- 1Psychosomatic Department, Hangzhou Traditional Chinese Medicine Hospital Affiliated to Zhejiang Chinese Medical University, Hangzhou, China
- 2State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China
- 3Affiliated Mental Health Center & Hangzhou Seventh People's Hospital, Zhejiang University School of Medicine, Hangzhou, China
Objective: There is growing evidence of a relationship between anti-seizure medication (ASM) use and the risk of dementia. This study examined this association using a meta-analysis approach.
Methods: PubMed, EMBASE, and Cochrane Library were systematically searched for peer-reviewed observational studies published up to February 2023. Study quality was evaluated using the Newcastle-Ottawa Scale, and an overall odds ratio (OR) was pooled using fixed or random-effects models.
Results: The analysis included 9 publications with 10 studies. The results showed that overall ASM exposure was associated with an increased risk of dementia [OR: 1.09, 95% confidence interval (CI): 1.03–1.15; P = 0.003] in general population. However, this association disappeared (OR: 1.02, 95% CI: 0.97–1.07; P = 0.361) when the study data adjusted for drug indications were pooled. Subgroup analysis based on individual drugs found only a positive association among those exposed to valproate, carbamazepine, and clonazepam. Furthermore, an increased risk was found in patients with bipolar disorder exposed to ASMs (OR: 1.43, 95% CI: 1.07–1.92; P = 0.015).
Conclusions: The statistically significant association between ASM and dementia in general population may be driven by unmeasured confounding or several individual first-generation ASMs. However, a higher risk of dementia was observed among bipolar disorder patients treated with ASMs. Given the few included studies and evidence of high heterogeneity, further larger, prospective studies that control for important confounders are needed to verify our findings.
1. Introduction
Dementia is a progressive neurodegenerative disease characterized by progressive cognitive and functional decline constituting one of the leading causes of disability worldwide (1). It mainly affects older people, especially those over 65 years old (2). With the growing aging population, the number of people with dementia is predicted to triple to an estimated 152 million worldwide by 2050 (3). Considering the lack of treatment options, recognition of the risk factors of dementia may help to prevent the disease and could also inform appropriate interventions. Modifiable risk factors, including hypertension, infection, mental disorders, diabetes, and smoking, account for around 35% of dementia cases (4). Therefore, decreases in the incidence of dementia are partially attributable to avoiding some of these risk factors (5).
Anti-seizure medication (ASM) are widely used to treat epilepsy and bipolar disorder (6). While effective, they have been linked to negative clinical outcomes, such as increased risks of cognitive decline (7), cardiovascular disease (8), and fracture (9). Increasing numbers of epidemiological studies (10–18) have investigated the risk of dementia in ASM users; however, the results have been controversial. Some found an increased risk of dementia with ASM exposure, whereas others revealed no association. In the earliest cohort study, Carter et al. (10) reported that ASM use was associated with an increased risk of dementia; in three other large studies (14, 15, 18), however, dementia was not associated with ASM use. The findings of three studies (11, 12, 17) focusing on patients with bipolar disorder also conflicted. Because the various factors associated with ASM exposure (i.e., type of ASM and participants) may alter the risk of dementia differentially, these factors should be evaluated. Due to the increasing use of ASMs, determining the long-term effects of these drugs on dementia is important. The purpose of this systematic literature review and meta-analysis is to assess whether ASMs exposure increases the incidence of dementia.
2. Methods
Preferred Reporting Items for Systematic Reviews and Meta-analysis framework guidelines (PRISMA) were followed for this meta-analysis.
2.1. Data sources and search strategy
A comprehensive literature search of the PubMed, EMBASE, and Cochrane Library databases was conducted on February 2, 2023, according to the PRISMA statement, with no year restrictions. The search incorporated index terms (Mesh) and free text words for the search concepts: (antiepileptic AND antiseizure AND anticonvulsant AND valproic acid AND paraldehyde AND phenobarbitone AND levetiracetam AND lorazepam AND carbamazepine AND phenytoin AND midazolam AND lidocaine AND fosphenytoin AND bumetanide) AND (dementia OR Alzheimer OR frontotemporal dementia OR cognitive dysfunction OR cognitive impair OR cognitive decline OR vascular dementia OR multiinfarct dementia OR neurodegenerative diseases OR neurocognitive disorders) AND (risk OR ratio OR prospective studies OR epidemiologic studies OR case-control studies OR cohort studies). An additional search was conducted in the bibliographies of relevant articles and relevant reviews.
2.2. Selection criteria
The studies were assessed by two independent reviewers who determined whether the studies met the inclusion criteria. Observational studies were included if they were: (1) a peer-reviewed study with a case–control or cohort design published in English, (2) included ASM exposure preceding a diagnosis of dementia, (3) included participants 18 years or older, (4) explored the association between ASM exposure and the risk of dementia, and (5) provided sufficient data to allow the calculation of risk estimates if adjusted data were not provided. Case reports, case series, animal studies, editorials, reviews, and meta-analyses were excluded. Studies that considered dementia as comorbidity and not as an outcome were also excluded.
2.3. Data extraction
Two authors extracted information from all selected studies using piloted data extraction sheets. Any discrepancies in the extracted data were resolved by a third author. The following information was collected from each study: author, publication year, study location, sample demographics, information on ASM exposure, diagnostic criteria for dementia, number of subjects in each group, statistical adjustments, and study quality.
