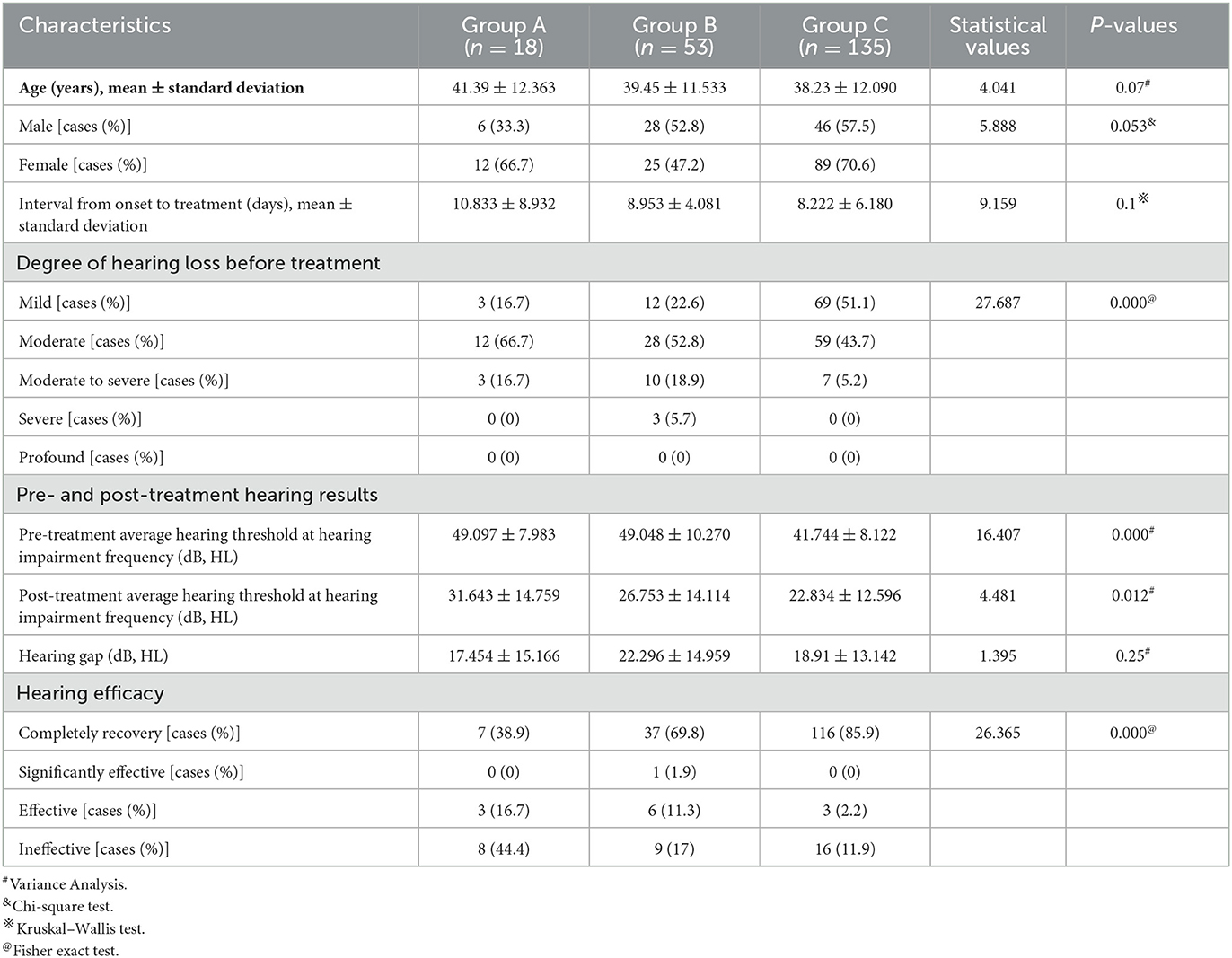
Table 4. Clinical characteristics and hearing efficacy of patients with ascending-type SSNHL in the three groups.
