
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 21 February 2023
Sec. Stroke
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1131250
This article is part of the Research TopicPost-Stroke Complications: Mechanisms, Diagnosis, and TherapiesView all 20 articles
Background: The issue of whether a stroke is causally related to gastrointestinal disorders was still not satisfactorily understood. Therefore, we investigated if there is a connection between stroke and the most prevalent gastrointestinal disorders, including peptic ulcer disease (PUD), gastroesophageal reflux disease (GERD), irritable bowel syndrome (IBS), and inflammatory bowel disease (IBD).
Methods: We applied two-sample Mendelian randomization to investigate relationships with gastrointestinal disorders. We obtained genome-wide association study (GWAS) summary data of any stroke, ischemic stroke, and its subtypes from the MEGASTROKE consortium. From the International Stroke Genetics Consortium (ISGC) meta-analysis, we acquired GWAS summary information on intracerebral hemorrhage (ICH), including all ICH, deep ICH, and lobar ICH. Several sensitivity studies were performed to identify heterogeneity and pleiotropy, while inverse-variance weighted (IVW) was utilized as the most dominant estimate.
Results: No evidence for an effect of genetic predisposition to ischemic stroke and its subtypes on gastrointestinal disorders were found in IVW. The complications of deep ICH are a higher risk for PUD and GERD. Meanwhile, lobar ICH has a higher risk of complications for PUD.
Conclusion: This study provides proof of the presence of a brain–gut axis. Among the complications of ICH, PUD and GERD were more common and associated with the site of hemorrhage.
Stroke is one of the leading causes of death and disability worldwide (1, 2). Based on neuropathology, there are two main categories of stroke: ischemic stroke (IS) and hemorrhagic stroke. Of the two major types of stroke, IS is the more frequent type (3). There are various subtypes of IS, such as large artery stroke, cardioembolic stroke, and small vessel stroke (4). Hemorrhagic stroke includes subarachnoid hemorrhage (SAH) and intracerebral hemorrhage (ICH). After a stroke, most patients will have varying degrees of motor impairment, cognitive impairment, speech dysphagia, depression, and other sequelae (5). In addition, up to 50% of patients usually experience gastrointestinal sequelae (6). The most common gastrointestinal disorders include PUD, GERD, IBS, and IBD. Among these four diseases, the prevalence of GERD is the highest, up to 18.1–27.8% in North America, followed by IBS and PUD, and the prevalence of IBD is lower. Patients with IBD commonly have abdominal pain, diarrhea, and bloody stools, while IBS has abdominal pain and altered bowel habits. GERD is usually characterized by regurgitation symptoms and heartburn, while PUD symptoms are not specific and abdominal pain is common (7–10). They sometimes have similar symptoms, such as abdominal pain, and the development of these disorders is all related to the brain–gut axis (11–13). Some observational studies have given attention to the relationship between stroke and peptic ulcer disease (PUD) (14) and also stroke and gastroesophageal reflux disease (GERD) (15). The study found that the GERD risk of patients with stroke is about 1.51 times that of patients without stroke (15). However, so far, it is not clear whether there is a causal relationship between the two diseases.
A growing number of observational studies have demonstrated complex interactions between stroke and gastrointestinal disorders (16–18). Furthermore, studies have shown that stroke promotes the destruction of the intestinal barrier and the imbalance of gut microbiota (19, 20). These proved that there is bidirectional communication between the brain and the gut, usually referred to as the brain–gut or gut–brain axis (21). After a stroke, the bidirectional communications between the brain and the gut may relate to the dysfunction of the autonomic nervous system, resulting in gastrointestinal disorders (22, 23). However, the exact mechanism accounting for the brain–gut axis is still widely considered as unsatisfactorily understood.