2.4. Risk of bias and quality assessment
The quality of the included observational studies was assessed using the Newcastle-Ottawa Scale (NOS) (19), which is recommended by the Cochrane Handbook for Systematic Reviews of Interventions. The assessment focuses on three major areas: the study population selection, the comparability between the two groups, and the ascertainment of exposure (for case-control studies) or the outcome of interest (for cohort studies).
2.5. Statistical analysis
We used the STATA ver.16.0 (StataCorp., College Station, TX, USA) to perform meta-analysis. A random-effects model was used to pool the odds ratios (ORs) and 95% confidence intervals (CI) of individual studies; such models are optimal in terms of allowing the results to be generalized because they can deal with potential heterogeneity (20). ORs were considered as approximations of relative risks (RRs) or hazard ratios (HRs) because the dementia outcome under study is rare in all populations and subgroups under review. Splitting one study into several estimates leads to substantially more weight being assigned to this study in the meta-analysis, especially in a random-effects model. Therefore, we used a fixed-effects model to produce a pooled OR if more than three estimates from one study were provided, and then included this pooled OR in the meta-analysis. The I2 statistic was used to assess between-study heterogeneity; The I2 values were classified into four groups: of 0–29%, 30–49%, 50–74%, and 75–100%, representing very low, low, medium, and high inconsistency, respectively (21). Funnel plots and Egger's test were used to test the presence of potential publication bias within this review (22, 23). All the statistical tests were bilateral, and P-values < 0.05 indicated considered significant.
3. Results
3.1. Search results
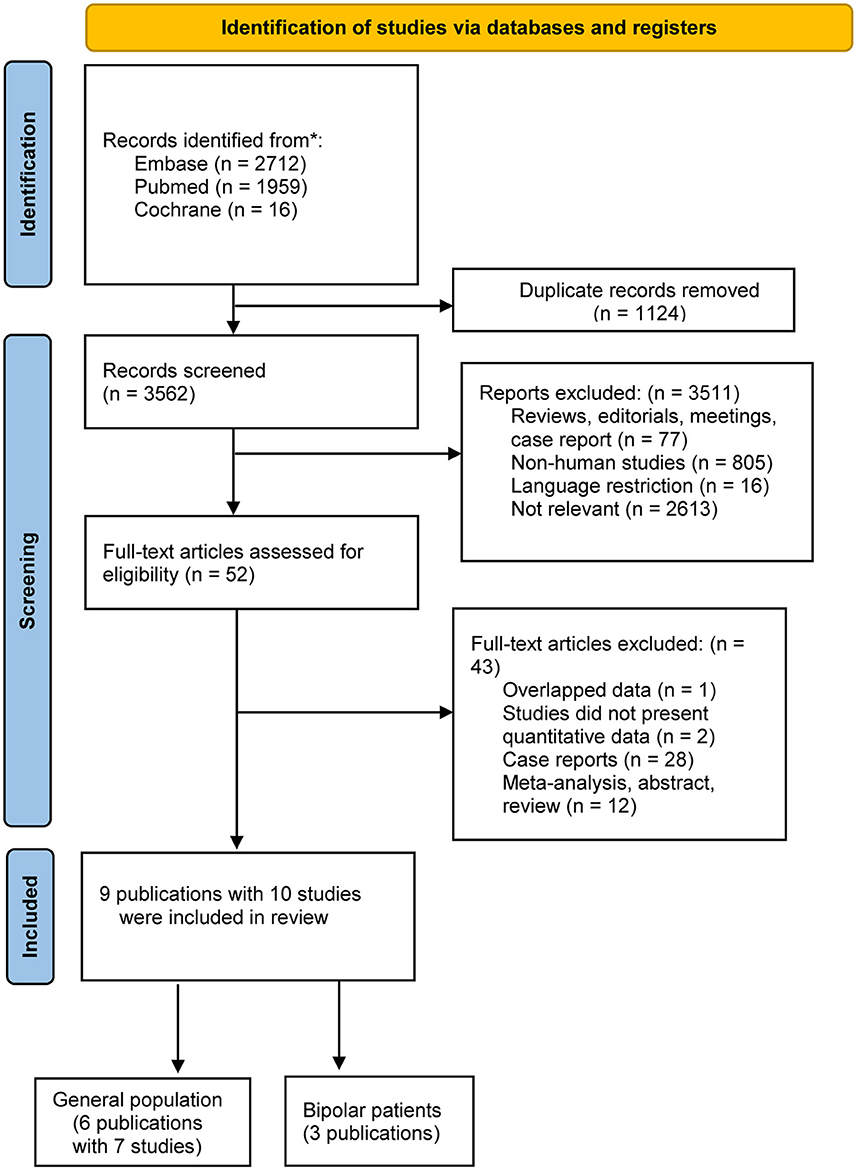
After using the keywords, 4,687 records were identified in the initial search. Of these, 1,124 were duplicates, and 3,511 records were not relevant to the Research Topic after title and abstract screening, leaving 52 potentially eligible studies for which the full text was reviewed. Based on the inclusion and exclusion criteria, 9 publications with 10 studies were eligible for inclusion; all nine (10–18) were observational studies. Figure 1 is a flow diagram of the literature search and selection process.