Efficacy of different local glucocorticoids combined with systemic glucocorticoid administration in patients with descending-type SSNHL
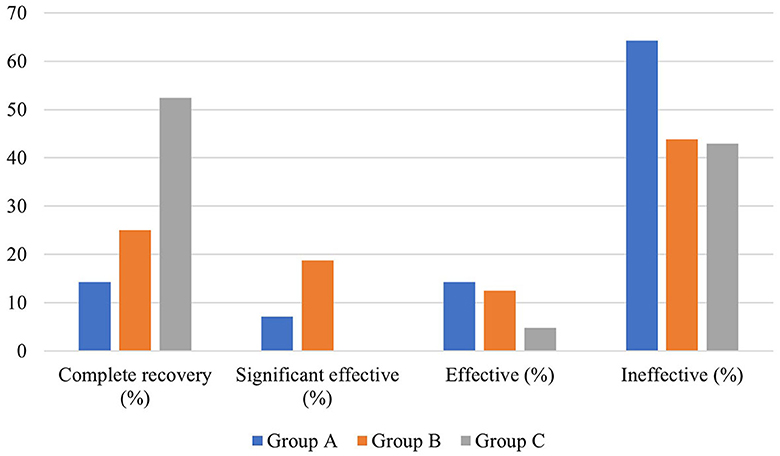
There was no significant difference in the average age, gender distribution, average interval from onset to treatment, and distribution of pre-treatment hearing loss degree among patients in the three groups (P > 0.05). In addition, no significant difference existed in the pre- and post-treatment average hearing threshold at the hearing impairment frequency among patients of the three groups (P > 0.05), as well as in the gap of the pre- and post-treatment average hearing threshold at the hearing impairment frequency between them (P > 0.05). Similarly, there was no significant difference in the proportion of patients with different hearing efficacy grading among patients in the three groups (P > 0.05) (Table 5, Figure 6).
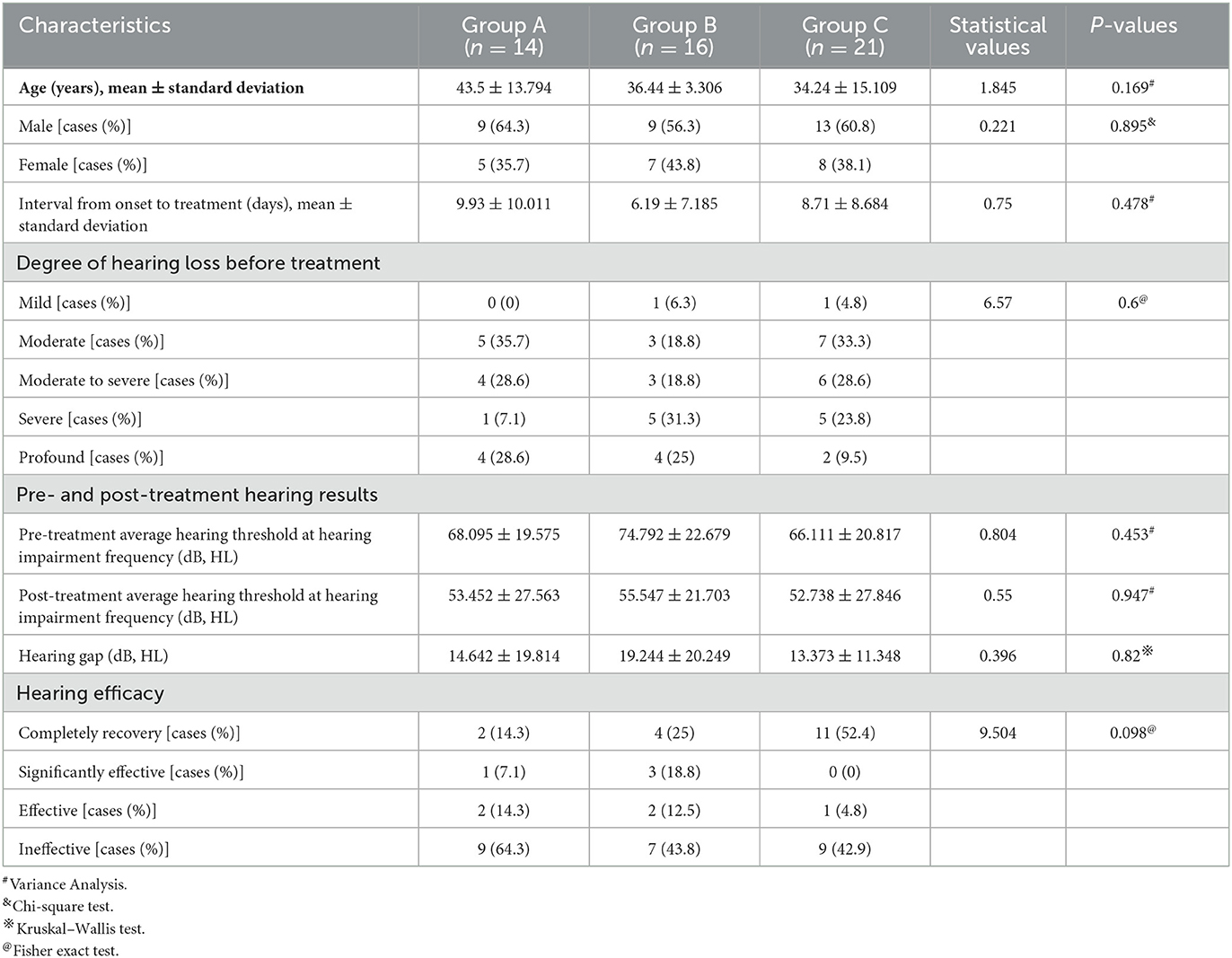
Table 5. Clinical characteristics and hearing efficacy of patients with descending-type SSNHL in the three groups.
