
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 12 May 2023
Sec. Stroke
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1131283
This article is part of the Research TopicPost-Stroke Complications: Mechanisms, Diagnosis, and TherapiesView all 20 articles
Objective: The aim of this study was to evaluate the effects of stereotactic minimally invasive puncture with different catheter placement positions when combined with urokinase thrombolysis for the treatment of small- and medium-volume basal ganglia hemorrhage. Our goal was to identify the best minimally invasive catheter placement position to enhance therapeutic efficacy for patients with cerebral hemorrhage.
Methods: The stereotactic minimally invasive thrombolysis at different catheter positions in the treatment of small- and medium-volume basal ganglia hemorrhage (SMITDCPI) was a randomized, controlled, and endpoint phase 1 trial. We recruited patients with spontaneous ganglia hemorrhage (medium-to-small and medium volume) who were treated in our hospital. All patients received stereotactic, minimally invasive punctures combined with an intracavitary thrombolytic injection of urokinase hematoma. A randomized number table method was used to divide the patients into two groups concerning the location of catheterization: a penetrating hematoma long-axis group and a hematoma center group. The general conditions of the two groups of patients were compared, and the data were analyzed, including the time of catheterization, the dosage of urokinase, the amount of residual hematoma, the hematoma clearance rate, complications, and the National Institute of Health stroke scale (NIHSS) score data at 1 month after surgery.
Results: Between June 2019 and March 2022, 83 patients were randomly recruited and assigned to the two groups as follows: 42 cases (50.60%) to the penetrating hematoma long-axis group and 41 cases (49.40%) to the hematoma center group. Compared with the hematoma center group, the long-axis group was associated with a significantly shorter catheterization time, a lower urokinase dose, a lower residual hematoma volume, a higher hematoma clearance rate, and fewer complications (P < 0.05). However, there were no significant differences between the two groups in terms of the NIHSS scores when tested 1 month after surgery (P > 0.05).
Conclusion: Stereotactic minimally invasive puncture combined with urokinase for the treatment of small- and medium-volume hemorrhage in the basal ganglia, including catheterization through the long axis of the hematoma, led to significantly better drainage effects and fewer complications. However, there was no significant difference in short-term NIHSS scores between the two types of catheterization.
Stroke is currently regarded as the second largest contributor to global disability-adjusted life years (DALYs) in developing countries (1). Although spontaneous intracerebral parenchymal hemorrhage (IPH) accounts for <20% of strokes, this condition is associated with high rates of morbidity and mortality. The bleeding site is usually located in the deep gray matter, including the basal ganglia and the thalamus (2). The recent results arising from the minimally invasive surgery plus alteplase for cerebral hemorrhage (MISTIE) III trial showed that minimally invasive surgery is safer than drug therapy and that thrombolysis after minimally invasive catheter evacuation reduces the size of hematomas by up to 15 ml, thus reducing mortality and improving prognosis (3). The MISTIE II trial demonstrated that the effects of surgery were directly associated with the position of the catheter. In this trial, the catheter was positioned along the entire longitudinal axis of the hematoma (defined as at least two-thirds of the longitudinal length). Stereotactic techniques for the placement of the drainage tube are known to be more accurate and controlled in the treatment of small-volume hemorrhage in the basal ganglia and have achieved effective outcomes (4, 5). Therefore, the purpose of this manuscript is to explore the effect of different catheter placement positions on the treatment of moderate to small amounts of hypertensive intracerebral hemorrhage.
In this trial, we recruited 95 neurosurgery patients from Yichang Three Gorges Central People's Hospital between June 2019 and March 2022 to receive stereotactic minimally invasive puncture and catheter placement combined with urokinase for the treatment of medium-to-small hemorrhages in the basal ganglia. Of these, 12 patients were surgical patients in the early stage of hemorrhages, and 83 patients were in the later stage of hemorrhages. The patients were divided into two groups by using a randomized number table method. According to the location of the catheter, the patients were divided into two groups as follows: those who were catheterized through the long axis of the hematoma (42 cases through the frontal approach) and those who received catheterization through the center of the hematoma (41 cases through the frontal–parietal approach). This trial complied with the relevant ethical standards and was approved by our hospital's ethics committee (KY-2022-0040). Signed and informed consent was obtained from all the patients or their legal representatives. The inclusion criteria were as follows: (1) all patients who were diagnosed with basal ganglia hemorrhage by head Computed Tomography (CT) and Computed Tomography Artery (CTA) examination on admission, according to the Guidelines for the Diagnosis and Treatment of Cerebral Hemorrhage in China (2019); (2) patients with a history of hypertension; (3) patients affected by basal ganglia hemorrhage for the first time and having no previous neurological dysfunction; and (4) patients with a bleeding volume in the basal ganglia of 20–40 ml. The exclusion criteria were as follows: (1) patients with severe coagulation dysfunction or severe basic diseases; (2) patients with surgical contraindications; (3) patients whose hemorrhage had broken into ventricles; and (4) patients whose hemorrhage was caused by brain tumors, cerebral aneurysms, cerebral vascular malformations, and other reasons (6).
