
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Neurol., 22 February 2023
Sec. Neuroinfectious Diseases
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1137024
This article is part of the Research TopicThe NeuroCOVID-19 Syndrome: Cognitive and Psychological Profiles, Physiopathology, and Impact on Neurologically Vulnerable PopulationsView all 11 articles
The COVID-19 pandemic and the associated post-acute sequelae of COVID-19 (PASC) have led to the identification of a complex disease phenotype that is associated with important changes in the immune system. Herein, we describe a unique case of Nocardia farcinica cerebral abscess in an individual with sudden immunodeficiency several months after mild COVID-19. Intravenous Bactrim and Imipenem were prescribed for 6 weeks. After this, a 12-month course of Bactrim and Clavulin was prescribed to be taken orally, given the N. farcinica infection at the level of the central nervous system. This case report highlights the need for future research into the pathophysiology of COVID-19 and PASC immune dysregulation in convalescent individuals. It also draws attention to the need for timely consideration of opportunistic infections in patients with a history of COVID-19.
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV2) is the causal agent of the coronavirus disease 2019 (COVID-19) global pandemic (1). Alarmingly, long COVID or post-acute sequelae of COVID-19 (PASC) can occur several weeks after infection in a subset of individuals and englobes a multitude of health problems (2, 3). This multisystem disease can arise after severe, mild, or even asymptomatic SARS-CoV2 infection (4, 5) and is characterized by the persistence or onset of new chronic symptoms lasting longer than what is ordinary in most cases of viral infection (6, 7). Indeed, this post-infectious syndrome draws a unique parallel with Ebola and SARS-CoV-1 (8, 9), wherein a long-lasting dysregulation of the immune system is observed long after the infection has cleared (10). Notably, flow cytometry analysis of COVID-19 convalescent individuals, both hospitalized and non-hospitalized, demonstrated that numerous adaptive and innate immune cells were decreased, and activation/exhaustion markers were elevated in T- and B-cell populations (11). Significant lymphopenia (CD4+ and CD8+ cells) in convalescent individuals were also identified by others (10, 12), and these changes in the peripheral immune system could potentially influence how individuals respond to other infections during this post-COVID-19 timeframe (10), potentially rendering some patients in a state of immunodeficiency.
Individuals may become immunocompromised secondary to underlying malignancies, cancer therapeutics, human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS), in situations of organ transplant, and after receiving a prolonged corticosteroid regimen (13, 14). Immunocompromised individuals are particularly vulnerable to infections, notably nocardiosis for which the causal agent is Nocardia species, a ubiquitous soil-dwelling Gram-positive bacteria. Nocardia asteroids are most associated with human disease (15); however, the less common Nocardia farcinica is associated with a higher risk of dissemination, drug resistance, and by extension, a higher mortality rate (16–18). The lungs are the primary site of Nocardia spp. infection and, when limited to the lung, can be treated with antibiotic treatment. This is however not the case when immunosuppression is prolonged, and secondary sites of infection are established (14); in such cases, bacteremia may later manifest as brain abscesses (19). Mortality rates in the central nervous system involvement range from 40 to 87%, despite therapeutic interventions (20, 21).
Herein, we describe a unique case of N. farcinica cerebral abscess in an individual with sudden immunodeficiency several months after mild COVID-19.
A male patient in his 50s with a history of high blood pressure, duodenal ulcer, dyslipidemia, and a history of smoking and alcoholism presented at the emergency with left-sided transient hemiparesis. Magnetic resonance imaging showed an enhancing lesion involving the high convexity on the right of the frontal lobe measuring ~1.9 cm × 1.7 cm, associated with marked adjacent vasogenic edema (Figures 1A, B). A biopsy of the lesion highlighted brain parenchyma with reactive gliosis and no significant findings except the growth of Propionibacterium acnes in broth cultures. On repeat imaging a couple of weeks later, there was an increase in the size (2.9 cm) of the ring-enhancing posterior right frontal/anterior parietal lobe, as well as a new ring-enhancing posterior right frontal lobe lesion measuring 1.1 cm, superior to the lesion described earlier (Figure 1B). There was also central restricted diffusion. A neurosurgical biopsy showed gliotic brain tissue, necrotic debris, neutrophils, and macrophages within the abscess. Beaded, filamentous bacilli were detected and later confirmed on growth media by Public Health Laboratory to be N. Farcinica (Figures 2A, B). Susceptibility results confirmed amoxicillin/clavulanic acid, Imipenem, and trimethoprim–sulfamethoxazole as potentially efficacious against the harvested strain (Supplementary Figure 1).