Efficacy of different local corticosteroids combined with systemic corticosteroid administration in patients with flat-type SSNHL
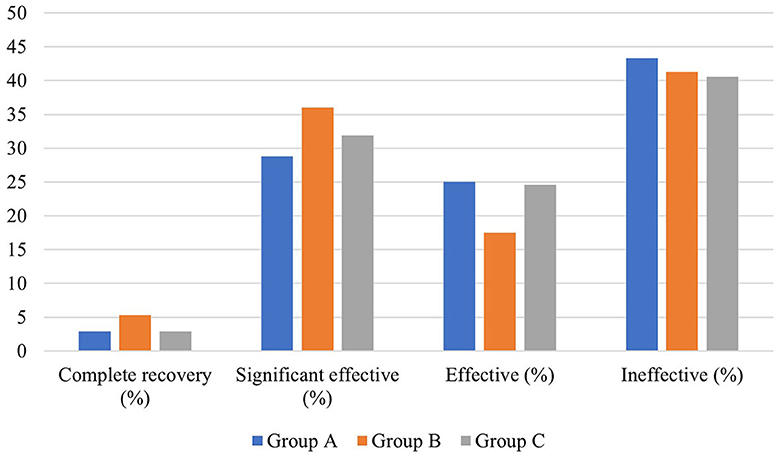
There was no significant difference in average age, gender distribution, and average interval from onset to treatment among patients in the three groups (P > 0.05), but there was a significant difference in the degree of hearing loss before treatment and pre- and post-treatment average hearing threshold at the hearing impairment frequency among patients in the three groups (P < 0.05). However, no significant difference was observed in the gap between the pre- and post-treatment average hearing threshold at the hearing impairment frequency (P > 0.05). Moreover, there was no significant difference in the proportion of patients with different hearing efficacy grading among patients in the three groups (P > 0.05) (Table 6, Figure 7).
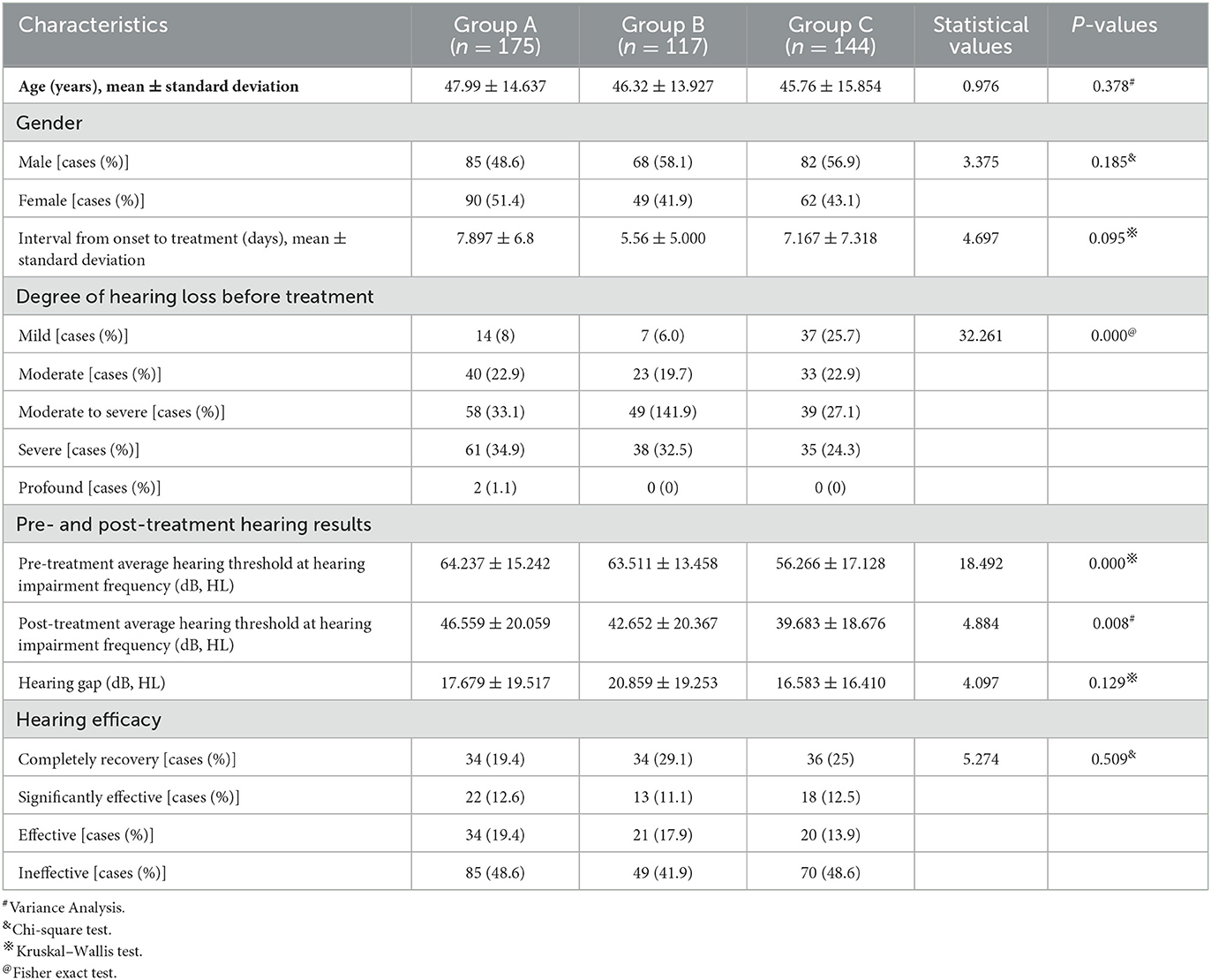
Table 6. Clinical characteristics and hearing efficacy of patients with flat-type SSNHL in the three groups.
Efficacy of different local corticosteroids combined with systemic corticosteroid administration in patients with profound SSNHL
There was no significant difference in average age, gender distribution, and average interval from onset to treatment among patients in the three groups (P > 0.05). Regarding the hearing result, there was a significant difference in the pre-treatment average hearing threshold at the hearing impairment frequency (P < 0.05), but no significant difference in the post-treatment average hearing threshold at the hearing impairment frequency, as well as the pre- and post-treatment gap at the hearing impairment frequency between the three groups (P > 0.05). Moreover, there was no significant difference in the proportion of patients with different hearing efficacy grading among patients in the three groups (P > 0.05) (Table 7, Figure 8).
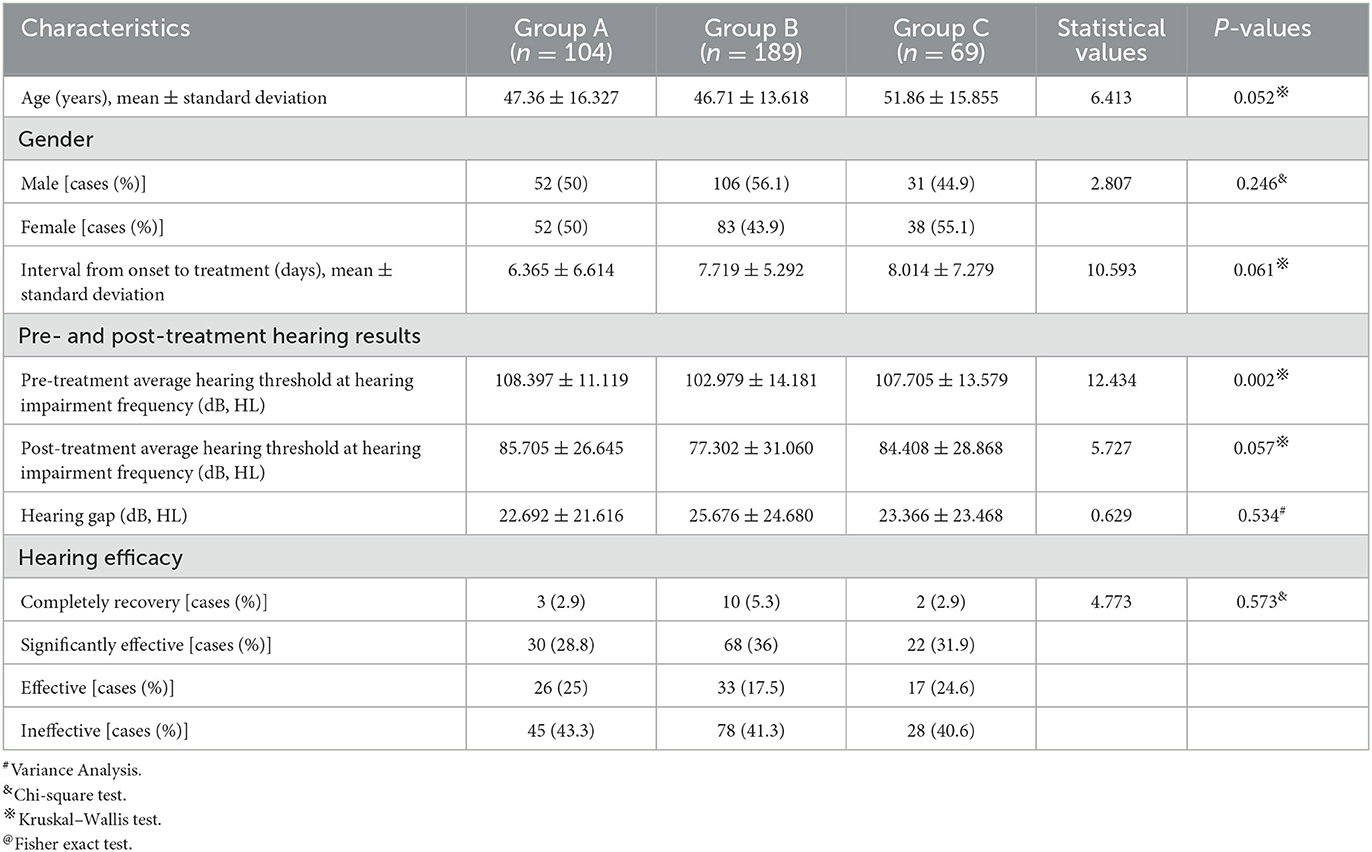
Table 7. Clinical characteristics and hearing efficacy of patients with profound SSNHL in the three groups.
Complications and follow-up results
