
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Neurol. , 30 March 2023
Sec. Epilepsy
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1135962
This article is part of the Research Topic Epilepsy Care During the COVID-19 Pandemic View all 3 articles
 Alekhya Lavu
Alekhya Lavu Donica Janzen
Donica Janzen Laila Aboulatta
Laila Aboulatta Payam Peymani
Payam Peymani Lara Haidar
Lara Haidar Brianne Desrochers
Brianne Desrochers Silvia Alessi-Severini
Silvia Alessi-Severini Sherif Eltonsy*
Sherif Eltonsy*Introduction: Given the lack of evidence on how the COVID-19 pandemic impacted antiseizure medication (ASM) use, we examined the trends of ASMs before and during COVID-19.
Methods: We conducted a population-based study using provincial-level health databases from Manitoba, Canada, between 1 June 2016 and 1 March 2021. We used interrupted time series autoregressive models to examine changes in the prevalence and incidence of ASM prescription rates associated with COVID-19 public health restrictions.
Results: Among prevalent users, the COVID-19 pandemic led to a significant increase in new-generation ASMs with a percentage change of 0.09% (p = 0.03) and a significant decrease in incidence use of all ASMs with a percentage change of −4.35% (p = 0.04). Significant trend changes were observed in the prevalent use of new-generation ASMs (p = 0.04) and incidence use of all (p = 0.04) and new-generation ASMs (p = 0.02). Gabapentin and clonazepam prescriptions contributed 37% of prevalent and 54% of incident use.
Conclusion: With the introduction of public health measures during COVID-19, small but significant changes in the incident and prevalent use of ASM prescriptions were observed. Further studies are needed to examine whether barriers to medication access were associated with potential deterioration in seizure control among patients.
Conference presentation: The results from this study have been presented as an oral presentation at the 38th ICPE, International Society of Pharmacoepidemiology (ISPE) annual conference in Copenhagen.
The COVID-19 pandemic has impacted the lives of patients with chronic diseases (1, 2). Physician activity in Canada decreased by ~30–40% in April 2020 compared to 2019, and more than 25% of Canadians reported that an appointment for healthcare services was canceled, rescheduled, or delayed (3). The preventive measures to contain COVID-19 affected the living conditions of patients with chronic disease resulting in reduced physical activity, changes in diet, changes in medical care, and availability of supplies (4, 5). Concerns of COVID-19 infection, prescription medication shortages, travel and gathering restrictions, financial restrictions, and substance use are some of the reported barriers to prescription medication access among patients with chronic disease (6–11). Epilepsy is a chronic neurological disorder prevalent in ~0.7–1.0% of the population (12). While published studies have reported changes in the prescriptions for chronic diseases such as hypertension and depression during the COVID-19 pandemic, there is a lack of evidence on prescription trends of antiseizure medications (ASMs) (7, 10, 13–20). In addition to their primary use to control seizures, ASMs are used off-label to treat both acute and chronic conditions such as pain, migraine, restless legs syndrome, some psychiatric disorders, and post-traumatic stress syndrome (21). Gabapentin (GBP) and clonazepam (CLZ) are the most used medications for the off-label use of ASMs. The present study aimed to study ASM prescription trends before and during the COVID-19 pandemic in a province-wide cohort from Manitoba, Canada.
We conducted a population-based quasi-experimental study to examine the trends of all ASM, new-generation ASM, and old-generation ASM utilization in pre-COVID-19 and during COVID-19 in Manitoba, Canada. We used the Manitoba Center for Health Policy (MCHP) repository, a provincial-level population database, to form our cohort. ASM quarterly dispensation rates were examined between 1 June 2016 and 1 March 2021, calculating the quarterly prevalence and incidence rate. As older adults have higher rates of antiseizure medication use, we dichotomized age into >65 and ≤ 65 years for descriptive results (22–26). We conducted interrupted time series analysis, using autoregressive models, to investigate changes in ASM prescriptions rates and trends before and during COVID-19, with the 2020 second quarter as the intervention point. We also calculated the relative percentage change as the relative change between the second quarter of 2020 and the first quarter of 2020 (Relative percentage change = (Percentage in Q2-2020 – Percentage in Q1-2020)/ Percentage in Q1-2020*100).