In systematic reviews and meta-analyses, their causal relationship is unclear or confusing. Mendelian randomization (MR) is a research method using a genetic variation to evaluate the causal relationship between exposures and outcomes based on Mendel's second law. MR overcomes the limitations of observational research by exposing potential causal links and has proved valuable in exploring the causality by using single-nucleotide polymorphisms (SNPs). SNPs are required to be associated with exposures and should not be independently associated with outcomes, except through exposures. Furthermore, SNPs must not be associated with confounders (24, 25). Moreover, we can further explore the outcomes of insufficient data in RCT through large samples in the genome-wide association study (GWAS). To our knowledge, there are relatively few studies on the causal relationship between stroke and gastrointestinal disorders, and gastrointestinal disorders have received less attention than other stroke complications, yet gastrointestinal disorders after stroke may lead to poor prognosis or even death (26). PUD, GERD, irritable bowel syndrome (IBS), and inflammatory bowel disease (IBD) are common diseases of the digestive system (27, 28). Therefore, we are committed to studying the causal effects of stroke and its subtypes and common gastrointestinal disorders by applying two-sample Mendelian randomization.
The conceptual MR framework is presented in Figure 1.
According to the Strengthening the Reporting of Observational Studies in Epidemiology-Mendelian Randomization (STROBE-MR) recommendations (29), the MR design was based on three hypotheses: (1) in this investigation, genetic variation was highly linked with the exposure of interest (stroke and its subtypes); (2) genetic variation was not associated with possible confounders; and (3) genetic variation solely had an impact on the outcome through the exposure of interest (gastrointestinal disorders in this study).
To investigate the potential causative relationship between stroke and gastrointestinal disorders such as PUD, GERD, IBS, and IBD, we used a two-sample MR method. The largest meta-analysis of genome-wide association studies (GWASs) produced by the MEGASTROKE consortium provided pooled statistics for any stroke, any ischemic stroke, and its subtypes (cardioembolic stroke, small vessel stroke, and large artery stroke) confirmed by clinical and imaging criteria (30). The International Stroke Genetics Consortium (ISGC), a group with European roots, provided the exposure dataset for hemorrhagic stroke (Table 1) (31). Regarding the outcome dataset, we selected the results according to Wu et al. (28). PUD, GERD, IBS, and IBD are common gastrointestinal diseases.
First, in line with the findings of Kwok et al. (32), we relaxed the correlation threshold with P <5 × 10−6 and linkage disequilibrium (LD) (r2 < 0.001) to obtain the top independent SNPs. This was done in light of the small number of SNPs (P < 5 × 10−8) that reached genome-wide significance. This strategy has been applied extensively in earlier MR investigations (33, 34). Second, the results of MR analysis are believed to be unaffected by weak instrumental bias if there is an F-statistic larger than 10. We used the following:
Third, we extracted the secondary phenotypes of each SNP from a PhenoScanner V2 (35) and the GWAS library to exclude any putative polymorphism effects. The radial MR and MR pleiotropy residual sum and outlier (MR-PRESSO) tests were used to eliminate outliers before each MR analysis.
Three methods, including MR-Egger, weighted median, and random effect inverse-variance weighting (IVW), were utilized in the MR analysis to evaluate robust effects. The primary analysis method was the IVW method with various models, depending on the heterogeneity. At least half of the data for the Mendelian randomization study must originate from reliable instruments to use the weighted median estimator (36, 37). The effectiveness of potential pleiotropic tools must be independent of their direct relationships with the outcome for MR-Egger regression to be valid. Radial MR-Egger was used to estimate the horizontal pleiotropy and to identify outlier variants (38). Heterogeneity was also assessed using Cochran's Q-test. With the Cochran Q test (statistics were deemed to be significant if P < 0.05) and the intercept from MR-Egger regression (statistics were deemed to be significant if P < 0.05), we evaluated heterogeneity between Mendelian randomization estimates. We also evaluated potential directional polymorphisms using funnel plots. We used fixed-effects IVW and limited our instrument selection for sensitivity analyses to a lower LD correlation threshold. In conclusion, we conducted a thorough investigation of causation using all these techniques. Given the 32 MR estimates, the Bonferroni-corrected P-value for the study of gastrointestinal disorders was set at 0.05/32 (1.563 × 10−3), and P < 0.05 was regarded as nominally significant. The statistical study was performed using R (version 4.2.0) and the “TwoSampleMR” and “RadialMR” packages.