In group A, 33 patients (10.6%) had a transient vertigo attack during tympanic injection, which lasted for ~1–3 min; 20 patients felt earache, lasting for 1–3 days. In group B, 18 patients (4.8%) complained of swelling and pain at the injection site, which disappeared within 2 days. No obvious complications such as tympanic membrane perforation or acute and chronic otitis media occurred in patients of group A during follow-up. In addition, there were no complications in all other patients during follow-up.
Discussion
The possible prognostic factors of SSNHL include the patient's age, the audiogram type, the degree of hearing loss, and the interval from onset to treatment (1, 16). The cofactors including the average age, gender distribution, and the distribution of auditory types and hearing degrees in the three groups were inequivalent, so we analyzed the efficacy of SSNHL patients with different audiogram-type SSNHL. It is reported that comparing patients with other types of audiometric curves, patients with low-frequency hearing loss may have a better prognosis (1, 17). In 2015, a Chinese multicenter study showed that the curative rate of patients with low-frequency hearing loss could be as high as 90.73%, whereas, in patients with high-frequency hearing loss, the number was only 65.96% (18).
The pathogenesis of SSNHL with various audiogram types may be different, which may explain this discrepancy. For example, the proposed pathogenesis of low-frequency hearing loss may be inner ear hydrops; the high-frequency hearing loss may be due to hair cell injury; the flat-type hearing loss is mostly caused by stria vascularis dysfunction or inner ear vasospasm; and the etiology of profound hearing loss is inner ear vascular embolism or thrombosis (14).
The effectiveness of intratympanic injection combined with systemic corticosteroids for treating SSNHL is still controversial. Several researchers showed that the effect of intratympanic combined with systemic corticosteroid therapy was better than that of systemic corticosteroid administration alone (19, 20). For example, a recent meta-analysis study result