3.2. Study characteristics
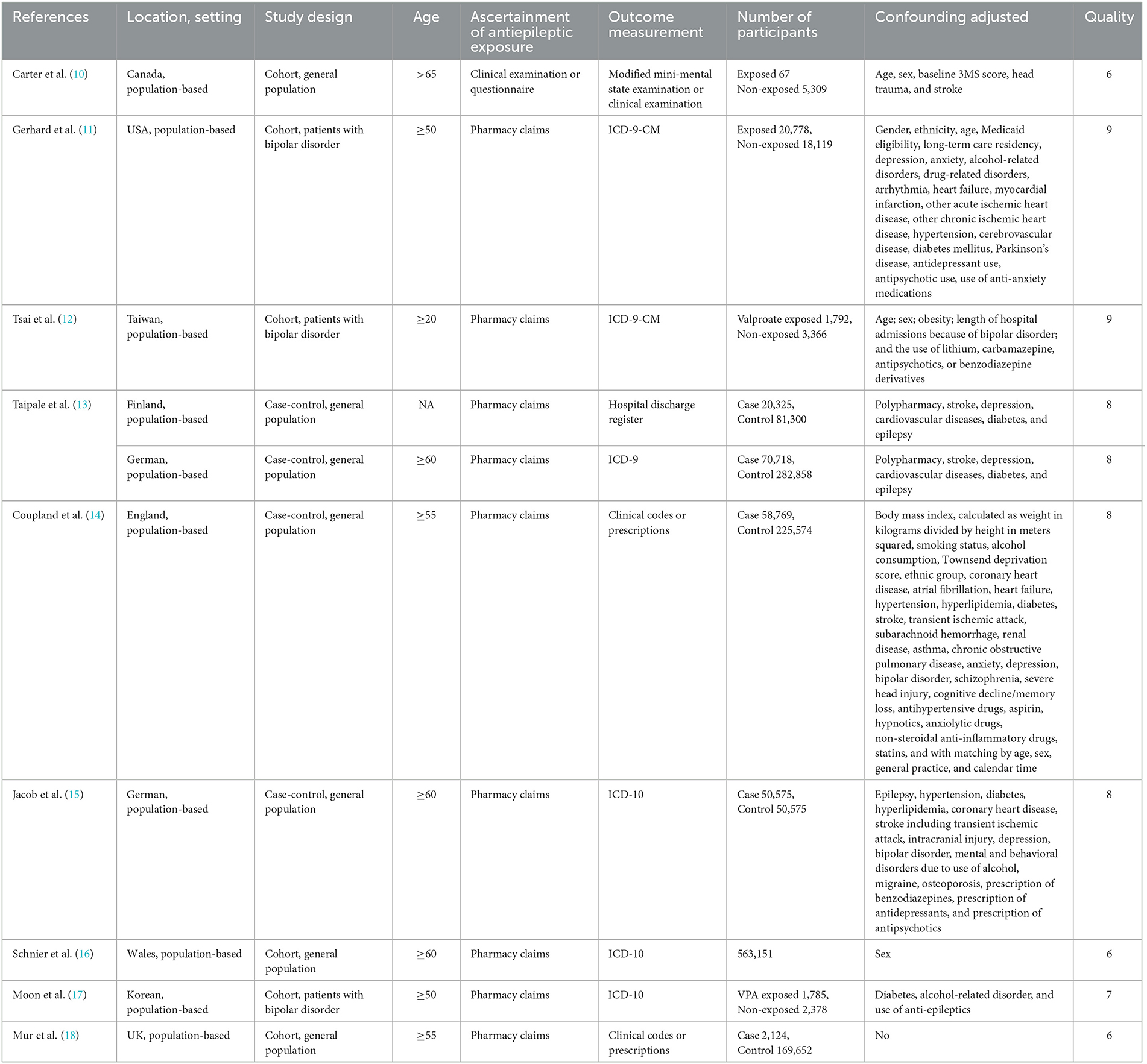
Table 1 summarizes the nine studies considered in this analysis. The studies included 1,629,213 participants from three different continents: five studies from Europe (13–16, 18), two from North America (10, 11), and two studies from Asia (12, 17). The publication year ranged from 2007 to 2022, and the sample sizes of the included studies ranged from 5,158 to 353,576. Exposure to ASMs was assessed using interviews or a drug prescription database. Three studies (11, 12, 17) assessed the use of ASMs and the development of dementia in individuals with bipolar disorder, and the remaining study evaluated this association in a general population. Regarding study quality, the mean NOS score for the nine studies was 8.3, indicating the high-quality of the included studies (Table 1). The score breakdown is given in Supplementary Tables S1, S2.