We collated a range of general clinical data for each patient (Table 1), including gender, age, hematoma volume at admission, time from onset to visit, blood pressure at admission, Glasgow Coma Scale (GCS) at admission, smoking history, compliance with hyperlipidemia drugs, antiplatelet therapy, diabetes, hypertension, random blood pressure, other cardiovascular diseases, NIHSS score, and the time from stroke to first CT at admission.
According to the maximum level of hematoma selected by the CT cross-section, the longest diameter of the hematoma (cm) and the widest diameter of the hematoma (cm) were measured on the imaging system. The layer thickness was determined from the CT film, and the number of layers was defined as the total number of layers showing all hematomas. The volume of cerebral hematoma was calculated by the Toda formula (7), where hematoma volume (ml) = 1/2 × the longest diameter of the hematoma (cm) × the widest diameter of the hematoma (cm) × slice thickness (cm) × layers.
Surgery can be completed using a frameless navigation system or a frame-based stereotactic system; in this trial, we used the Leskell G-frame system (Elekta Instrument AB, Box 7593,Kungstensgatan 18, SE-103 93 Stockholm, Sweden). Patients underwent surgery if CT reexamination indicated that the hematoma was stable after 6 h or more. All patients received stereotactic minimally invasive catheter drainage surgery. For patients in the long-axis group, the incision was 2–3 cm above the eyebrow arch on the same side of the hematoma and 2–2.5 cm beside the midline. The planned puncture approach and target point before surgery were through the hematoma (Figures 1A–E). The incision was made 9–11 cm above the eyebrow arch on the same side of the hematoma and 3–3.5 cm beside the midline for patients in the central group. The planned puncture approach and target were in the center of the hematoma before surgery (Figures 2A–E). The first step was to install a square Leksell headrest on the patient's head under local anesthesia. The second step was to perform 64 rows of Siemens head 3D CT scanning. Third, according to the preoperative surgical plan, we needed to directly measure and calculate the target coordinates (X, Y, and Z space coordinates) using a CT three-dimensional film reading system. Fourth, for patients in the long-axis group, we removed a fixed strut at the surgical side of the forehead; this action was not required for patients in the center group. The Leskell headstand was connected and fixed to a special neurosurgery operating table by an adapter. Next, surgery was performed under local anesthesia and ECG monitoring. The size of the incision was generally 2 cm. After drilling the skull, a 5-mm incision was made in the dura mater to avoid the excessive outflow of cerebrospinal fluid. The Leskell guide arc was then installed, and the drainage tube was inserted under the guidance of the stereotactic instrument to reach the target point. During surgery, 1 ml of negative pressure was used to draw ~5 ml of blood; the same volume of normal saline was injected for replacement. When the drainage was smooth, the surgical procedure was completed, and the drainage tube was led out through another subcutaneous tunnel.
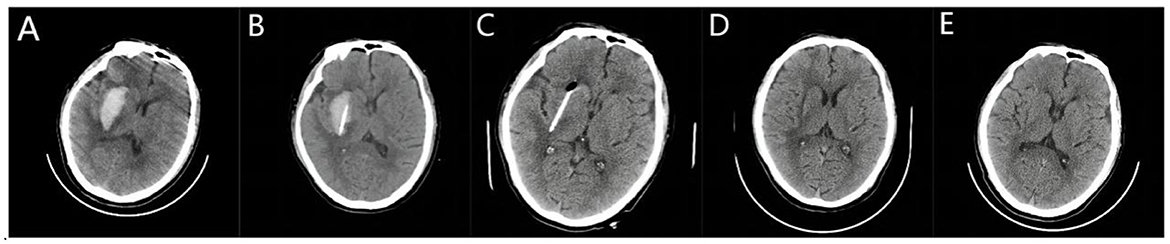
Figure 1. Stereotactic catheterization through the long axis of hematoma for basal ganglia hemorrhage. (A) A preoperative head Computed Tomography (CT) showed that the bleeding volume was ~29 ml. (B) A three-dimensional CT performed 6 h after surgery confirmed the position of the drainage tube along the long axis of the hematoma, with a hematoma volume of ~20 ml. (C) Following the injection of urokinase, the hematoma was completely drained by the third day after surgery. The high-density area indicates the drainage tube with a hematoma volume of 0 ml. (D) The bleeding site 7 days after surgery was slightly softer. (E) On the 20th day post-surgery, the softening lesion at the bleeding site was reduced.