showed that the treatment effects ranked from high to low were as follows: intravenous, intratympanic corticosteroid, intravenous combined with an oral corticosteroid, intratympanic corticosteroid, intravenous corticosteroid, oral corticosteroid, and placebo (19). The results of animal experiments demonstrated that through intratympanic corticosteroid route, more drugs could be delivered into the inner ear, longer therapeutic window and more effective result could be achieved than by intravenous or intratympanic injection route alone in the pharmacokinetics (21). Therefore, researchers concluded that the curative effect of the combination of corticosteroid might be better than that of intravenous or intratympanic corticosteroid therapy alone. Indeed, theoretically, a blood–labyrinth barrier may limit the therapeutic agents permeating into the inner ear, and combined corticosteroid administration may reach higher drug concentrations and better effects. However, a prospective randomized study showed that the recovery rates in the patients with combined treatment and intratympanic injection treatment were 70 and 73%, respectively, and no significant difference existed between the patients of the two groups (3). In addition, another systematic meta-analysis also confirmed that the effect of combined therapy was equivalent to that of systemic or intratympanic injection of corticosteroid alone, and the hearing effect of intratympanic injection was similar to systemic corticosteroid among patients with moderate and severe SSNHL (22). Furthermore, our study results demonstrated that there was no significant difference between the efficacy of intratympanic injection combined with systemic corticosteroid treatment and the control group, suggesting that systemic corticosteroid treatment can achieve a similar efficacy as combined treatment as an initial treatment. We speculate that the reason for this phenomenon is that inner ear hormone receptors are saturated after patients receive a sufficient dose of systemic corticosteroid treatment, so the additional local medication has no additional effect.