We examined the prescription trends of (1) all ASMs, (2) old-generation ASMs, and (3) new-generation ASMs. We classified the medications included in the analysis into the old and new generations according to previously established criteria reflecting the year of introduction to the markets, where the old generation included first-generation ASMs while the new generation included both second- and third-generation ASMs (see Supplementary Table 1 for medication list). We further conducted a subgroup analysis examining all ASMs, old-generation ASMs, and new-generation ASMs, while excluding clonazepam and gabapentin, given their increase in use for indications other than epilepsy.
We studied ~1.3 million prescriptions between 1 June 2016 to 1 March 2021, representing the incident and prevalent trends of ASM use. The population above 65 years had ASM prescriptions 2-fold higher than the < 65-year-old group, and women had a higher prevalence of ASM prescriptions than men (see Supplementary Figures 1, 2). Similar trends for age and sex were observed for incident use. During the period examined, an average of 5,709 incident ASM prescriptions/quarter and an average of 52,178 prevalent ASM prescriptions/quarter were filled. During our study period (1 June 2016 to 1 March 2021), we observed an increase in the prevalent use of new-generation ASMs and a decrease in old-generation ASM use; however, both were relatively minimal (Figure 1A). We did not observe any major changes in the incident use of all or new-generation ASMs; however, we observed a minor decrease in old-generation ASMs (Figure 1B). After excluding gabapentin and clonazepam from both incident and prevalent prescriptions, we observed a minor increase in all and new-generation ASMs and a stable rate of old-generation ASM use (Figures 1C, D).
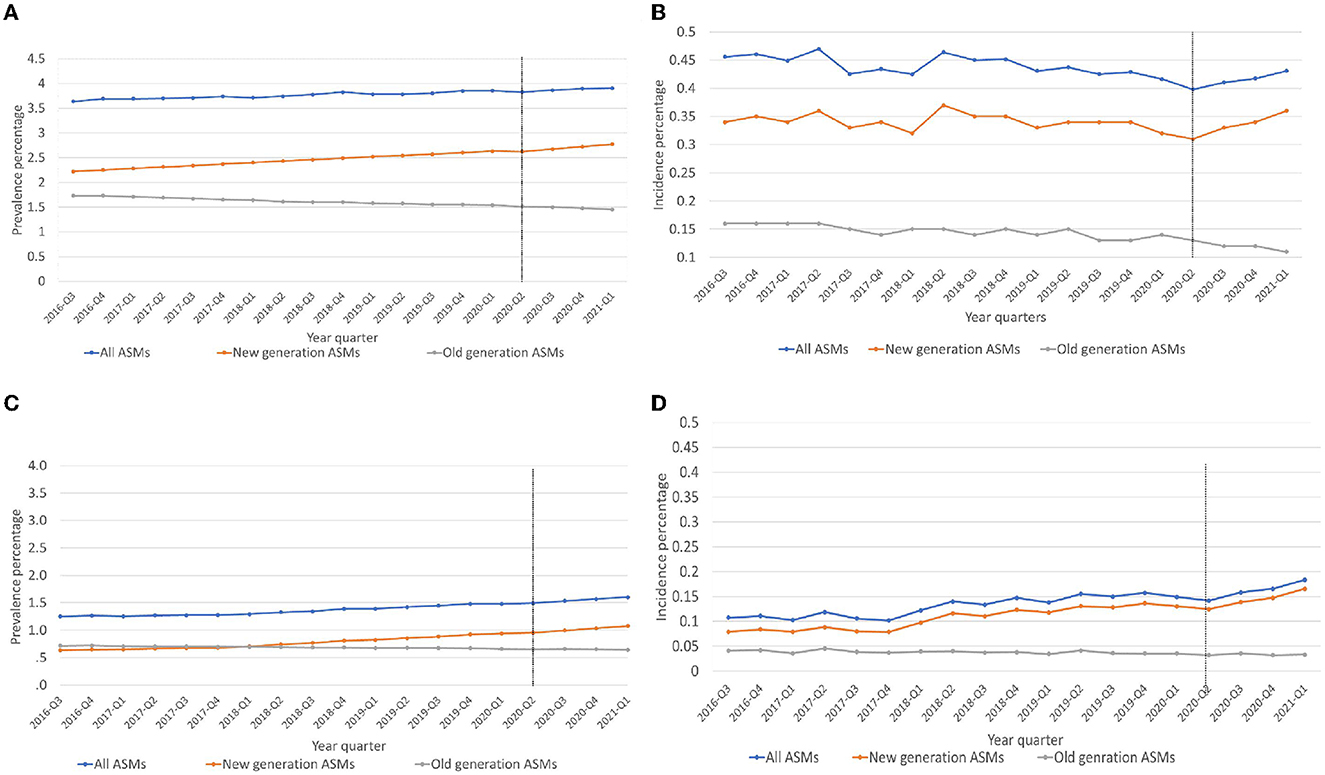
Figure 1. (A–D) Trends of prevalent and incident antiseizure medication prescriptions used. (A) Prevalent use of ASMs, (B) Incident use of ASMs. (C) Prevalent use of ASMs excluding Gabapentin and Clonazepam. (D) Incident use of ASMs excluding Gabapentin and Clonazepam, ASMs, antiseizure medications, 2020-Q2, Intervention point (COVID-19 pandemic).