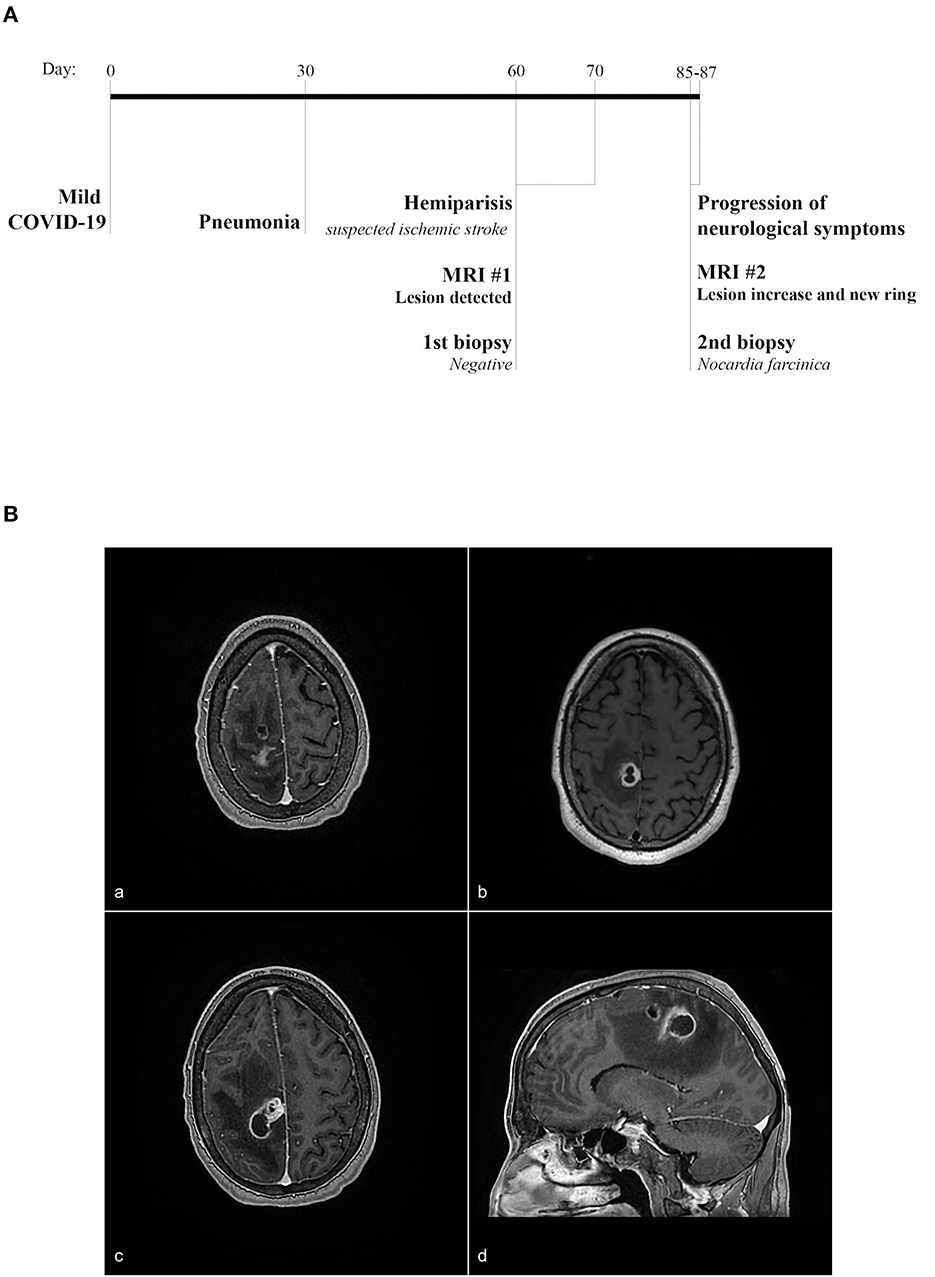
Figure 1. (A) Timeline of an episode of care. The patient contracted COVID-19 with mild symptomatology. Thirty days later, the patient was diagnosed with pneumonia and at day 60, presented with hemiparesis. MRI revealed a cerebral lesion. At day 85, the patient presented worsening neurological symptoms and a second MRI showed an additional lesion as well as an increased size of the primary cerebral lesion. At this time, a second biopsy confirmed N. farcinica. (B) MRI imaging showing an abscess involving the high convexity on the right of the frontal lobe measuring ~1.9 cm × 1.7 cm associated with marked adjacent vasogenic edema (a, b). On repeat imaging, an increase in the size (2.9 cm) of the ring enhancing posterior right frontal/anterior parietal lobe is seen, as well as a new ring-enhancing posterior right frontal lobe lesion measuring 1.1 cm, superior to the lesion described prior (c, d).