The SNPs of stroke subtypes on gastrointestinal disorders are listed in Supplementary Tables 1–8. Looking over the Phenoscanner, three SNPs (rs10850001, rs10774624, and rs3184504) were associated with smoking and were removed when analyzing PUD-associated SNPs. A total of 10 SNPs (rs12932445, rs1537375, rs2107595, rs2466455, rs4444878, rs4932370, rs6536024, rs6838973, rs72700114, and rs2634074) were related to the anticoagulant use, which was analyzed for PUD-related SNPs removed during the analysis. A total of 10 SNPs (rs10774624, rs1549758, rs1975161, rs2107595, rs2284665, rs34416434, rs42039, rs616154, rs78893982, and rs8103309) were associated with obesity and were removed in the analysis of GERD-related SNPs.
We performed a comprehensive MR study of stroke and its subtypes on gastrointestinal diseases (Supplementary Table 9). Among them, using IVW as the primary analysis, it could be seen that genetics predicted that any ischemic stroke had a normal significance with GERD (P < 0.05). All ICHs had normal significance with PUD and IBD (P < 0.05). Meanwhile, deep ICH had signed with the PUD and GERD (P < 1.563 × 10−3). Lobar ICH had signed with the PUD and IBS (P < 1.563 × 10−3). A bubble plot was used to show the statistical significance of the analysis (Figure 2). After that, the MR analyses with significant P-values were demonstrated in a forest plot (Figure 3). For ischemic stroke, there was no significant causal relationship with gastrointestinal disorders. For hemorrhagic stroke, the result of IVW showed that deep ICH [odds ratio (OR): 1.020; 95% confidence interval (CI): 1.010–1.030; P = 4.740 × 10−5] was associated with an increased risk of PUD and greater disease severity with the weight median method (OR: 1.020; 95% CI: 1.010–1.030; P = 4.740 × 10−5). The results of the MR-Egger method showed consistent directions but were not statistically significant (OR: 0.997; 95% CI: 0.905–1.099; P = 0.954). In addition, similar causal estimates of lobar ICH on PUD were obtained, and IVW (OR: 1.026; 95% CI: 1.016–1.037; P = 9.018 × 10−7) and weight median (OR: 1.042; 95% CI: 1.027–1.057; P = 4.077 × 10−8) were included, while the same result was observed using the MR-Egger method but without any statistical difference (OR: 1.008, 95% CI: 0.968–1.049, P = 0.700). Deep ICH was associated with an increased risk of GERD with the IVW (OR: 1.028; CI: 1.022–1.034; P = 1.663 × 10−21) and weight median (OR: 1.032; CI: 1.024–1.030; P = 9.994 × 10−17); however, there was no statistical difference in the MR-Egger method (OR: 1.036; CI: 0.971–1.106; P = 0.290), where all p > 0.05 for the MR-Egger intercept test, except for the MR analysis of lobar ICH on the IBS of lingual without weight median, indicated no horizontal pleiotropy. For significance and nominal significance estimates, Cochran's Q-test, the MR-Egger intercept test, the leave-one-out analysis, and the funnel plot were used to assess horizontal multiplicity (Supplementary Figures 1–4). Finally, we determined that deep ICH and labor ICH were causally related to PUD, and deep ICH was causally related to GERD.