Intratympanic corticosteroid injection has its own advantages. The first one is that it can avoid the side effects of systemic corticosteroid administration, which is contraindicated in patients with diabetes, hypertension, tumor, infection, and acute stage of peptic ulcer. Second, intratympanic injection is easy to operate. However, this technology is an invasive operation and may result in some local complications, such as pain, dizziness, and secondary acute or chronic otitis media. Therefore, it is necessary to select treatment strategies according to the patient's own circumstances. For example, for patients with contraindications of systemic administration of corticosteroids, local administration of corticosteroids can be taken as a priority.
Regarding the type of corticosteroid selected for intratympanic injection, dexamethasone, methylprednisolone, and prednisone are most used by medical institutions. Animal experiment results demonstrated that methylprednisolone has the highest permeability after intratympanic injection compared with dexamethasone and prednisone (5). However, the degree and incidence of pain response in patients after intratympanic methylprednisolone injection were significantly higher than those of patients who underwent intratympanic dexamethasone injection (23). Before we carried out this study, we also initially applied intratympanic methylprednisolone injection for SSNHL patients and found that many patients complained of unbearable earache. Some patients even suffered from tympanic perforation and ear pus. In contrast, a recent meta-analysis study demonstrated that as a salvage treatment for SSNHL, intratympanic injection of dexamethasone was more effective than methylprednisolone (24). Therefore, we chose dexamethasone as the intratympanic injection agent for treating SSNHL. Referring to the therapeutic schedule reported in the previous literature and recommended by the guidelines, we performed an intratympanic injection of 5 mg dexamethasone once a day, four times in total for SSNHL patients.
As a new treatment scheme for SSNHL, postauricular corticosteroid injection has been more and more valued by otologists. This scheme is recommended by the 2015 Chinese guideline for the diagnosis and treatment of SSNHL (1) but is not widely applied worldwide. Both animal and clinical studies have verified the effectiveness of the postauricular injection route. Wang et al. (25) explored the cochlear concentration and distribution of dexamethasone after administration by intratympanic, post-aural, and intraperitoneal methods. They found that intratympanic and post-aural administration could result in higher dexamethasone concentrations in the Organ of Corti than systemic administration, but systemic administration could produce higher dexamethasone concentrations in the stria vascularis than the other administration methods. Li et al. (13) conducted an animal study by using 7.0 Testa magnetic resonance imaging for guinea pigs after postauricular and intravenous injection of gadopentetate dimeglumine (Gd). They measured the relative signal intensity in the scala tympani of the basal turn to evaluate indirectly the dynamic Gd uptake in the perilymph. They found a delayed time to peak enhancement, prolonged elimination half-life, extended mean residence time, and a greater area under the signal–time curve among postauricularly treated guinea pigs. This study's results indicate that the bioavailability of drugs may increase to a certain extent and achieve a better effect through the postauricular injection route than systemic administration. In clinical studies, a retrospective study that enrolled 63 refractory SSNHL patients has revealed that the patients who underwent postauricular corticosteroid administration as a salvage treatment demonstrated better results than untreated patients. The most frequent adverse event was injection pain; other major adverse events included sleep change, increase in blood glucose, and headache (26). Moreover, another multicenter clinical study that enrolled 173 SSNHL patients showed that the clinical efficacy of intratympanic steroid perfusion and postauricular steroid injection was similar for refractory severe and profound SSNHL patients (27). In contrast, compared with intratympanic injection, the postauricular injection has its own advantages. It is simple to operate and less likely to generate complications. However, due to the small number of literature reporting the effect of this technology currently, its effectiveness is uncertain. In future, more studies are needed to further investigate the efficacy and safety of this technology in SSNHL treatment. Although complications might be more likely to occur by applying the intratympanic injection route, our clinical practice shows that this technology is a safe procedure, since no patient suffered from severe complications during the follow-up period. In order to ensure the effectiveness of the medication, for patients with contraindications to systemic medication, intratympanic injection route is still a top priority currently.