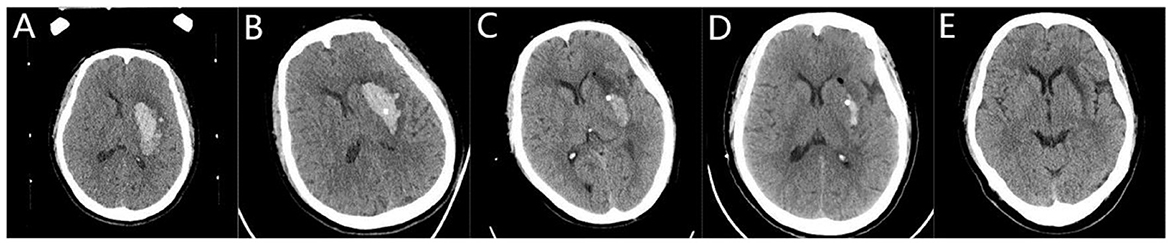
Figure 2. Stereotactic central catheterization for basal ganglia hemorrhage. (A) A preoperative head Computed Tomography (CT) showed that the volume of bleeding was ~26 ml. (B) A three-dimensional CT performed 6 h after surgery showed the drainage tube located in the center of the hematoma with a hematoma volume of ~17 ml. (C) Following the injection of urokinase, there was a small amount of residual hematoma on the third day after the operation, with a hematoma volume of 5 ml. (D) On the 7th day after surgery, the hematoma volume was 1.2 ml. (E) On the 20th day after surgery, the bleeding site softened.
We performed another three-dimensional CT scan of the head 6 h after surgery to determine the position of the drainage tube, the size of the hematoma, and the extent of bleeding in the puncture tunnel. If the drainage tube position was satisfactory, we fully dissolved 50,000 units of urokinase in 5 ml of normal saline. After surgery, the dissolved urokinase was injected into the drainage tube 1–2 times a day, and the drainage tube was closed for 3 h before natural drainage. According to the residual situation of the hematoma, urokinase was injected multiple times into the hematoma cavity, as required. The CT reexamination showed that the drainage tube cannot effectively drain the hematoma, which was the extubation index. The catheterization time did not exceed 7 days.
We compared the general clinical data from the two groups of patients. A range of statistical data was collated, including the time of catheter insertion, the total dose of urokinase, the clearance rate of hematoma before extubation, the amount of residual hematoma, and the NIHSS score 1 month after surgery. We also recorded complications experienced by the two groups of patients during treatment, including intracranial infection, pulmonary infection, urinary system infection, secondary intracranial hemorrhage, lower limb vein thrombosis, stress ulcer, anemia, hypoproteinemia, and mortality within 1 month.
The SPSS version 25.0 statistical software (IBM, International Business Machines Corporation, New York, USA) was used for data analysis. Measurement data were expressed as mean ± standard deviation (x ± s). Comparisons between groups of normally distributed data were performed using the independent sample t-test and analysis of variance (ANOVA). The Mann–Whitney U-rank sum test was used for the data that were not normally distributed. Numerical data were expressed as the number of cases and as percentages (%), and the data were compared using the chi-square test. A P-value of < 0.05 (bilateral) was considered to be statistically significant.
From June 2019 to March 2022, we recruited 95 patients, including 12 who underwent surgery in the early stage. In this trial, 83 patients with medium-to-small volumes of hemorrhage in the basal ganglia were included for final analysis (Figure 3). Of these patients, 42 of them were catheterized through the long axis of the hematoma, and 41 were catheterized through the center of the hematoma. Both patient groups were injected with urokinase through the drainage tube after surgery, as shown in Table 1.
Compared with the central group, patients in the long-axis group had a significantly shorter catheterization time, a lower dose of urokinase, less residual hematoma, and a higher hematoma clearance rate (P < 0.05). There was no significant difference between the two groups in terms of NIHSS scores 1 month after surgery (P > 0.05) as shown in Table 2.
There was no significant difference between the two groups in terms of pulmonary infection, intracranial hemorrhage, urinary system infection, stress ulcer, anemia, and mortality within 1 month of surgery (P > 0.05). Compared with the central hematoma group, the incidence of intracranial infection, deep vein thrombosis, and hypoproteinemia in the long-axis group was significantly lower (P < 0.05), as shown in Table 3.