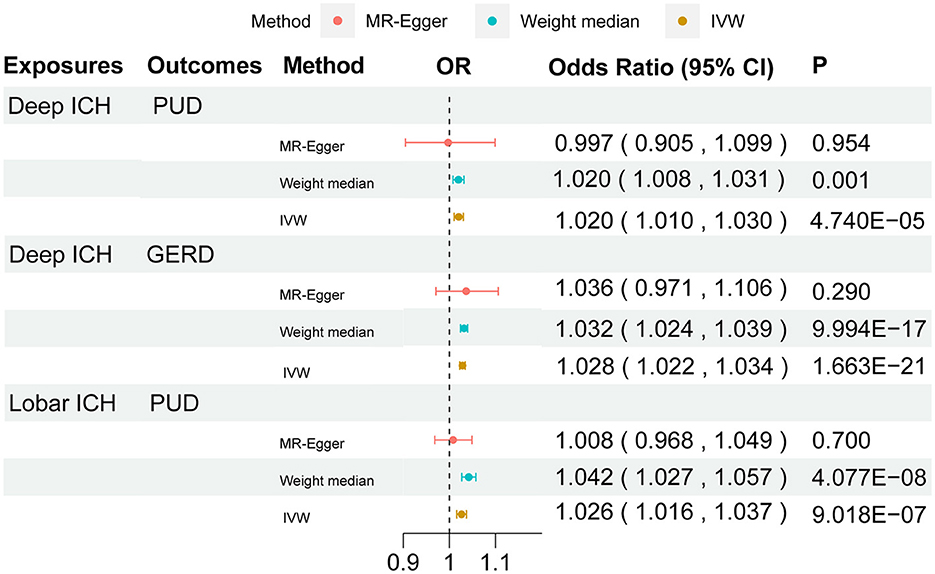
Figure 3. Forest plot for the causal effect of stroke on the risk of gastrointestinal disorders. OR, odds ratio; CI, confidence interval.
Previous studies have not found a clear causal relationship between stroke and gastrointestinal diseases. In our study, the relationship between stroke and its subtypes of gastrointestinal disorders was determined by the MR analysis. It is reported that obesity, smoking, anticoagulant, and other risk factors are often related to gastrointestinal diseases (39–41). The GWAS of GERD and PUD found genetic overlapping with the identified aforementioned hazardous factors (42, 43). We cannot rule out that SNP affects the outcome through other related variables. Therefore, we should try our best to reduce the bias caused by pleiotropy. To reduce pleiotropy, we look over the PhenoScanner and eliminate those pleiotropic genetic variants. Thus, we successfully removed SNPs that were highly correlated with possible confounders such as obesity, smoking, and anticoagulation therapy. In addition, we also conducted some sensitivity analyses, such as a leave-one-out analysis and a funnel plot, and other methods such as Cochran's Q-test and the MR-Egger intercept test to assess horizontal multiplicity.
Stroke is often associated with PUD. A retrospective review including 808 cases found that the incidence of gastrointestinal bleeding caused by PUD in patients with ICH was 26.7% (18). Moreover, the incidence of gastrointestinal bleeding was significantly higher in patients who often use stress ulcer prophylaxis (SUP) for stress ulcer prevention compared with patients not receiving SUP (18). Another observational study examined 177 patients with acute stroke by gastroscopy, of which 92 (52%) had gastric changes, 10 of which were acute ulcers (44). For patients with severe ICH, an observational study found that 28.0% of 715 patients with severe ICH developed stress-related gastrointestinal bleeding (SGIB) or stress ulcers during hospitalization (45). Regrettably, none of these observational studies had a large sample size. Our MR study suggested that stroke has a causal impact on PUD but only on deep ICH and lobar ICH and not ischemic stroke.