The limitation of this study is that after stratification, the sample size of subjects in each subgroup is limited, although our study has a large sample size. Therefore, it is difficult to ensure the equivalence of the baseline such as age or pre-treatment hearing level between each group, which may generate methodological bias and affect the accuracy of the results. Nevertheless, since the baselines of most subgroups are equivalent, our results are convincing. In contrast, in order to ensure the treatment effect, patients received a combination of multiple therapies such as antioxidants, neurotrophic agents, and defibrinogen, which may also result in heterogeneity. In addition, a randomized blind placebo control multicenter study is necessary before we make a clear conclusion.
Conclusion
The exact role of intratympanic and postauricular corticosteroids requires additional trials. The addition of intratympanic or postauricular corticosteroid to systemic steroids did not result in a significant effect on hearing recovery in SSNHL. However, for patients with contraindications to systemic corticosteroid administration, intratympanic corticosteroid or postauricular corticosteroid may be safe and efficacious alternatives.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Second Affiliated Hospital of Nanchang University Institutional Review Board. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
MD and YL conceived and designed the study. WX, NK, JL, and HP performed the experiments. CL and ZZ collected data. WX, NK, and MD wrote the manuscript. All authors contributed to the writing and final approval of the manuscript.
Funding
This study was supported by the project of the Science and Technology Department of Jiangxi Province (20202BBGL73017).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery; Society of Otorhinolaryngology Head and Neck Surgery CMA. Guideline of diagnosis and treatment of sudden deafness (2015). Zhonghua er bi Yan Hou Tou Jing Wai ke Za Zhi. (2015) 50:443–7.
2. Chandrasekhar SS, Tsai Do BS, Schwartz SR, Bontempo LJ, Faucett EA, Finestone SA, et al. Clinical practice guideline: sudden hearing loss (update). Otolaryngol Head Neck Surg. (2019) 161:S1–45. doi: 10.1177/0194599819859885
3. Ahn JH, Yoo MH, Yoon TH, Chung JW. Can intratympanic dexamethasone added to systemic steroids improve hearing outcome in patients with sudden deafness? Laryngoscope. (2008) 118:279–82. doi: 10.1097/MLG.0b013e3181585428
4. Haynes DS, O'Malley M, Cohen S, Watford K, Labadie RF. Intratympanic dexamethasone for sudden sensorineural hearing loss after failure of systemic therapy. Laryngoscope. (2007) 117:3–15. doi: 10.1097/01.mlg.0000245058.11866.15
5. Parnes LS, Sun AH, Freeman DJ. Corticosteroid pharmacokinetics in the inner ear fluids: an animal study followed by clinical application. Laryngoscope. (1999) 109:1–17. doi: 10.1097/00005537-199907001-00001
6. Chandrasekhar SS. Intratympanic dexamethasone for sudden sensorineural hearing loss: clinical and laboratory evaluation. Otol Neurotol. (2001) 22:18–23. doi: 10.1097/00129492-200101000-00005
7. Hargunani CA, Kempton JB, DeGagne JM, Trune DR. Intratympanic injection of dexamethasone: time course of inner ear distribution and conversion to its active form. Otol Neurotol. (2006) 27:564–9. doi: 10.1097/01.mao.0000194814.07674.4f
8. Rauch SD, Halpin CF, Antonelli PJ, Babu S, Carey JP, Gantz BJ, et al. Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss: a randomized trial. JAMA. (2011) 305:2071–9. doi: 10.1001/jama.2011.679
9. Doyle KJ, Bauch C, Battista R, Beatty C, Hughes GB, Mason J, et al. Intratympanic steroid treatment: a review. Otol Neurotol. (2004) 25:1034–9. doi: 10.1097/00129492-200411000-00031
10. Hu A, Parnes LS. Intratympanic steroids for inner ear disorders: a review. Audiol Neurootol. (2009) 14:373–82. doi: 10.1159/000241894
11. Choi JW, Lee CK, Kim SB, Lee DY, Ko SC, Park KH, et al. Potential benefits of salvage intratympanic dexamethasone injection in profound idiopathic sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol. (2020) 277:2219–27. doi: 10.1007/s00405-020-05967-z
12. Amarillo E, Hernando M, Eisenberg G, Granda M, Plaza G. Efficacy of intratympanic corticosteroid as a salvage treatment in idiopathic sudden sensorineural hearing loss. Acta Otorrinolaringol Espanola. (2019) 70:207–14. doi: 10.1016/j.otoeng.2018.04.006
13. Li J, Yu L, Xia R, Gao F, Luo W, Jing Y. Postauricular hypodermic injection to treat inner ear disorders: experimental feasibility study using magnetic resonance imaging and pharmacokinetic comparison. J Laryngol Otol. (2013) 127:239–45. doi: 10.1017/S0022215113000017
14. Ganzer U, Albegger KW, Arnold W. Leitlinie ‘Hörsturz'. Konsensusbericht in Auftrag des präsidium der Deutschen Gesellschaft für Hals-Nasen-Ohren-Heikunde, Kopf-und Hals-Chirurgie. HNO Information. (2004) 4:302–8.