Hypertensive intracerebral hemorrhage (HICH) accounts for 21–48% of all patients with stroke. The most common type of HICH is basal ganglia hemorrhage (8), which accounts for 50% of patients with cerebral hemorrhage. In addition, patients often experience symptoms such as hemianopsia, hemiplegia, and hemiparesia due to damage to the cystic conduction tract in the basal ganglia region. It is very important to evaluate the neurological function of patients with cerebral hemorrhage in a timely fashion and determine whether surgical treatment is needed. There is no definite conclusion on the effects of surgical treatment for supratentorial intracerebral hemorrhage, although surgery is an option for patients with a large hematoma, with severe neurological dysfunction, or in coma. The surgical methods involve craniotomy and minimally invasive surgery. The mortality rate associated with craniotomy is very high. The surgical trials in intracerebral hemorrhage (STICH) and STICH II trials showed that patients with spontaneous supratentorial intracerebral hemorrhage did not have any overall benefit from early surgery when compared with conservative treatment (9, 10). However, 98% of patients in the STICH study underwent craniotomy; the researchers thus proposed that minimally invasive technology is more conducive for deep brain hematoma. Studies have reported that minimally invasive surgery is better than craniotomy (11–13). Minimally invasive surgical methods involve keyhole craniotomy, neuroendoscopy hematoma removal, and minimally invasive catheter drainage. In the MISTIE II trial, a stereotactic catheter was used for catheter placement and continuous thrombolysis; this method achieved accurate catheter placement. This procedure is highly reproducible and can safely and accurately drain a cerebral hemorrhage; the effects of hematoma drainage are closely associated with the accuracy of catheter placement. Currently, most minimally invasive catheters for intracerebral hemorrhage are placed at the midpoint of the largest hematoma layer in the brain as the puncture sites (14–16). However, few studies have investigated the effects of different catheter positions on hematoma drainage. In this study, we describe the results of our recent trial that examined the effects of different catheter positions for intracerebral hemorrhage.
The target error for stereotactic technology was previously determined to be 2.02 mm (17). This type of technology is widely adopted in minimally invasive neurosurgery, such as deep brain electrical stimulation in Parkinson's disease, epileptic electrode implantation, brain tumor biopsy, stereotactic puncture, and the aspiration of cerebral hemorrhage (18, 19). Compared with conservative treatment, the rebleeding rate of minimally invasive stereotactic catheterization in 20–40 ml HICH was significantly lower than that for conservative medical treatment. Furthermore, a hematoma can be cleared quickly, thus reducing compression caused by the hematoma on the brain tissue and the risk of secondary damage; this is conducive to the recovery of a patient's neural function, thus reducing hospital stay (20). In previous studies, when the amount of cerebral hemorrhage was >30 ml, craniotomy or endoscopic surgery was mostly used. Compared with craniotomy, stereotactic catheterization for an intracerebral hemorrhage of more than 30 ml is safe and effective and has fewer complications (21). The clinical effect of this treatment is better than that of traditional craniotomy and can thus avoid secondary cranioplasty. In our research, we found that the hematoma volume was more than 30 ml in some patients and 20–30 ml in others. The preoperative hematoma volume was 26.64 ± 3.48 ml in the long-axis group and 25.41 ± 3.78 ml in the central group. We found a relationship between hematoma volume and the enrollment data. Currently, minimally invasive surgery for intracerebral hemorrhage is regarded as a trend, but there is no specific standard for endoscopic surgery and stereotactic minimally invasive puncture (19). For 40–60 ml of bleeding, we prioritize craniotomy or endoscopic hematoma evacuation. Endoscopic intracerebral hemorrhage clearance has also advanced significantly. Endoscopic surgery can enhance the neurological function of patients with more than 40 ml of intracerebral hemorrhage at 6 months after surgery and can reduce the mortality of patients with a GCS score of 3–8 (22). Endoscopic surgery can quickly remove a hematoma, although the trauma incurred is relatively significant, thus requiring high levels of surgical skill. Moreover, the operation takes a long period and requires general anesthesia. Stereotactic minimally invasive catheterization is minimally invasive and can be performed under local anesthesia. Furthermore, the surgical time is reduced, and the operation is reproducible; however, the disadvantage of this technique is that a hematoma cannot be quickly removed, and the rate of rebleeding can be high (23). Stereotactic guidance can clear a hematoma along the long axis of the hematoma, combined with endoscopic removal of the intracerebral hemorrhage. Most blood clots can be cleared only once through the endoscopic sheath; this can minimize damage to normal brain tissue (8). Some studies have shown that the disability rate associated with stereotactic catheterization for 20–50 ml superficial and deep hemorrhage is lower than that of small bone window craniotomy and endoscopic surgery (19). Some elderly patients have a strong tolerance to increased intracranial pressure due to the presence of brain atrophy. We have attempted to use stereotactic minimally invasive catheterization in elderly patients with cerebral hemorrhage volumes exceeding 40 ml. Currently, only 10 cases of surgery have been attempted, although the final treatment effect is very good.