The pathogenesis of ICH complicated by peptic ulcers is still unclear and may be related to the damage to the thalamus and the subthalamus. To summarize various studies, the possible mechanisms are as follows: first, patients with acute ICH often experience intracranial hypertension and cerebral edema, which directly or indirectly causes damage to the brain stem, the hypothalamus, and other parts and finally affects their normal physiologic functions, leading to a dysfunction of the autonomic nervous system and gastric hyperchlorhydria. It lessens the blood flow of gastrointestinal mucosa and damages the gastric mucosal barrier, resulting in stress gastrointestinal ulcer peptic ulcers as well as peptic ulcer bleeding (46). According to previous studies, the development of stress ulcers in patients with ICH can be better predicted by the hematoma volume of ICH (47, 48). Mechanistically, larger hematomas in the case of cerebral hemorrhage are more likely to lead to increased intracranial pressure (45). As mentioned earlier, elevated intracranial pressure may cause strong sympathetic excitation and gastrointestinal vasoconstriction, causing a decrease in gastrointestinal blood flow, which subsequently leads to mucosal ischemia and increased gastric acid secretion. Second, post-stroke sepsis plays a very important role in the development of stress ulcers induced by severe ICH (45). Inflammatory cytokines are released in large amounts in the development of sepsis, thus exacerbating the ischemia of the gastrointestinal mucosa caused by intracerebral hemorrhage and driving the development of stress ulcers (49, 50). In an observational study, the incidence of gastrointestinal bleeding in patients with ischemic stroke was 7.8%, 74% of which were caused by peptic ulcers (51). Combined with our findings, it is clear that hemorrhagic strokes are more likely to develop peptic ulcers than ischemic strokes. The development of peptic ulcers in ischemic stroke may be associated with vagal hyperactivity, stress, and neuroendocrine dysregulation (51, 52). However, the trigger for gastrointestinal bleeding in most patients with ischemic stroke is not stress, and stress ulcers due to acute ischemic brain injury may be very rare (52, 53). One possible explanation for the aforementioned results is that compared to ischemic strokes, hemorrhagic strokes are a more devastating subtype of stroke (54). Hemorrhagic stroke may have a stronger effect on the brain–gut axis than ischemic stroke. The study finding that hemorrhagic stroke disrupts the gut microbiota more than ischemic stroke may prove this (55). The incidence of stress ulcer bleeding in patients with brain injury is closely related to the severity of the injury (56). In our MR study, the risk of lobar ICH is more associated with an increased risk of PUD compared to the risk of deep ICH. The size of the hematoma, sepsis, and prognosis have been reported to be the strongest predictors of gastrointestinal bleeding in patients with ICH in previous research (48). A Japanese observational study found a higher rate of poor prognosis in patients with lobar ICH than in those with non-lobar ICH (57). Even lobar ICH is associated with more severe cognitive impairment (58). It suggests clinical vigilance for PUD for hemorrhagic stroke.
Several studies have proposed an association between stroke and GERD. A population-based Taiwanese cohort study including 18,412 patients with stroke and 18,412 without stroke found that the risk of GERD in patients with stroke was 1.51 times higher than that in patients without stroke (95% CI, 1.40–1.67) (15). Moreover, they separated the stroke cohort into two subgroups: hemorrhagic stroke and ischemic stroke. Compared with the subjects without stroke, the HRs for GERD in the intracerebral hemorrhage and ischemic stroke cohorts were 1.45 and 1.52 (95% CI, 1.22–1.71 and 95% CI, 1.39–1.67) (15). Our MR study found that the higher risk of GERD is complicated by the risk of deep ICH, and there is a positive causal relationship between them. We reviewed the relevant literature to explain the mechanisms by which stroke leads to the development of GERD. For ischemic stroke, GERD may be induced by drugs used to treat IS, such as aspirin. One of the independent risk factors associated with the clinical symptoms of GERD is NSAIDs. The study also found an increased incidence of GERD in patients with stroke treated with antiplatelet therapy (15, 59). In addition, ischemic stroke may disrupt the neural regulation of oropharyngeal, esophageal, and gastrointestinal motility, resulting in an extensive impairment of oropharyngeal and gastrointestinal motility and a reduced tone of the lower esophageal sphincter (52). ICH has a similar effect on the vagus nerve, resulting in the malfunction of esophageal peristalsis, gastrointestinal motility, and the lower esophageal sphincter (48, 60). Parasympathetic dysfunction in patients with stroke may lead to impaired esophageal motility, the abnormal transmission of food, and the abnormal relaxation of the lower esophageal sphincter (15, 61). Hypertension is one of the most important risk factors for stroke, and treatment to lower blood pressure to prevent stroke, including the use of calcium channel blockers, often leads to lower esophageal sphincter (LES) pressure and eventually GERD (62, 63). A community study found that calcium channel blockers were independently associated with GERD symptoms as a risk factor (63). To explain why deep ICH is more prone to GERD than other subtypes of stroke, we looked through many studies. Deep ICH is often thought to be closely associated with hypertension, while lobar ICH is often thought to be caused by cerebral amyloid angiopathy (CAA) (64). Among the drugs used to treat hypertension, calcium channel blockers have the effect of lowering the pressure of the LES and impeding gastric emptying, thus inducing GERD (65). Therefore, compared to lobar ICH, deep ICH is more prone to GERD.