At 1 month preceding N. farcinica cerebral abscess, the patient had contracted pneumonia (inferior right lobe) and was treated with amoxicillin/clavulanic acid, taken orally, twice daily for 10 days. Although the causal agent for the pneumonia was not confirmed to be N. farcinica, this route of dissemination (from the lungs to the brain) is very likely as 58% of central nervous system niches originate from the lungs (16). The patient had also contracted COVID-19 1 month before pneumonia, which was confirmed by bedside testing. The patient was fully immunized against COVID-19 (three doses of mRNA vaccine) before contracting the disease.
The patient first presented to emergency services for temporary hemiparesis. Given the patient's cardiovascular history, temporary hemiparesis was initially suspected to be the result of an ischemic stroke. After this initial visit, the patient presented three additional times to emergency services over the course of 3 weeks (Figure 1A). Upon the second admission, an MRI was performed, which indicated a cerebral abscess rather than an ischemic stroke (Figures 1A, B). Upon the third admission, a neurological biopsy was negative for bacterial growth, and precautionary antibiotic treatment was prescribed. On the fourth and final visit to emergency services, neurological symptoms had progressed. The growth of the abscess prompted a second neurological biopsy and Public Health Laboratory testing, which revealed the presence of N. farcinica (Figure 1B). As cases of N. farcinica are mostly found in immunocompromised individuals, immunological flow cytometry analysis of peripheral blood was performed. Indeed, the patient had an immunodeficient profile, but immunosuppression was neither the result of corticosteroid use nor HIV/AIDS. More specifically, lymphopenia was documented in populations CD3+CD4+, CD3+CD8+, and CD3−CD16+CD56+, albeit CD19-labeled cells (B lymphocytes) were increased (Table 1). Serum immunoglobulins were all reported to be within the reference range for IgG, IgA, and IgM. Flow cytometry was performed 15 weeks after the patient experienced mild COVID-19 (Table 1).
Intravenous Bactrim and Imipenem were prescribed for 6 weeks. After this, a 12-month course of Bactrim and Clavulin was prescribed to be taken orally, given the N. farcinica infection at the level of the central nervous system, especially because of the immunosuppressive state.
Antibiotic treatment was effective, and no other issues with infection were experienced afterward. The patient followed a 6-week rehabilitation plan for neurological sequelae and is doing well, despite some residual neuropathy of the left leg.