16. Kang WS, Yang CJ, Shim M, Song CI, Kim TS, Lim HW, et al. Prognostic factors for recovery from sudden sensorineural hearing loss: a retrospective study. J Audiol Otol. (2017) 21:9–15. doi: 10.7874/jao.2017.21.1.9
17. Tripathi P, Deshmukh P. Sudden sensorineural hearing loss: a review. Cureus. (2022) 14:e29458. doi: 10.7759/cureus.29458
18. Chinese Sudden Hearing Loss Multi-Center Clinical Study Group.Prospective clinical multi-center study on the treatment of sudden deafness with different typings in China. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2013) 48:355–61.
19. Ahmadzai N, Kilty S, Cheng W, Esmaeilisaraji L, Wolfe D, Bonaparte JP, et al. A systematic review and network meta-analysis of existing pharmacologic therapies in patients with idiopathic sudden sensorineural hearing loss. PLoS ONE. (2019) 14:e0221713. doi: 10.1371/journal.pone.0221713
20. Li J, Ding L. Effectiveness of steroid treatment for sudden sensorineural hearing loss: a meta-analysis of randomized controlled trials. Ann Pharmacother. (2020) 1060028020908067. doi: 10.1177/1060028020908067
21. Li Y, Kanzaki S, Shibata S, Nakamura M, Ozaki M, Okano H, et al. Comparison of inner ear drug availability of combined treatment with systemic or local drug injections alone. Neurosci Res. (2020) 155:27–33. doi: 10.1016/j.neures.2019.07.001
22. Mirian C, Ovesen T. Intratympanic vs systemic corticosteroids in first-line treatment of idiopathic sudden sensorineural hearing loss: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. (2020) 146:1–8. doi: 10.1001/jamaoto.2020.0047
23. Demirhan H, Gökduman AR, Hamit B, Yürekli Altindag MF, Yigit Ö. Contribution of intratympanic steroids in the primary treatment of sudden hearing loss. Acta Otolaryngol. (2018) 138:648–51. doi: 10.1080/00016489.2018.1438660
24. Ng JH, Ho RC, Cheong CS, Ng A, Yuen HW, Ngo RY. Intratympanic steroids as a salvage treatment for sudden sensorineural hearing loss? A meta-analysis. Eur Arch Otorhinolaryngol. (2015) 272:2777–82. doi: 10.1007/s00405-014-3288-8
25. Wang Y, Han L, Diao T, Jing Y, Wang L, Zheng H, et al. A comparison of systemic and local dexamethasone administration: from perilymph/cochlea concentration to cochlear distribution. Hear Res. (2018) 370:1–10. doi: 10.1016/j.heares.2018.09.002
26. Ren G, Xu J, Lan L, Ma B, Zhang Q. Postauricular injection of methylprednisolone sodium succinate as a salvage treatment for refractory sudden sensorineural hearing loss. Ir J Med Sci. (2021) 190:1165–72. doi: 10.1007/s11845-021-02610-6
Keywords: sudden sensorineural hearing loss, intratympanic injection, postauricular injection, corticosteroid, treatment
Citation: Xie W, Karpeta N, Liu J, Peng H, Li C, Zhang Z, Liu Y and Duan M (2023) Efficacy of intratympanic or postauricular subperiosteal corticosteroid injection combined with systemic corticosteroid in the treatment of sudden sensorineural hearing loss: A prospective randomized study. Front. Neurol. 14:1138354. doi: 10.3389/fneur.2023.1138354
Received: 05 January 2023; Accepted: 13 March 2023;
Published: 06 April 2023.
Edited by:
Kathrine Jauregui-Renaud, Mexican Social Security Institute (IMSS), MexicoReviewed by:
Haibo Shi, Shanghai Jiao Tong University, ChinaNorma De Oliveira Penido, Federal University of São Paulo, Brazil
Daogong Zhang, Shandong Provincial ENT Hospital, China
Copyright © 2023 Xie, Karpeta, Liu, Peng, Li, Zhang, Liu and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuehui Liu, bGl1eXVlaHVpY2xhcmtAMjFjbi5jb20=; Maoli Duan, bWFvbGkuZHVhbkBraS5zZQ==
 Wen Xie
Wen Xie Niki Karpeta
Niki Karpeta Jiali Liu
Jiali Liu Haisen Peng
Haisen Peng Chunhua Li
Chunhua Li Zhiling Zhang
Zhiling Zhang Yuehui Liu
Yuehui Liu Maoli Duan
Maoli Duan