According to our MR results, intracerebral hemorrhage is more likely to cause gastrointestinal disease than ischemic stroke, and we are thinking about the reasons for this result. It is well-known that ischemic stroke and intracerebral hemorrhage do not occur by similar mechanisms, and their degree of criticality is different. ICH is the most severe subtype of stroke. Furthermore, the most devastating type of pathology among the subtypes of stroke is ICH (54). In general, ICH produces more severe strokes than cerebral infarct (66, 67). ICH typically manifests as elevated intracranial pressure, hematoma compression, and serious cerebral edema, which can cause many negative effects, such as neuroinflammation, mitochondrial dysfunction, and apoptosis, resulting in a sudden disruption of the blood–brain barrier (68). Contrary to ICH, the structural stability of brain cells and the blood–brain barrier is retained for a longer length of time following the beginning of symptoms in ischemic stroke (69). One possible explanation for our findings is that compared to ischemic stroke, ICH is more damaging to the brain–gut axis, causing a more severe dysbiosis in the gut microbiota, abnormal gastrointestinal motor function, and impaired gastrointestinal motility, which leads to gastrointestinal disorders. Compared to patients with ischemic stroke, patients with ICH have more severe gut microbiota destruction (70). ICH causes rapid damage to astrocytes and the blood–brain barrier in patients (69). Contemporary genetics considers stroke not as a disease but as a syndrome. Stroke is an acute manifestation of a range of chronic cerebrovascular diseases (71). Another possible explanation for our findings is that some subtypes of ischemic stroke present additional phenotypic dilemmas, such as the cardiogenic stroke subtype, whereas the phenotype of ICH is more uniform (71). Moreover, genetic factors are important in the pathogenesis of ICH (72). It is estimated that up to 44% of cases of ICH are heritable, and possessing an ICH first-degree relative increases the risk of developing the condition by a factor of six (73).