Corticosteroids are often used in COVID-19-related pneumonia and may lead to an immunocompromised state (22–25) and opportunistic N. farcinica infection (26). However, in this case, the patient had not been prescribed any such treatment or other immunomodulators. The immunocompromised state was therefore presumed to have been SARS-CoV2-related. Furthermore, the occurrence of the ailment extended beyond the habitual course of infection and well into PASC territory. The immune response to SARS-CoV2 is believed to be responsible for the enduring symptoms in PASC, potentially through a persisting inflammatory process (27). In this case report, although the immunocompromised state was not typically so severe for opportunistic infection, we believe that the altered immune state in PASC indeed may have enabled N. farcinica infection. T-cell lymphopenia was documented, albeit with an accompanying rise in B lymphocytes, as previously documented (10–12). Therefore, we hypothesize that N. farcinica infection may potentially have been facilitated by an exhausted immune system; it is becoming increasingly apparent that COVID-19 may lead to an altered immune state and lymphopenia (27–32). Indeed, the immune response to Nocardia spp. is mediated by CD8+ T cells, whereas B lymphocytes and humoral immunity do not appear to be as important (33), such that the immunocompromised host will be susceptible to such infections (34). Analogously, mucormycosis and links to abnormalities in immune cells after a bout of asymptomatic COVID-19 have also been documented (31, 35). The patient in this study had not received corticosteroid treatments, was not HIV positive, and had similarly contracted COVID-19 but remained mildly symptomatic (31). Hence, it is possible that delayed recovery of T cells may lead to an increased risk of life-threatening infections. Little is currently known about T-cell modulation in mildly symptomatic and asymptomatic disease, as most studies have been carried out in more severe cases of COVID-19.
Furthermore, the considerable systemic inflammation during COVID-19 can lead to endothelitis and disruption of the blood–brain barrier (36, 37), which may have facilitated the entry of N. farcinica into the brain. Taken together, both the immunocompromised state and the potential disruption in the blood–brain barrier may have created a propitious environment for the growth and dissemination of the bacterium.
The patient underwent two neurological biopsies to detect bacterial growth. The first biopsy came with the growth of P. acnes in the broth only. The second biopsy was sent to a Public Health Laboratory, which identified the causal agent. Nocardia farcinica cultures are fastidious, and so, laboratory testing may be negative even in the event of nocardiosis (14); hence, failure to ensure proper growth conditions for an adequate amount of time may fail to reveal growth. Furthermore, if N. farcinica had originated from the lungs, the 10-day amoxicillin/clavulanic acid treatment during pneumonia was not sufficient to fully treat the infection as treatment is recommended to last several months (38). A limitation of this case report remains the absence of confirmation of the lungs being the primary site of N. farcinica infection. Another important limitation is the lack of understanding regarding the molecular and cellular mechanisms leading to PASC and susceptibility to opportunistic infections. This case emphasizes the importance of early consideration of opportunistic infection in patients with a known history of COVID-19.
This case report highlights the need for future research into the pathophysiology of COVID-19 and PASC immune dysregulation in convalescent individuals. It also draws attention to the need for timely consideration of opportunistic infections for patients with a history of COVID-19.
This unique case presentation strengthens the notion of immunomodulation after mild COVID-19 and well after the viral infection has cleared. Recognizing these features might prompt considering and testing for infection early on.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Vitalité Health Network Research Ethics Board. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
NB prepared the ethical submission and paperwork to obtain patient consent. NB and DC wrote and corrected the manuscript. CB and NS coordinated the clinical investigations, patient management, and interpreted the clinical data. All authors reviewed, provided feedback, and approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1137024/full#supplementary-material
Supplementary Figure 1. Antibiogram for N. farcinica.
1. Ramos-Casals M, Brito-Zerón P, Mariette X. Systemic and organ-specific immune-related manifestations of COVID-19. Nat Rev Rheumatol. (2021) 17:315–32. doi: 10.1038/s41584-021-00608-z
2. National Institute for Health and Care Excellence. COVID-19 Rapid Guideline: Managing the Long-term Effects of COVID-19 NICE Guideline [NG188]. (2020). AVailale online at: https://www.nice.org.uk/guidance/ng188 (accessed July 12, 2022).