COVID-19 restrictions led to a small but significant increase in the prevalent use of new-generation ASMs by 0.09 % (p = 0.03). No significant change was observed among all ASMs (−0.68%, p = 0.12) or old-generation ASMs (−2.26%, p = 0.51). A significant change in prescription trends was observed in the prevalent use of new-generation ASMs (β3) = 0.018 (p = 0.04; Table 1, Figure 1).
Excluding clonazepam and gabapentin lowered prevalent prescriptions by 37%. We observed a non-significant change in the prevalent use of all ASMs, 0.69% (p = 0.61), old-generation ASMs, −0.81% (p = 0.52), and new-generation ASMs 1.70% (p = 0.68; Table 1).
We observed a significant decrease in the incidence use of all ASMs by 4.35% (p = 0.04) and a non-significant decrease in new-generation ASM prescriptions by 1.59% (p = 0.06) and old-generation ASM prescriptions by 8.69% (p = 0.79). Significant trend changes in the incident prescriptions of all ASMs (β3) = −0.013 (p = 0.04) and new-generation ASMs (β3) = 0.016 (p = 0.02) were found. There was no significant trend change in the incidence use of old-generation ASM prescriptions (Table 1, Figure 1).
After excluding gabapentin and clonazepam, we observed a 54% decrease in the incident ASM prescriptions. We found a significant decrease in the incident prescriptions of all ASMs by 5.11% (p = 0.02) and new-generation ASMs by 4.34 (p = 0.02), while the decrease in old-generation ASM use was non-significant (−9.38%, p = 0.46). We observed a significant change in the incident prescription trends of all ASMs (β3) = 0.009 (p = 0.03) and new-generation ASMs (β3) = 0.009 (p = 0.02; Table 1).
In the current study, we found that restrictions due to the COVID-19 pandemic were associated with a small (0.09%) but significant immediate increase in new-generation ASM prescriptions among prevalent users. However, after excluding gabapentin from new-generation ASMs, there was a non-significant increase in prevalent use by 1.70%. We also found a significant 4.35% decline in all ASM incident prescriptions, and the results were consistent with a 5.11% decrease in all ASM incident prescriptions after excluding gabapentin and clonazepam. With the exclusion of gabapentin from new-generation ASMs, we found a significant decline in incident ASM prescriptions by 4.34%.
Hospital visits in Manitoba were fully suspended on March 18, reducing access to in-person care (22). Reduction in access to physician care might have had a potential impact on the diagnosis of new cases of epilepsy and short-term prescription of ASMs for non-epilepsy conditions (e.g., gabapentin or clonazepam). Therefore, these measures possibly contributed to the significant decrease in incident prescriptions of ASMs.
Different jurisdictions in Canada have reported changes in medication use during the early periods of the pandemic, with a significant increase in claims for some drugs (e.g., cardiovascular drugs and oral antidiabetic drugs) but a slight decrease in claims for controlled drugs such as opioids and benzodiazepines (16). Similar changes in chronic medication use due to the COVID-19 pandemic have been observed in other parts of the world (3, 4, 10, 12–14). For example, a study from eight European countries reported a decrease in antibiotics, COPD, and asthma medication (26). Another study among the populations of England, Scotland, and Wales reported a significant decrease in hypertensive medications due to COVID-19 (27). Moreover, Maeda et al. reported a decrease in antidiabetic medication use in Japan during the first wave of COVID-19 (28). These trends can be attributed to medication stockpiling amid fears of drug shortages and anticipated supply chain disruptions (28). We did not observe a similar pattern of stockpiling of ASMs in Manitoba, as the observed increase in new-generation ASMs was relatively small and absent among old-generation users.