3.3. Meta-analysis
3.3.1. Association between ASM use and dementia among general population
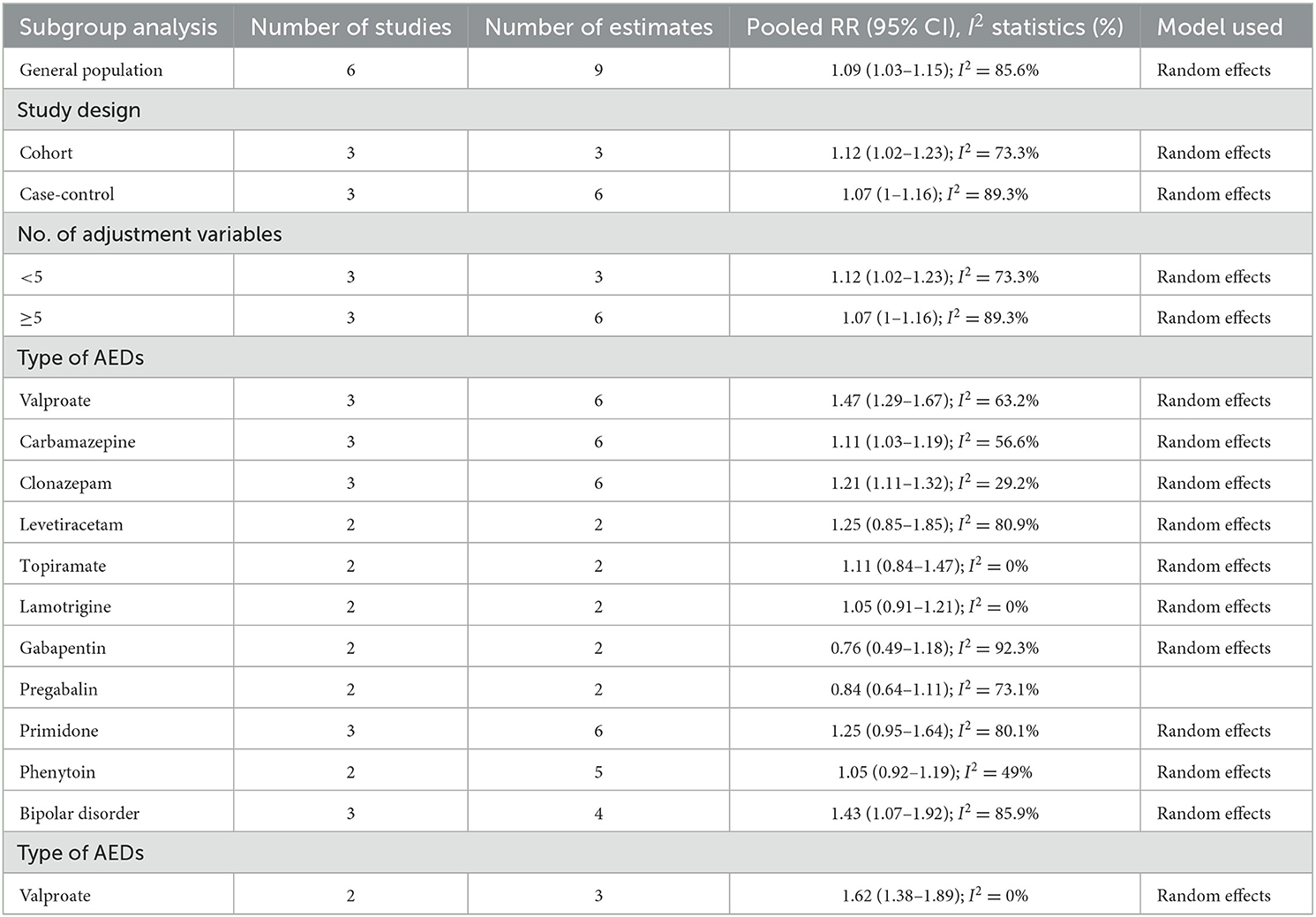
The results of all analyses are listed in Table 2. Six studies measured the relationship between overall ASM exposure and the risk of dementia among the general population. A meta-analysis of these studies with 9 estimates indicated that overall ASM exposure was significantly associated with an increased risk of dementia (OR: 1.09, 95% CI: 1.03–1.15; P = 0.003) (Figure 2). High heterogeneity was observed among these studies (I2 = 85.6%). As shown in Supplementary Figure S1, we did not find any evidence of publication bias (Begg's test, P = 0.3).