3. Rando HM, Bennett TD, Byrd JB, Bramante C, Callahan TJ, Chute CG, et al. Challenges in defining Long COVID: striking differences across literature, electronic health records, and patient-reported information. medRxiv. (2021). doi: 10.1101/2021.03.20.21253896
4. Greenhalgh T, Knight M. A'Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. (2020) 370:m3026. doi: 10.1136/bmj.m3026
5. Stavem K, Ghanima W, Olsen MK, Gilboe HM, Einvik G. Persistent symptoms 15-6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax. (2021) 76:405–7. doi: 10.1136/thoraxjnl-2020-216377
6. Mahase E. Covid-19: what do we know about “long covid”? BMJ. (2020) 370:m2815. doi: 10.1136/bmj.m2815
7. Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. (2021) 397:220–32. doi: 10.1016/S0140-6736(20)32656-8
8. Clark DV, Kibuuka H, Millard M, Wakabi S, Lukwago L, Taylor A, et al. Long-term sequelae after Ebola virus disease in Bundibugyo, Uganda: a retrospective cohort study. Lancet Infect Dis. (2015) 15:905–12. doi: 10.1016/S1473-3099(15)70152-0
9. Ngai JC, Ko FW, Ng SS, To KW, Tong M, Hui DS. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. (2010) 15:543–50. doi: 10.1111/j.1440-1843.2010.01720.x
10. Ryan FJ, Hope CM, Masavuli MG, Lynn MA, Mekonnen ZA, Yeow AEL, et al. Long-term perturbation of the peripheral immune system months after SARS-CoV-2 infection. BMC Med. (2022) 20:26. doi: 10.1186/s12916-021-02228-6
11. Files JK, Boppana S, Perez MD, Sarkar S, Lowman KE, Qin K, et al. Sustained cellular immune dysregulation in individuals recovering from SARS-CoV-2 infection. J Clin Invest. (2021) 131:e140491. doi: 10.1172/JCI140491
12. Wen W, Su W, Tang H, Le W, Zhang X, Zheng Y, et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. (2020) 6:31. doi: 10.1038/s41421-020-00187-5
13. Ambrosioni J, Lew D, Garbino J. Nocardiosis: updated clinical review and experience at a tertiary center. Infection. (2010) 38:89–97. doi: 10.1007/s15010-009-9193-9
14. Lederman ER, Crum NF. A case series and focused review of nocardiosis: clinical and microbiologic aspects. Medicine. (2004) 83:300–13. doi: 10.1097/01.md.0000141100.30871.39
15. Arabi Y, Fairfax MR, Szuba MJ, Crane L, Schuman P. Adrenal insufficiency, recurrent bacteremia, and disseminated abscesses caused by Nocardia asteroides in a patient with acquired immunodeficiency syndrome. Diagn Microbiol Infect Dis. (1996) 24:47–51. doi: 10.1016/0732-8893(95)00249-9
16. Torres OH, Domingo P, Pericas R, Boiron P, Montiel JA, Vázquez G. Infection caused by Nocardia farcinica: case report and review. Eur J Clin Microbiol Infect Dis. (2000) 19:205–12. doi: 10.1007/s100960050460
17. Wallace RJ, Tsukamura M, Brown BA, Brown J, Steingrube VA, Zhang YS, et al. Cefotaxime-resistant Nocardia asteroides strains are isolates of the controversial species Nocardia farcinica. J Clin Microbiol. (1990) 28:2726–32. doi: 10.1128/jcm.28.12.2726-2732.1990
18. Wenger PN, Brown JM, McNeil MM, Jarvis WR. Nocardia farcinica sternotomy site infections in patients following open heart surgery. J Infect Dis. (1998) 178:1539–43. doi: 10.1086/314450
19. Peters BR, Saubolle MA, Costantino JM. Disseminated and cerebral infection due to Nocardia farcinica: diagnosis by blood culture and cure with antibiotics alone. Clin Infect Dis. (1996) 23:1165–7. doi: 10.1093/clinids/23.5.1165
20. Hoeprich PD, Brandt D, Parker RH. Nocardial brain abscess cured with cycloserine and sulfonamides. Am J Med Sci. (1968) 255:208–16. doi: 10.1097/00000441-196803000-00008
21. Smego RA, Moeller MB, Gallis HA. Trimethoprim-sulfamethoxazole therapy for Nocardia infections. Arch Intern Med. (1983) 143:711–8. doi: 10.1001/archinte.143.4.711
22. Ross SH, Cantrell DA. Signaling and function of interleukin-2 in T lymphocytes. Annu Rev Immunol. (2018) 36:411–33. doi: 10.1146/annurev-immunol-042617-053352
23. Lanza L, Scudeletti M, Puppo F, Bosco O, Peirano L, Filaci G, et al. Prednisone increases apoptosis in in vitro activated human peripheral blood T lymphocytes. Clin Exp Immunol. (1996) 103:482–90. doi: 10.1111/j.1365-2249.1996.tb08306.x
24. Fauci AS, Dale DC, Balow JE. Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med. (1976) 84:304–15. doi: 10.7326/0003-4819-84-3-304
25. Paliogianni F, Ahuja SS, Balow JP, Balow JE, Boumpas DT. Novel mechanism for inhibition of human T cells by glucocorticoids. Glucocorticoids inhibit signal transduction through IL-2 receptor. J Immunol. (1993) 151:4081–9. doi: 10.4049/jimmunol.151.8.4081
26. DiMeglio M, Shaikh H, Newman J, Vazsquez-Rubio G. Nocardiosis of the central nervous system: a rare complication of COVID management? IDCases. (2022) 29:e01599. doi: 10.1016/j.idcr.2022.e01599
27. Ramakrishnan RK, Kashour T, Hamid Q, Halwani R, Tleyjeh IM. Unraveling the mystery surrounding post-acute sequelae of COVID-19. Front Immunol. (2021) 12:686029. doi: 10.3389/fimmu.2021.686029
28. Wynants L, Van Calster B, Collins GS, Riley RD, Heinze G, Schuit E, et al. Prediction models for diagnosis and prognosis of covid-19: systematic review and critical appraisal. BMJ. (2020) 369:m1328.