Manitoba pharmacists were directed to limit dispensations of all medications to a maximum 1-month supply early in the pandemic, but pharmacies remained open as essential services throughout the pandemic (22, 29). These policies minimized the impact of COVID-19 restrictions on existing users of ASMs (i.e., prevalent users). In contrast, reduced access to in-person care and patient-reported delays in seeking care likely contributed to our observed decrease in the incident use of ASMs. However, a rapid shift to virtual care and a return of physician care activity to pre-pandemic levels by the end of the first wave may have mitigated this impact on incident prescriptions. Further studies should compare the results with other parameters of care for people with epilepsy (e.g., seizure frequency or frequency of physician contacts) before and during the pandemic.
Some limitations in our study should be acknowledged. We did not study a specific epilepsy population using a definition, since our interest was the ASM utilization and not epilepsy itself. However, by excluding clonazepam and gabapentin—the most used ASMs for non-seizure-related indications, we were able to capture a closer exclusive epilepsy population (30, 31). However, it must be noted that other ASMs may also be prescribed for non-epilepsy conditions (for example, topiramate for headaches) (32). In our study, there might be factors (e.g., age, sex, and socioeconomic status) that could act as effect modifiers for the associations observed (33). However, our sample sizes did not allow for interaction models. The study data are limited to Manitoba province in Canada from 2016 to 2021. However, the results are generalizable to similar populations of developed countries.
We found small but significant changes in the prescriptions of ASMs due to the COVID-19 pandemic measures. A significant volume of prescriptions was for gabapentin and clonazepam. Further studies are needed to monitor the trend changes through the pandemic waves and examine whether such changes have had any effect at the patient level.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Health Research Ethics Board (HREB) at the University of Manitoba and Manitoba Health Information Privacy Committee (HIPC). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
AL and SE: study conception, analysis, and manuscript writing. DJ, LA, PP, LH, BD, and SA-S: analysis and manuscript editing. All authors contributed to the article and approved the submitted version.
This study was supported by Research Manitoba.
The authors acknowledge the Manitoba Centre for Health Policy for use of data contained in the Manitoba Population Research Data Repository. The results and conclusions are those of the authors and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health, or other data providers is intended or should be inferred.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1135962/full#supplementary-material
1. Arif M, Saqib N, Siddiqui S, Qasim M, Sohail M. Effect of COVID-19 lockdown on patients with chronic diseases. Diabetes Metab Syndr Clin Res Rev. (2020) 2020:1621–3. doi: 10.1016/j.dsx.2020.08.028
2. Statistics Canada. Survey on Access to Health Care Pharmaceuticals During the Pandemic, March (2020) to May 2021. Dly. (2021). Available online at: https://www.statcan.gc.ca/n/daily-quotidien//dqb-eng.hm (accessed October 1, 2022).
3. Information CI for H. COVID-19's Impact on Physician Services. (2020). Available online at: https://www.cihi.ca/en/covid–resources/impact-ofcovid–on-canadas-health-care-systems/physicianservices (accessed November 10, 2022).