In the MISTIE III trial, three methods of catheterization were adopted. The first method was to cut the forehead and insert a catheter along the longitudinal axis of the hematoma. The second method was to cut the parietal–occipital part and place a tube along the longitudinal axis of the hematoma. The third method was to cut the temporal part and place a tube along the transverse axis of the hematoma (3). The highest proportion of patients with hematomas smaller than 15 ml was the highest (79.3%) at the end of tube insertion when using the first method. When the drainage tube is located in the center of the hematoma, theoretically, it can make urokinase contact the hematoma more evenly, thus improving the dissolution effect of the hematoma; this can improve the hematoma clearance rate when the tube is withdrawn (24). In fact, in most cases, we found that when the catheter was placed in the center of the hematoma, although the effect of early hematoma dissolution was very good, there was a residual hematoma at the back when the catheter was removed. In our 41 patients, we found that the hematoma clearance rate was 90.83 ± 5.80%. We analyzed the causes of residual hematoma in the posterior regions. Most patients with intracerebral hemorrhage had their heads raised by 30° during surgery. Due to positional factors and the gravity of the brain tissue itself, the hematoma around and in front of the drainage tube hole can often provide easy contact with urokinase. Most of the hematomas were successfully drained, thus resulting in the drainage tube hole being located at the edge of the posterior residual hematoma. Without further surgery, the drainage tube hole cannot be adjusted to contact the hematoma again; therefore, the urokinase injected through the catheter cannot fully contact the posterior hematoma because of the residue. When inserting the tube through the long axis of the hematoma, the catheter hole is located at the rear of the hematoma, and the urokinase can dissolve the hematoma around the drainage tube hole (25). The direction of hematoma dissolution occurs from back to front. When the current hematoma dissolution and drainage were poor, we rechecked the CT to investigate the relationship between the drainage tube and the hematoma. Because the tube is placed through the long axis of the hematoma, this was performed by withdrawing part of the drainage tube to make the tube hole make full contact with the hematoma again, thus reducing residuals. The clearance rate of hematoma for the 42 patients was 93.71 ± 4.33%. In four cases, the drainage tube was partially withdrawn to achieve a satisfactory drainage effect. According to the MISTIE III trial and other research results, we recommend transfrontal long-axis catheterization for hematomas. This method has a good drainage effect, can reduce the number of urokinase injections, and can reduce the risk of intracranial infection.
The survival rate of patients with intracerebral hemorrhage and the recovery of neurological function are related to the location of the hematoma, space-occupying effects, and intracranial pressure; these factors are also related to the long-term neurological dysfunction caused by neurotoxicity or inflammatory brain edema around the hematoma (26, 27). Numerous preclinical and clinical studies have shown that perihematoma edema (PHE) is a quantifiable marker for secondary brain injury after intracerebral hemorrhage (ICH) and is associated with poor prognosis (28). In ~30% of patients, the volume of PHE 2–3 weeks after ICH was 3 ml larger than that 1 week after ICH; this increase in volume was reported to represent an independent risk factor for poor prognosis (29). Minimally invasive surgery may change the course of PHE. The extent of edema in PHE was associated with hematoma clearance, and a higher percentage of hematoma clearance has been shown to result in a slower increase in PHE (30). In a previous study, 28% of patients with a residual hematoma volume of >15 ml had an Modified Rankin Scale (mRS) of ≤ 3 at 180 days, and 49% of patients with a residual hematoma volume of ≤ 15 ml had an mRS of ≤ 3 at 180 days (31). The increase in PHE at 72 h may have had adverse effects on the neurological recovery of ICH, especially in patients with small-to-medium volume hematomas (32). Increasing the clearance rate of residual hematomas after surgery is conducive to reducing brain injury after ICH (33). Our study showed that there was no significant difference in short-term neurological recovery between the two groups; this may be related to the reduction in a residual hematoma or the short follow-up time of the two groups of patients during extubation. There was a certain difference in the residual amount of hematoma between the two different methods of catheter insertion, although the hematoma was < 15 ml in volume when the catheter was removed. Common medical complications after intracerebral hemorrhage include pneumonia (24%), acute renal injury (9%), ventricular inflammation (5%), asymptomatic rebleeding (4%), ischemic stroke (3%), surgical infection (1%), and others (31, 34). In the acute phase of intracerebral hemorrhage, the reduction in hematoma volume is conducive to reducing potential medical complications (35). Our study also confirmed that patients with a higher hematoma clearance rate had fewer complications in the short term.