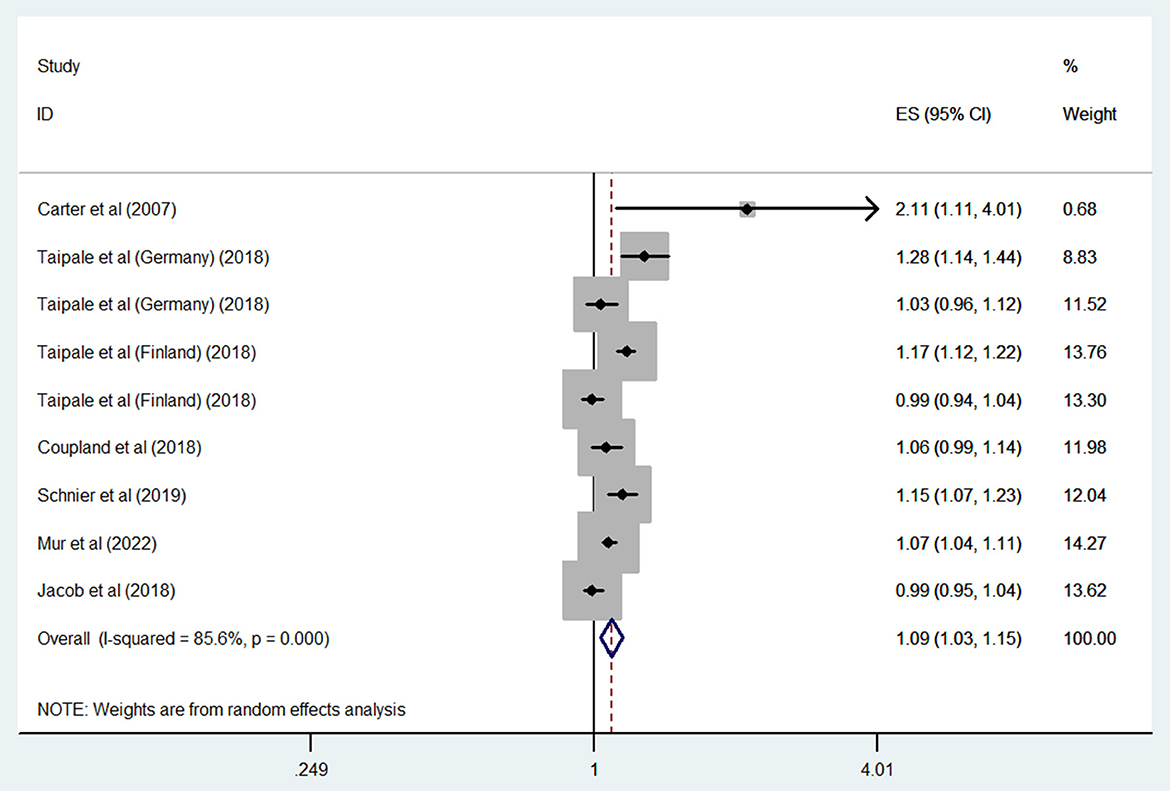
Figure 2. Forest plot of the overall risk of dementia in relation to ASMs use among the general population.
A subgroup analysis by study design found a significant association in cohort studies (OR: 1.12, 95% CI: 1.02–1.23; P = 0.02; I2 = 73.3%), but a non-significant trend toward an increased risk of dementia in case–control studies (OR: 1.07, 95% CI: 1–1.16; P = 0.059; I2 = 89.3%).
Considering the number of adjustment variables revealed a significantly increased dementia risk in those studies adjusting for fewer than five variables (OR: 1.12, 95% CI: 1.02–1.23; P = 0.02; I2 = 73.3%), but no significant association in those adjusting for more than five (OR: 1.07, 95% CI: 1–1.16; P = 0.059; I2 = 89.3%).
When we grouped studies by ASM type, significant associations were observed for those using valproate (OR: 1.47, 95% CI: 1.29–1.67; P < 0.001; I2 = 63.2%), carbamazepine (OR: 1.11, 95% CI: 1.03–1.19; P = 0.004; I2 = 56.6%), or clonazepam (OR: 1.21, 95% CI: 1.11–1.32; P < 0.001; I2 = 29.2%), but no significant association was observed for those using levetiracetam (OR: 1.25, 95% CI: 0.85–1.85; P = 0.253; I2 = 80.9%), topiramate (OR: 1.11, 95% CI: 0.84–1.47; P = 0.452; I2 = 0%), lamotrigine (OR: 1.05, 95% CI: 0.91–1.21; P = 0.527; I2 = 0%), gabapentin (OR: 0.76, 95% CI: 0.49–1.18; P = 0.225; I2 = 92.3%), pregabalin (OR: 0.84, 95% CI: 0.64–1.11; P = 0.227; I2 = 73.1%), primidone (OR: 1.25, 95% CI: 0.95–1.64; P = 0.11; I2 = 80.1%), or phenytoin (OR: 1.05, 95% CI: 0.92–1.19; P = 0.465; I2 = 32.3%).
3.3.2. Association between ASM use and dementia among patients with bipolar disorder
Three studies compared the risk of dementia in bipolar disorder patients who were and were not exposed to ASMs; the combined OR of dementia was 1.43 (95% CI: 1.07–1.92; P = 0.015; I2 = 85.9%) (Figure 3). When our analysis limited to studies only evaluated valproate; the combined OR of dementia was 1.62 (95% CI: 1.38–1.89; P < 0.001; I2 = 0%).