4. Di Renzo L, Gualtieri P, Pivari F, Soldati L, Attinà A, Cinelli G, et al. Eating habits and lifestyle changes during COVID-19 lockdown: An Italian survey. J Transl Med. (2020) 18:1–15. doi: 10.1186/s12967-020-02399-5
5. Gualano MR, Lo Moro G, Voglino G, Bert F, Siliquini R. Effects of COVID-19 lockdown on mental health and sleep disturbances in Italy. Int J Environ Res Public Health. (2020) 17:1–13. doi: 10.3390/ijerph17134779
6. Gul ZB, Atakli HD. Effect of the COVID-19 pandemic on drug compliance and stigmatization in patients with epilepsy. Epilepsy Behav. (2020) 114:107610. doi: 10.1016/j.yebeh.2020.107610
7. Costantino F, Bahier L, Tarancón LC, Leboime A, Vidal F, Bessalah L, et al. COVID-19 in French patients with chronic inflammatory rheumatic diseases: Clinical features, risk factors and treatment adherence. Jt Bone Spine. (2021) 88:105095. doi: 10.1016/j.jbspin.2020.105095
8. Shimels T, Kassu RA, Bogale G, Bekele M, Getnet M, Getachew A, et al. Magnitude and associated factors of poor medication adherence among diabetic and hypertensive patients visiting public health facilities in Ethiopia during the COVID-19 pandemic. PLoS ONE. (2021) 16:e0249222. doi: 10.1371/journal.pone.0249222
9. Subathra GN, Rajendrababu SR, Senthilkumar VA, Mani I, Udayakumar B. Impact of COVID-19 on follow-up and medication adherence in patients with glaucoma in a tertiary eye care centre in south India. Indian J Ophthalmol. (2021) 69:1264–70. doi: 10.4103/ijo.IJO_164_21
10. Zhang HQ, Lin JY, Guo Y, Pang S, Jiang R, Cheng QJ. Medication adherence among patients with chronic obstructive pulmonary disease treated in a primary general hospital during the COVID-19 pandemic. Ann Transl Med. (2020) 8:1179. doi: 10.21037/atm-20-6016
11. Ruksakulpiwat S, Zhou W, Niyomyart A, Wang T, Kudlowitz A. How does the COVID-19 pandemic impact medication adherence of patients with chronic disease? A systematic review. Chronic Illn. (2022) 2022:17423953221110151. doi: 10.1177/17423953221110151
12. Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon CS, Dykerma J, et al. Prevalence and incidence of epilepsy: A systematic review and meta-analysis of international studies. Neurology. (2017) 88:296–303. doi: 10.1212/WNL.0000000000003509
13. Leong C, Kowalec K, Eltonsy S, Bolton JM, Enns MW, Tan Q, et al. Psychotropic medication use before and during COVID-19: A population-wide study. Front Pharmacol. (2022) 13:1–10. doi: 10.3389/fphar.2022.886652
14. Aboulatta L, Peymani P, Vaccaro C, Leong C, Kowalec K, Delaney J, et al. Drug utilization patterns before and during COVID-19 pandemic in Manitoba, Canada: A population-based study. PLoS ONE. (2022) 17:1–15. doi: 10.1371/journal.pone.0278072
15. Nikolaus P, Noha S. The effect of SARS-CoV-2 on the prescribing of antimicrobials and analgesics by NHS general dental practitioners in England. Br Dent J. (2021) 2:1–6. doi: 10.1038/s41415-020-2595-2
16. Network. ODPR. COVID-19 Ontario Prescription Drug Utilization Tool. (2020). Available online at: https://odprn.ca/covid-ontario-prescription-drugutilization-tool/ (accessed October 20, 2022).
17. Rose AN, Baggs J, Wolford H, Neuhauser MM, Srinivasan A, Gundlapalli AV, et al. Trends in antibiotic use in United States hospitals during the coronavirus disease (2019) pandemic. Open Forum Infect Dis. (2021) 8:ofab236. doi: 10.1093/ofid/ofab236
18. Hernandez I, Tadrous M, Magnani JW, Guo J, Suda KJ. Impact of the COVID-19 pandemic on global anticoagulant sales: A cross-sectional analysis across 39 countries. Am J Cardiovasc Drugs. (2021) 2021:19–21. doi: 10.1007/s40256-021-00475-9
19. Gouin KA, Creasy S, Beckerson M, Wdowicki M, Hicks LA, Lind JN, et al. Trends in prescribing of antibiotics and drugs investigated for coronavirus disease (2019) (COVID-19) treatment in US nursing home residents during the COVID-19 pandemic. Clin Infect Dis. (2021) 2019:1–9. doi: 10.1093/cid/ciab225