Because the stereotactic technique was not adopted in the MISTIE III trial, the rate of good catheter placement was 62%, although 6.8% of patients with poor catheter placement needed replacement of the drainage tube. We adopted the stereotactic technique to insert the catheter in 83 cases; proper placement of the drainage tube was achieved in 97.59% of cases. No cases required replacement of the drainage tube; this instance depends on the improvement of the method used to replace the catheter during surgery. To improve the accuracy of stereotactic catheterization, the following points should be considered: (1) avoiding the implantation of soft drainage tubes through the broken suction needle path in the hematoma; (2) selecting a drainage tube that matches the aperture of the guide sub; (3) directly using a soft drainage tube with a hard tube core for puncture; (4), when the drainage tube is located at the target position, the first suction of blood does not exceed 5 ml; and (5) adjusting the position of the Leksell head frame bone nail according to the hematoma location to avoid the influence of artifacts of skull bone nails during CT scanning. The following points should also be considered to reduce surgical-related complications: (1) reducing the number of urokinase injections can reduce the risk of infection; (2) as some patients may experience complete drainage of a hematoma on the second day, it is important to avoid removal of the drainage tube at this time. Removal of the drainage tube too early may increase the risk of bleeding; (3) the retention time of the drainage tube shall not exceed 7 days. Even if there is residual hematoma at this time, the drainage tube should be removed. If the drainage tube is left for too long, it will increase the risk of intracranial infection; and (4) if the costs are not considered, we would recommend the use of alteplase as this drug has a lower risk of bleeding. However, the clinical application of alteplase has proven that it is safe, effective, and controls the amount of urokinase used for each hematoma dissolution (36).
For the surgical treatment of small and medium volumes of ICH in the basal ganglia, patients can undergo stereotactic catheter drainage (37). We found that the placement of the catheter was accurate, and the residual amount of hematoma after drainage was < 15 ml. There was no significant difference in short-term neurological function scores between hematoma long-axis catheterization and hematoma central catheterization. However, we recommend inserting a tube through the long axis of the hematoma through the forehead approach, because the hematoma clearance rate is high and the dose of urokinase required is small, thus reducing medical complications. One limitation of this study is that it was a single-center study with a small number of cases and a short follow-up time. Currently, multicenter case data are being collected for further analysis.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Human Ethics Committee of People's Hospital of China Three Gorges University (KY-2022-0040). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
LJ and ZY prepared Tables 1, 2. All authors made substantial contributions to the conception and design, the acquisition of data, analysis and interpretation of data, drafting, critical revision, and approval of the final version of this manuscript.
This study was funded by the Hubei Natural Science Foundation Project (Reference: 2022CFC044) and the Science and Technology Research Project of the Hubei Provincial Department of Education (Reference: B2021029).
We thank SC for revising the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Feigin VL, Krishnamurthi RV, Parmar P, Norrving B, Mensah GA, Bennett DA, et al. Update on the global burden of ischemic and hemorrhagic stroke in 1990–2013: the GBD 2013 study. Neuroepidemiology. (2015) 3:161–76. doi: 10.1159/000441085
2. Guo W, Liu H, Tan Z, Zhang X, Gao J, Zhang L, et al. Comparison of endoscopic evacuation, stereotactic aspiration, and craniotomy for treatment of basal ganglia hemorrhage. J Neurointerv Surg. (2020)12:55–61. doi: 10.1136/neurintsurg-2019-014962
3. Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K, McBee N, et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. (2019) 393:1021–32. doi: 10.1016/S0140-6736(19)30195-3