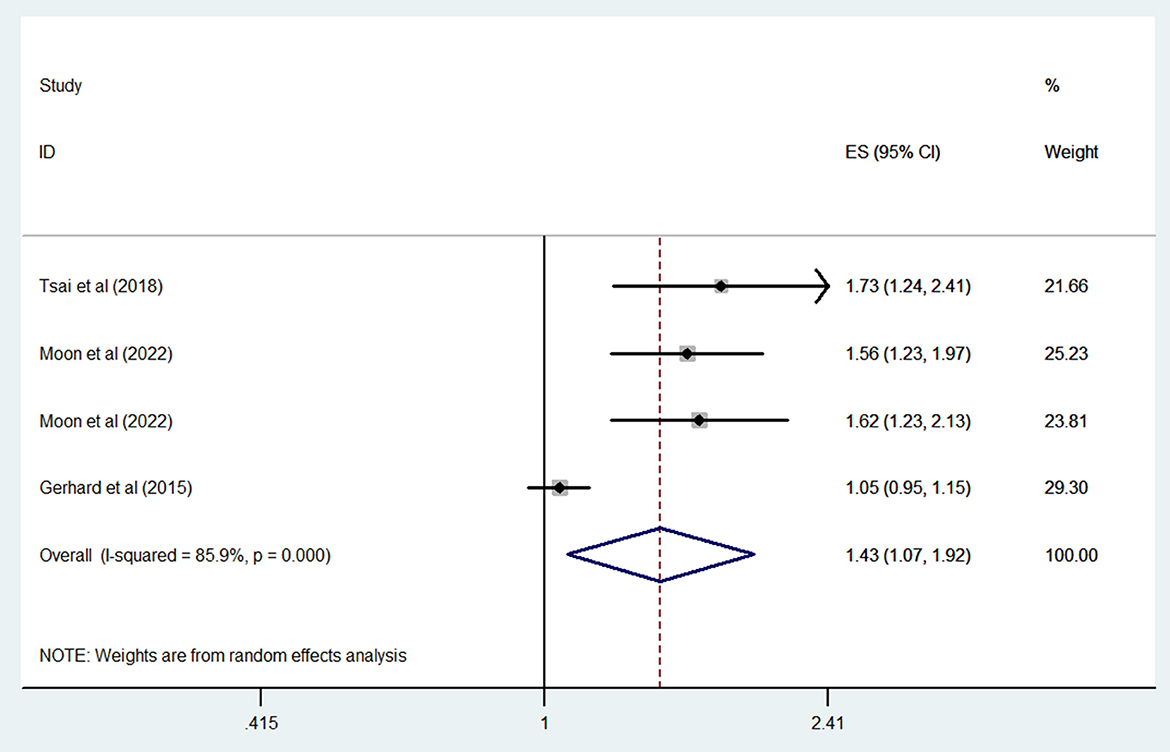
Figure 3. Forest plot of the overall risk of dementia in relation to ASMs use among patients with bipolar disorder.
4. Discussion
This meta-analysis of current observational evidence suggests that the statistically significant association between ASM use and dementia in general population can be partially explained by unmeasured confounding. However, subgroup analyses based on individual ASMs found that only valproate, carbamazepine, and clonazepam were associated with an increased risk of dementia. Furthermore, we found that bipolar disorder patients who were prescribed ASM showed an increased risk of dementia.
The impact of ASM use on cognitive function is controversial. Theoretically, ASMs can adversely affect cognitive functions by suppressing neuronal excitability or enhancing inhibitory neurotransmission (7, 24); however, several studies (25–27) have shown that exposure to several ASMs was associated with improved cognitive function because they also induce the neurogenesis of neural progenitor/stem cells both in vitro and in vivo (28). Consistent with the findings of these preclinical studies, the results of clinical studies that assessed the effects of ASMs on cognitive function or dementia varied. Furthermore, previous reviews (7, 24) have summarized this relationship, but failed to provide an overall estimate of the effects of ASMs on cognitive function or dementia. The authors noted that first-generation drugs had negative effects on cognitive function, but they were not found to increase the risk of dementia.
Although these modifying effects of ASMs on dementia are biologically plausible, the results of the included studies were discordant, as reflected in the high heterogeneity in the overall meta-analysis. This heterogeneity could not be accounted for in the subgroup analyses based on study design, location, or quality; number of adjustments; drug indications; and individual drugs. The existence of clinical heterogeneity should lead to a degree of statistical heterogeneity in the results.
Most of the studies in our overall analysis drew conclusions based on general-population data and did not consider the drug indications. However, epilepsy was shown to be associated with an increased risk of dementia (29). It is reasonable to speculate that this association may be overestimated if the studies did not adjust for this potential confounder. To minimize the effect of indication, we conducted a subgroup analysis based on the number of adjustment variables and found no significant association after we combined the estimates from the included studies adjusted for the drug indication. In addition to epilepsy, ASMs are commonly prescribed to treat bipolar disorder, depression, and other mental disorders (6). Previous meta-analysis have demonstrated that bipolar disorder is associated with an increased risk of dementia (30). Three included studies (11, 12, 17) focused on patients with bipolar disorder and used non-exposed patients as negative controls to minimize the effects of indication. In our meta-analysis, we observed an ~ 1.43-fold increase in the risk of dementia in patients with bipolar disorder who were exposed to ASMs.
The high heterogeneity of the overall analysis may also arise from the types of ASM. In our subgroup analysis of individual ASMs, only valproate, carbamazepine, and clonazepam, which are first-generation ASMs, were found to increase the risk of dementia. Previous studies demonstrated that the main cognitive effects of ASM use were impaired attention, vigilance, and psychomotor speed. One double-blind, placebo-controlled study (31) reported convincing evidence of improved motor skills after discontinuing valproate in patients with epilepsy. Two double-blind, placebo-controlled studies (32, 33) involving epilepsy patients on ASM monotherapy (mainly carbamazepine or valproate) observed that drug discontinuation significantly improved performance in tests that required complex cognitive processing under time pressure. However, most studies (25, 34, 35) tend to report little or no cognitive impairment associated with pregabalin or gabapentin in people with partial epilepsy. Consistent with the cognitive findings in epilepsy patients, our individual ASM analysis found that newer ASMs act more favorably on dementia risk compared with first-generation drugs. Recently, preclinical studies demonstrated a protective effect of levetiracetam on cognitive function. In the transgenic mice models of Alzheimer's disease, a low dose of levetiracetam could alleviate cognitive decline, through suppression of proinflammatory cytokines expression and inhibition of abnormal tau hyperphosphorylation (36, 37). In clinical study, levetiracetam improved performance on spatial memory and executive function tasks in patients with Alzheimer's disease (38). However, the beneficial role of levetiracetam on dementia was not detected in our analysis. Hence, our results of individual ASM on risk of dementia may be limited by sample size and need further investigation to clarify this issue.