20. Abelenda-Alonso G, Padullés A, Rombauts A, Gudiol C, Pujol M, Alvarez-Pouso C, et al. Antibiotic prescription during the COVID-19 pandemic: A biphasic pattern. Infect Control Hosp Epidemiol. (2020) 41:1371–2. doi: 10.1017/ice.2020.381
21. Rogawski MA, Löscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. (2004) 5:553–64. doi: 10.1038/nrn1430
22. Aboulatta L, Kowalec K, Delaney J, Alessi-Severini S, Leong C, Falk J, et al. Trends of COVID-19 incidence in Manitoba and public health measures: March (2020) to February 2022. BMC Res Notes. (2022) 15:1–6. doi: 10.1186/s13104-022-06049-5
23. Silva TM, Estrela M, Gomes ER, Piñeiro-Lamas M, Figueiras A, Roque F, et al. The impact of the covid-19 pandemic on antibiotic prescribing trends in outpatient care: A nationwide, quasi-experimental approach. Antibiotics. (2021) 10 :91040. doi: 10.3390/antibiotics10091040
24. Krulichová IS, Selke GW, Bennie M, Hajiebrahimi M, Nyberg F, Fürst J, et al. Comparison of drug prescribing before and during the COVID-19 pandemic: A cross-national European study. Pharmacoepidemiol Drug Saf. (2022) 31:1046–55. doi: 10.1002/pds.5509
25. Vaduganathan M, van Meijgaard J, Mehra MR, Joseph J, O'Donnell CJ, Warraich HJ. Prescription fill patterns for commonly used drugs during the COVID-19 pandemic in the United States. Ann Intern Med. (2020) 172:577–82. doi: 10.1001/jama.2020.9184
26. King LM, Lovegrove MC, Shehab N, Tsay S, Budnitz DS, Geller AI, et al. Trends in US outpatient antibiotic prescriptions during the coronavirus disease (2019) pandemic. Clin Infect Dis an Off Publ Infect Dis Soc Am. (2021) 73:e652–60. doi: 10.1093/cid/ciaa1896
27. Mian M, Teoh L, Hopcraft M. Trends in dental medication prescribing in Australia during the COVID-19 pandemic. JDR Clin Transl Res. (2021) 6:145–52. doi: 10.1177/2380084420986766
28. Elbeddini A, Botross A, Gerochi R, Gazarin M, Elshahawi A. Pharmacy response to COVID-19: lessons learnt from Canada. J Pharm Policy Pract. (2020) 13:1–8. doi: 10.1186/s40545-020-00280-w
29. Manitoba Health Seniors Active Living (MHSAL). Health Care Order for Pharmacist During Pandemic. Available online at: https://www.gov.mb.ca/health/pharmacare/profdocs/covid19_30days.pdf
30. Shouman W, Delaney JA, Kowalec K, Ng M, Ruth C, Falk J, et al. Trends of utilization of antiseizure medications among pregnant women in Manitoba, Canada: A 20-year population-based study. Front Pharmacol. (2022) 13:871136. doi: 10.3389/fphar.2022.871136
31. Leong C, Mamdani MM, Gomes T, Juurlink DN, Macdonald EM, Yogendran M. Antiepileptic use for epilepsy and nonepilepsy disorders: A population-based study (1998-2013). Neurology. (2016) 86:939–46. doi: 10.1212/WNL.0000000000002446
32. Cereza G, Pedrós C, Garcia N, Laporte JR. Topiramate in non-approved indications and acute myopia or angle closure glaucoma. Br J Clin Pharmacol. (2005) 60:578–9. doi: 10.1111/j.1365-2125.2005.02470.x
Keywords: antiseizure medications, epilepsy, seizures, COVID-19, antiepileptic drugs, drug utilization, prescription patterns
Citation: Lavu A, Janzen D, Aboulatta L, Peymani P, Haidar L, Desrochers B, Alessi-Severini S and Eltonsy S (2023) Prescription trends of antiseizure medications before and during the COVID-19 pandemic. Front. Neurol. 14:1135962. doi: 10.3389/fneur.2023.1135962
Received: 02 January 2023; Accepted: 06 March 2023;
Published: 30 March 2023.
Edited by:
Randi Von Wrede, University Hospital Bonn, GermanyReviewed by:
Brin Freund, Mayo Clinic Florida, United StatesCopyright © 2023 Lavu, Janzen, Aboulatta, Peymani, Haidar, Desrochers, Alessi-Severini and Eltonsy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sherif Eltonsy, c2hlcmlmLmVsdG9uc3lAdW1hbml0b2JhLmNh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.