29. Mahmoodpoor A, Hosseini M, Soltani-Zangbar S, Sanaie S, Aghebati-Maleki L, Saghaleini SH, et al. Reduction and exhausted features of T lymphocytes under serological changes, and prognostic factors in COVID-19 progression. Mol Immunol. (2021) 138:121–7. doi: 10.1016/j.molimm.2021.06.001
30. De Biasi S, Meschiari M, Gibellini L, Bellinazzi C, Borella R, Fidanza L, et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat Commun. (2020) 11:3434. doi: 10.1038/s41467-020-17292-4
31. Mandke C, Divekar R, Pradhan V, Arora S. Quantitative B and T cell abnormalities in four patients presenting with mucormycosis and prior asymptomatic COVID-19 infection. BMJ Case Rep. (2022) 15:e247893. doi: 10.1136/bcr-2021-247893
32. Hanna SJ, Codd AS, Gea-Mallorqui E, Scourfield DO, Richter FC, Ladell K, et al. T cell phenotypes in COVID-19 - a living review. Oxf Open Immunol. (2021) 2:iqaa007. doi: 10.1093/oxfimm/iqaa007
33. Rico G, Ochoa R, Oliva A, Gonzalez-Mendoza A, Walker SM, Ortiz-Ortiz L. Enhanced resistance to Nocardia brasiliensis infection in mice depleted of antigen-specific B cells. J Immunol. (1982) 129:1688–93. doi: 10.4049/jimmunol.129.4.1688
34. Beaman BL, Beaman L. Nocardia species: host-parasite relationships. Clin Microbiol Rev. (1994) 7:213–64. doi: 10.1128/CMR.7.2.213
35. Patel A, Agarwal R, Rudramurthy SM, Shevkani M, Xess I, Sharma R, et al. Multicenter epidemiologic study of coronavirus disease-associated mucormycosis, India. Emerg Infect Dis. (2021) 27:2349–59. doi: 10.3201/eid2709.210934
36. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
37. Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. (2020) 41:3038–44. doi: 10.1093/eurheartj/ehaa623
Keywords: nocardiosis, Nocardia farcinica, coronavirus disease 2019 (COVID-19), post-acute sequelae of COVID-19 (PASC), brain abscess
Citation: Bouhamdani N, Comeau D, Bourque C and Saulnier N (2023) Encephalic nocardiosis after mild COVID-19: A case report. Front. Neurol. 14:1137024. doi: 10.3389/fneur.2023.1137024
Received: 03 January 2023; Accepted: 02 February 2023;
Published: 22 February 2023.
Edited by:
Thorsten Rudroff, The University of Iowa, United StatesCopyright © 2023 Bouhamdani, Comeau, Bourque and Saulnier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nadia Bouhamdani,  bmFkaWEuYm91aGFtZGFuaUB2aXRhbGl0ZW5iLmNh
bmFkaWEuYm91aGFtZGFuaUB2aXRhbGl0ZW5iLmNh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.