To our knowledge, this meta-analysis is the first to explore the association between ASM use and dementia risk. The strengths of this work are the comprehensive search and the rigorous systematic review and meta-analysis of all relevant reports to date. We also performed several additional analyses to test the robustness of the results. Nonetheless, there are several limitations to this meta-analysis. First, residual confounders are always a concern in epidemiological observational studies. Second, all of the included studies considered Western populations and not subjects from Asia or Africa, which may have affected the generalizability of our results. Third, information on the dose of ASM used in the included studies could not be extracted; therefore, any exposure parameter possibly associated with dementia could not be defined.
In summary, this systematic review and meta-analysis only observed a greater risk of dementia with the use of valproate, carbamazepine, or clonazepam in general population. We also found that ASMs are associated with an increased risk of dementia in bipolar disorder. However, large, well-designed, prospective cohort studies that consider a greater number of confounding factors are warranted to verify our findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LZ and H-yJ searched the library, wrote the manuscript text, extracted data, and reviewed all articles. W-jL designed the manuscript. All authors reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1133816/full#supplementary-material
References
1. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. (2020) 396:413–46. doi: 10.1016/S0140-6736(20)30367-6
2. GBD 2017 US Neurological Disorders Collaborators, Feigin VL, Vos T, Alahdab F, Amit AML, Bärnighausen TW, et al. Burden of neurological disorders across the US from 1990–2017: a global burden of disease study. JAMA Neurol. (2021) 78:165–76. doi: 10.1001/jamaneurol.2020.4152
3. GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. (2022) 7:e105–25. doi: 10.1016/S2468-2667(21)00249-8
4. GBD 2016 Dementia Collaborators. Global, regional, and national burden of Alzheimer's disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. (2019) 18:88–106. doi: 10.1016/S1474-4422(18)30403-4
5. Tsai FJ, Shen SW. Concepts of dementia prevention in the health promotion among older adults: a narrative review. Medicine. (2022) 101:e32172. doi: 10.1097/MD.0000000000032172
6. Cacabelos R. Pharmacogenomics of cognitive dysfunction and neuropsychiatric disorders in dementia. Int J Mol Sci. (2020) 21:3059. doi: 10.3390/ijms21093059
7. Beghi E, Beghi M. Epilepsy, antiepileptic drugs and dementia. Curr Opin Neurol. (2020) 33:191–7. doi: 10.1097/WCO.0000000000000802
8. Pan Y, Davis PB, Kaebler DC, Blankfield RP, Xu R. Cardiovascular risk of gabapentin and pregabalin in patients with diabetic neuropathy. Cardiovasc Diabetol. (2022) 21:170. doi: 10.1186/s12933-022-01610-9
9. Cheng HH, Huang WC, Jeng SY. Anti-epileptic drugs associated with fractures in the elderly: a preliminary population-based study. Curr Med Res Opin. (2019) 35:903–7. doi: 10.1080/03007995.2018.1541447
10. Carter MD, Weaver DF, Joudrey HR, Carter AO, Rockwood K. Epilepsy and antiepileptic drug use in elderly people as risk factors for dementia. J Neurol Sci. (2007) 252:169–72. doi: 10.1016/j.jns.2006.11.004
11. Gerhard T, Devanand DP, Huang C, Crystal S, Olfson M. Lithium treatment and risk for dementia in adults with bipolar disorder: population-based cohort study. Br J Psychiatry. (2015) 207:46–51. doi: 10.1192/bjp.bp.114.154047
12. Tsai P-S, Liu I-C, Chiu C-H, Huang C-J, Wang M-Y. Effect of valproic acid on dementia onset in patients with bipolar disorder. J Affect Disord. (2016) 201:131–6. doi: 10.1016/j.jad.2016.05.010
13. Taipale H, Gomm W, Broich K, Maier AM W, Tolppanen, Tanskanen A, et al. Use of antiepileptic drugs and dementia risk-an analysis of finnish health register and german health insurance data. J Am Geriatr Soc. (2018) 66:1123–9. doi: 10.1111/jgs.15358
14. Coupland CAC, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J. Anticholinergic drug exposure and the risk of dementia: a nested case-control study. JAMA Intern Med. (2019) 179:1084–93. doi: 10.1001/jamainternmed.2019.0677
15. Jacob L, Bohlken J, Kostev K. Is there an association between antiepileptic drug use and dementia risk? A case-control study. J Alzheimers Dis. (2019) 68:97–103. doi: 10.3233/JAD-181194
16. Schnier C, Duncan S, Wilkinson T, Mbizvo GK, Chin RFM. A nationwide, retrospective, data-linkage, cohort study of epilepsy and incident dementia. Neurology. (2020) 95:e1686–93. doi: 10.1212/WNL.0000000000010358
17. Moon W, Ji E, Shin J, Kwon JS, Kim KW. Effect of valproate and lithium on dementia onset risk in bipolar disorder patients. Sci Rep. (2022) 12:14142. doi: 10.1038/s41598-022-18350-1
18. Mur J, Russ TC, Cox SR, Marioni RE, Muniz-Terrera G. Association between anticholinergic burden and dementia in UK Biobank. Alzheimers Dement. (2022) 8:e12290. doi: 10.1002/trc2.12290
19. Higgins JP. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane collaboration (2014). Available online at: www.cochrane-handbook.org (accessed December 06, 2014).
20. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
21. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
22. Irwig L, Macaskill P, Berry G, Glasziou P. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
23. Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. (2006) 333:597–600. doi: 10.1136/bmj.333.7568.597
24. Eddy CM, Rickards HE, Cavanna AE. The cognitive impact of antiepileptic drugs. Ther Adv Neurol Disord. (2011) 4:385–407. doi: 10.1177/1756285611417920
25. Mortimore C, Trimble M, Emmers E. Effects of gabapentin on cognition and quality of life in patients with epilepsy. Seizure. (1998) 7:359–64. doi: 10.1016/S1059-1311(05)80003-2
26. Seidel WT, Mitchell WG. Cognitive and behavioral effects of carbamazepine in children: data from benign rolandic epilepsy. J Child Neurol. (1999) 14:716–23. doi: 10.1177/088307389901401106
27. Kälviäinen R, Aikiä M, Saukkonen AM, Mervaala E, Sr PJR. Vigabatrin vs carbamazepine monotherapy in patients with newly diagnosed epilepsy. A randomized, controlled study. Arch Neurol. (1995) 52:989–96. doi: 10.1001/archneur.1995.00540340081016
28. Zhang XZ, Li XJ, Zhang HY. Valproic acid as a promising agent to combat Alzheimer's disease. Brain Res Bull. (2010) 81:3–6. doi: 10.1016/j.brainresbull.2009.09.003
29. Tang T, Zhang R, Pan X. Meta-analysis of the risk of dementia in elderly patients with late-onset epilepsy. Clin Neurol Neurosurg. (2022) 223:107499. doi: 10.1016/j.clineuro.2022.107499
30. Stafford J, Chung WT, Sommerlad A, Kirkbride JB, Howard R. Psychiatric disorders and risk of subsequent dementia: systematic review and meta-analysis of longitudinal studies. Int J Geriatr Psychiatry. (2022) 11:37. doi: 10.1002/gps.5711
31. Duncan JS, Shorvon SD, Trimble MR. Discontinuation of phenytoin, carbamazepine, and valproate in patients with active epilepsy. Epilepsia. (1990) 31:324–33. doi: 10.1111/j.1528-1157.1990.tb05383.x
32. Hessen E, Lossius MI, Reinvang I, Gjerstad L. Influence of major antiepileptic drugs on attention, reaction time, and speed of information processing: results from a randomized, double-blind, placebo-controlled withdrawal study of seizure-free epilepsy patients receiving monotherapy. Epilepsia. (2006) 47:2038–45. doi: 10.1111/j.1528-1167.2006.00805.x
33. Hessen E, Lossius MI, Gjerstad L. Antiepileptic monotherapy significantly impairs normative scores on common tests of executive functions. Acta Neurol Scand. (2009) 119:194–8. doi: 10.1111/j.1600-0404.2008.01109.x
34. Valentin A, Moran N, Hadden R, Oakes A, Elwes R, Delamont R, et al. Pregabalin as adjunctive therapy for partial epilepsy: an audit study in 96 patients from the South East of England. Seizure. (2009) 18:450–2. doi: 10.1016/j.seizure.2009.01.001
35. French JA, Kugler AR, Robbins JL, Knapp LE, Garofalo EA. Dose-response trial of pregabalin adjunctive therapy in patients with partial seizures. Neurology. (2003) 60:1631–7. doi: 10.1212/01.WNL.0000068024.20285.65
36. Zheng X-Y, Zhang H-C, Lv Y-D, Jin F-Y, Wu X-J, Zhu J, et al. Levetiracetam alleviates cognitive decline in Alzheimer's disease animal model by ameliorating the dysfunction of the neuronal network. Front Aging Neurosci. (2022) 14:888784. doi: 10.3389/fnagi.2022.888784
37. Silva JC, Shen Y, Chan J, Kwan P, Jones NP. Anti-epileptogenic effects of synaptic vesicle protein 2A modulation in a mouse model of Alzheimer's disease. Epilepsy Res. (2022) 186:106994. doi: 10.1016/j.eplepsyres.2022.106994
Keywords: anti-seizure, second generation, cognitive, systematic, meta-analysis
Citation: Zhang L, Jiang H-y and Liu W-j (2023) Anti-seizure medication exposure and the risk of dementia: A meta-analysis of observational studies. Front. Neurol. 14:1133816. doi: 10.3389/fneur.2023.1133816
Received: 29 December 2022; Accepted: 28 February 2023;
Published: 22 March 2023.
Edited by:
Pamela J. Lein, University of California, Davis, United StatesReviewed by:
Sanjeev Kumar, University of Toronto, CanadaAlina Arulsamy, Jeffrey Cheah School of Medicine and Health Sciences, Monash University, Malaysia
Copyright © 2023 Zhang, Jiang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen-juan Liu, bGl1d2VuanVhbjIwMjIyMDIyQDE2My5jb20=
 Lei Zhang1
Lei Zhang1 Hai-yin Jiang
Hai-yin Jiang Wen-juan Liu
Wen-juan Liu