4. Huang X, Jiang L, Chen S, Li G, Pan W, Peng L, et al. Comparison of the curative effect and prognosis of stereotactic drainage and conservative treatment for moderate and small basal ganglia haemorrhage. BMC Neurol. (2021) 21:268. doi: 10.1186/s12883-021-02293-7
5. Brouwers HB, Goldstein JN. Therapeutic strategies in acute intracerebral hemorrhage. Neurotherapeutics. (2012) 9:87–98. doi: 10.1007/s13311-011-0091-8
6. Hanley DF, Lane K, McBee N, Ziai W, Tuhrim S, Lees KR, et al. Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet. (2017) 389:603–11. doi: 10.1016/S0140-6736(16)32410-2
7. Khan M, Baird GL, Elias R, Rodriguez-Srednicki J, Yaghi S, Yan S, et al. Comparison of intracerebral hemorrhage volume calculation methods and their impact on scoring tools. J Neuroimaging. (2017) 27:144–8. doi: 10.1111/jon.12370
8. Dye JA, Dusick JR, Lee DJ, Gonzalez NR, Martin NA. Frontal bur hole through an eyebrow incision for image-guided endoscopic evacuation of spontaneous intracerebral hemorrhage. J Neurosurg. (2012) 117:767–73. doi: 10.3171/2012.7.JNS111567
9. Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. (2005) 365:387–97. doi: 10.1016/S0140-6736(05)70233-6
10. Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. (2013) 382:397–408. doi: 10.1016/S0140-6736(13)60986-1
11. Xu X, Chen X, Li F, Zheng X, Wang Q, Sun G, et al. Effectiveness of endoscopic surgery for supratentorial hypertensive intracerebral hemorrhage: a comparison with craniotomy. J Neurosurg J Neurosurg. (2018) 128:553–9. doi: 10.3171/2016.10.JNS161589
12. Eroglu U, Kahilogullari G, Dogan I, Yakar F, Al-Beyati ESM, Ozgural O, et al. Surgical management of supratentorial intracerebral hemorrhages: endoscopic versus open surgery. World Neurosurg. (2018) 114:e60–5. doi: 10.1016/j.wneu.2018.02.056
13. Zhou X, Chen J, Li Q, Ren G, Yao G, Liu M, et al. Minimally invasive surgery for spontaneous supratentorial intracerebral hemorrhage: a meta-analysis of randomized controlled trials. Stroke. (2012) 43:2923–30. doi: 10.1161/STROKEAHA.112.667535
14. Hanley DF, Thompson RE, Muschelli J, Rosenblum M, McBee N, Lane K, et al. Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral haemorrhage evacuation (MISTIE): a randomised, controlled, open-label, phase 2 trial. Lancet Neurol. (2016) 15:1228–37. doi: 10.1016/S1474-4422(16)30234-4
15. Wu J, Zhang S. Analysis of the therapeutic effect and prognostic factors of 126 patients with hypertensive cerebral hemorrhage treated by soft-channel minimally invasive puncture and drainage. Front Surg. (2022) 9:885580. doi: 10.3389/fsurg.2022.885580
16. Zhou H, Zhang Y, Liu L, Huang Y, Tang Y, Su J, et al. Minimally invasive stereotactic puncture and thrombolysis therapy improves long-term outcome after acute intracerebral hemorrhage. J Neurol. (2011) 258:661–9. doi: 10.1007/s00415-011-5902-7
17. Zhang D, Cui X, Zheng J, Zhang S, Wang M, Lu W, et al. Neurosurgical robot-assistant stereoelectroencephalography system: operability and accuracy. Brain Behav. (2021) 11:e2347. doi: 10.1002/brb3.2347
18. Habets JGV, Heijmans M, Kuijf ML, Janssen MLF, Temel Y, Kubben PL. An update on adaptive deep brain stimulation in Parkinson's disease. Mov Disord. (2018) 33:1834–43. doi: 10.1002/mds.115
19. Chi F-L, Lang T-C, Sun S-J, Tang X-J, Xu S-Y, Zheng H-B, et al. Relationship between different surgical methods, hemorrhage position, hemorrhage volume, surgical timing, and treatment outcome of hypertensive intracerebral hemorrhage. World J Emerg Med. (2014) 5:203–8. doi: 10.5847/wjem.j.issn.1920-8642.2014.03.008
20. Zhang X, Zhou S, Zhang Q, Fu X, Wu Y, Liu J, et al. Stereotactic aspiration for hypertensive intracerebral haemorrhage in a Chinese population: a retrospective cohort study. Stroke Vasc Neurol. (2019) 4:14–21. doi: 10.1136/svn-2018-000200
21. Shi J, Cai Z, Han W, Dong B, Mao Y, Cao J, et al. Stereotactic catheter drainage versus conventional craniotomy for severe spontaneous intracerebral hemorrhage in the basal ganglia. Cell Transplant. (2019) 28:1025–32. doi: 10.1177/0963689719852302
22. Du Y, Gao Y, Liu HX, Zheng LL, Tan ZJ, Guo H, et al. Long-term outcome of stereotactic aspiration, endoscopic evacuation, and open craniotomy for the treatment of spontaneous basal ganglia hemorrhage: a propensity score study of 703 cases. Ann Transl Med. (2021) 9:1289. doi: 10.21037/atm-21-1612