To explain the causal relationship between hemorrhagic stroke and several gastrointestinal diseases, we have found several possible mechanisms. Intracerebral smoke can affect the function of the autonomic nervous system. Through the enteric nervous system, the extrinsic and autonomic nervous systems can regulate the motor, sensory, and secretory functions of the gastrointestinal tract. ICH affects gastrointestinal function in this way, mainly with motor dysfunction (74). For example, strokes are often complicated by dysphagia, which may be due to cranial nerve involvement in the region of the vertebrobasilar artery (75). This is one of the possible causes of stroke complicating gastrointestinal motility disorder-related disease. Moreover, the change in gut microbes caused by intracerebral hemorrhage may be one of the causes of some gastrointestinal diseases (68). A prospective case–control study found that compared with the control group, the intestinal microbiota composition of both patients with ischemic stroke and patients with intracerebral hemorrhage changed (55). More specifically, compared with the control group, the abundance of invasive aerobic bacterial genera (Enterococcus species and Escherichia/Shigella species) in all patients with stroke increased, while obligate anaerobic genera decreased (55). The authors observed that the extent of gut microbiota destruction was positively associated with the severity of stroke. An intracerebral hemorrhage causes more severe disruption of the gut microbiota than an ischemic stroke (55). The autonomic nervous system abnormally releases norepinephrine to the intestine, which may change the intestinal microbiota (23). Another study found that the immune system of model mice is disturbed after intracerebral hemorrhage. Furthermore, the gut barrier function of model mice was impaired, and intestinal permeability increased (70). In addition, experimental studies also found that inflammatory cytokines were upregulated in the intestine, malondialdehyde (MDA) levels were elevated, the superoxide (SOD) dismutase activity was reduced, severe intestinal mucosal damage and plasma endotoxin levels were elevated 2 h after intracerebral hemorrhage in model mice, and intestinal propulsion was reduced 12 h later, and these symptoms persisted for 7 days after the appearance of the above symptoms (76). These suggest that intracerebral hemorrhage significantly increases inflammatory cytokine levels and myeloperoxidase activity, which, in turn, promotes an inflammatory response in the intestine, leading to gastrointestinal disorders associated with intestinal motility and barrier dysfunction. In contrast, elevated malondialdehyde levels and reduced superoxide dismutase also suggest that intracerebral hemorrhage induced excessive oxygen radical production in the intestine during ischemia-reperfusion. The pathological imbalance of the intestinal oxidative–antioxidant system may also be involved in the pathogenesis of gastrointestinal disorders after intracerebral hemorrhage (76). In a word, intracerebral hemorrhage may lead to impaired communication between the brain and intestinal axis, which may directly result in gastrointestinal motility dysfunction or intestinal flora disorders. Although there are many studies on how the brain–gut axis interacts, the exact mechanism has not been clarified.
Our MR study has some strengths. First, compared with one-sample MR, our research has a larger sample size and higher statistical efficiency. Second, our research overcame the shortcomings of traditional causal inference. Since the alleles followed the principle of random assignment, we obtained results independent of the confounding factors and reversed causal associations found in traditional epidemiological studies. Furthermore, there is Cochran's Q-test, the MR-Egger intercept test, and sensitivity analysis to test the pleiotropy of instrumental variables, which enhances the reliability of the results. At the same time, our analysis has some limitations. First of all, the estimates mentioned in our MR study cannot be directly compared with those of other observational studies. Second, we have selected only four common gastrointestinal disorders, and it is unknown whether a stroke has a causal effect on other gastrointestinal disorders. Third, the dataset on which our study is primarily based includes only individuals of European ancestry and thus may not be applicable to other humans, which would make our findings not generalizable. Finally, because of the limitation of the number of SNPs, the p-value limits were adjusted in our article.
Our MR study provides evidence for a causal relationship between deep ICH on PUD and GERD and a causal relationship between lobar ICH on PUD, and our results add to the gap in observational studies in this regard and warrant further research for the prevention of gastrointestinal disorders after deep ICH and lobar ICH.
Our research supports a possible causal link between stroke and its subtypes and gastrointestinal disorders. Early gastrointestinal disease risk assessment and prevention in hemorrhagic stroke is crucial and could aid in the introduction of tailored treatment as soon as possible.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JS contributed to the methodology and wrote the manuscript. WC contributed to conceptualization and investigation. WY contributed to the funding, writing, reviewing, and editing. All authors contributed to the article and approved the submitted version.
This study was supported by Hangzhou Medical Key Cultivation Discipline (Spleen and Gastroenterology, Grant No. 2020SJZDXK13) and Zhejiang Provincial TCM Science and Technology Program Project (Grant No. 2021ZA107).