23. Fu C, Wang N, Chen B, Wang P, Chen H, Liu W, et al. Surgical management of moderate basal ganglia intracerebral hemorrhage: comparison of safety and efficacy of endoscopic surgery, minimally invasive puncture and drainage, and craniotomy. World Neurosurg. (2019) 122:e995–e1001. doi: 10.1016/j.wneu.2018.10.192
24. Malinova V, Stockhammer F, Atangana EN, Mielke D, Rohde V. Catheter placement for lysis of spontaneous intracerebral hematomas: is a navigated stylet better than pointer-guided frameless stereotaxy for intrahematomal catheter positioning? Transl Stroke Res. (2014) 5:407–14. doi: 10.1007/s12975-014-0326-1
25. Malinova V, Schlegel A, Rohde V, Mielke D. Catheter placement for lysis of spontaneous intracerebral hematomas: does a catheter position in the core of the hematoma allow more effective and faster hematoma lysis? Neurosurg Rev. (2017) 40:397–402. doi: 10.1007/s10143-016-0792-x
26. Zheng F, Zhou J, Wang C, Hu W, Krischek B. Reader response: location-specific risk factors for intracerebral hemorrhage: systematic review and meta-analysis. Neurology. (2021) 96:1010–1. doi: 10.1212/WNL.0000000000012001
27. Gross BA, Jankowitz BT, Friedlander RM. Cerebral intraparenchymal hemorrhage: a review. JAMA. (2019) 321:1295–303. doi: 10.1001/jama.2019.2413
28. Chen Y, Chen S, Chang J, Wei J, Feng M, Wang R. Perihematomal edema after intracerebral hemorrhage: an update on pathogenesis, risk factors, and therapeutic advances. Front Immunol. (2021) 12:740632. doi: 10.3389/fimmu.2021.740632
29. Peng W-J, Li Q, Tang J-H, Reis C, Araujo C, Feng R, et al. The risk factors and prognosis of delayed perihematomal edema in patients with spontaneous intracerebral hemorrhage. CNS Neurosci Ther. (2019) 2510:1189–94. doi: 10.1111/cns.13219
30. Horowitz ME, Ali M, Chartrain AG, Allen OS, Scaggiante J, Glassberg B, et al. Definition and time course of pericavity edema after minimally invasive endoscopic intracerebral hemorrhage evacuation. J Neurointerv Surg. (2022) 14:149–54. doi: 10.1136/neurintsurg-2020-017077
31. Kellner CP, Song R, Pan J, et al. Long-term functional outcome following minimally invasive endoscopic intracerebral hemorrhage evacuation. J Neurointerv Surg. (2020) 12:489–94. doi: 10.1136/neurintsurg-2019-015528
32. Murthy SB, Moradiya Y, Dawson J, Lees KR, Hanley DF, Ziai WC, et al. Perihematomal edema and functional outcomes in intracerebral hemorrhage: influence of hematoma volume and location. Stroke. (2015) 46:3088–92. doi: 10.1161/STROKEAHA.115.010054
33. Jing C, Bian L, Wang M, Keep RF Xi G, Hua Y. Enhancement of hematoma clearance with CD47 blocking antibody in experimental intracerebral hemorrhage. Stroke. (2019) 50:1539–47. doi: 10.1161/STROKEAHA.118.024578
34. Gong S, Lin C, Zhang D, Kong X, Chen J, Wang C, et al. Effects of intensive blood pressure reduction on acute intracerebral hemorrhage: a systematic review and meta-analysis. Sci Rep. (2017) 7:10694. doi: 10.1038/s41598-017-10892-z
35. LoPresti MA, Bruce SS, Camacho E, Kunchala S, Dubois BG, Bruce E, et al. Hematoma volume as the major determinant of outcomes after intracerebral hemorrhage. J Neurol Sci. (2014) 345:3–7. doi: 10.1016/j.jns.2014.06.057
36. rd JCH, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2015) 46:2032–60. doi: 10.1161/STR.0000000000000069
Keywords: stereotactic, minimally invasive, catheter location, hypertensive cerebral hemorrhage, small to medium volume basal ganglia hemorrhage
Citation: Huang X, Yan Z, Jiang L, Chen S and Liu Y (2023) The efficacy of stereotactic minimally invasive thrombolysis at different catheter positions in the treatment of small- and medium-volume basal ganglia hemorrhage (SMITDCP I): a randomized, controlled, and blinded endpoint phase 1 trial. Front. Neurol. 14:1131283. doi: 10.3389/fneur.2023.1131283
Received: 24 December 2022; Accepted: 12 April 2023;
Published: 12 May 2023.
Edited by:
Yinong Huang, Sun Yat-sen University, ChinaReviewed by:
Jefferson W. Chen, University of California, Irvine, United StatesCopyright © 2023 Huang, Yan, Jiang, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yifei Liu, bHlmZ2YxMDIyQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.