The authors gratefully thank the MEGASTROKE Consortium for providing summary statistics data. All MEGASTROKE Consortium authors are listed online (https://www.megastroke.org/authors.html). The MEGASTROKE project received funding from sources specified at https://www.megastroke.org/acknowledgements.html. For the source of data on hemorrhagic stroke, the authors thank the ISGC genome-wide association study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1131250/full#supplementary-material
1. Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet. (2018) 392:2052–90. doi: 10.1016/S0140-6736(18)31694-5
2. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
3. Petro M, Jaffer H, Yang J, Kabu S, Morris VB, Labhasetwar V. Tissue plasminogen activator followed by antioxidant-loaded nanoparticle delivery promotes activation/mobilization of progenitor cells in infarcted rat brain. Biomaterials. (2016) 81:169–80. doi: 10.1016/j.biomaterials.2015.12.009
4. Regenhardt RW, Das AS, Lo EH, Caplan LR. Advances in understanding the pathophysiology of lacunar stroke: a review. JAMA Neurol. (2018) 75:1273–81. doi: 10.1001/jamaneurol.2018.1073
5. Rost NS, Brodtmann A, Pase MP, van Veluw SJ, Biffi A, Duering M, et al. Post-Stroke cognitive impairment and dementia. Circ Res. (2022) 130:1252–71. doi: 10.1161/CIRCRESAHA.122.319951
6. Pluta R, Januszewski S, Czuczwar SJ. The role of gut microbiota in an ischemic stroke. Int J Mol Sci. (2021) 22:915. doi: 10.3390/ijms22020915
7. Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. (2017) 390:2769–78. doi: 10.1016/S0140-6736(17)32448-0
8. Sandhu DS, Fass R. Current trends in the management of gastroesophageal reflux disease. Gut Liver. (2018) 12:7–16. doi: 10.5009/gnl16615
9. Lanas A, Chan FKL. Peptic ulcer disease. Lancet. (2017) 390:613–24. doi: 10.1016/S0140-6736(16)32404-7
10. Defrees DN, Bailey J. Irritable bowel syndrome: epidemiology, pathophysiology, diagnosis, and treatment. Prim Care. (2017) 44:655–71. doi: 10.1016/j.pop.2017.07.009
11. Tache Y. The peptidergic brain-gut axis: influence on gastric ulcer formation. Chronobiol Int. (1987) 4:11–7. doi: 10.1080/07420528709078504
12. Ancona A, Petito C, Iavarone I, Petito V, Galasso L, Leonetti A, et al. The gut-brain axis in irritable bowel syndrome and inflammatory bowel disease. Dig Liver Dis. (2021) 53:298–305. doi: 10.1016/j.dld.2020.11.026
13. Yadlapati R, Gyawali CP, Pandolfino JE. Aga clinical practice update on the personalized approach to the evaluation and management of gerd: expert review. Clin Gastroenterol Hepatol. (2022) 20:984–94.e1. doi: 10.1016/j.cgh.2022.01.025
14. Xu Z, Wang H, Lin Y, Zhai Q, Sun W, Wang Z, et al. The impacts of peptic ulcer on functional outcomes of ischemic stroke. J Stroke Cerebrovasc Dis. (2019) 28:311–6. doi: 10.1016/j.jstrokecerebrovasdis.2018.09.056
15. Chang CS, Chen HJ, Liao CH. Patients with cerebral stroke have an increased risk of gastroesophageal reflux disease: a population-based cohort study. J Stroke Cerebrovasc Dis. (2018) 27:1267–74. doi: 10.1016/j.jstrokecerebrovasdis.2017.12.001
16. Satou Y, Oguro H, Murakami Y, Onoda K, Mitaki S, Hamada C, et al. Gastroesophageal reflux during enteral feeding in stroke patients: a 24-hour esophageal ph-monitoring study. J Stroke Cerebrovasc Dis. (2013) 22:185–9. doi: 10.1016/j.jstrokecerebrovasdis.2011.07.008
17. Kristensen SL, Lindhardsen J, Ahlehoff O, Erichsen R, Lamberts M, Khalid U, et al. Increased risk of atrial fibrillation and stroke during active stages of inflammatory bowel disease: a nationwide study. Europace. (2014) 16:477–84. doi: 10.1093/europace/eut312
18. Yang TC, Li JG, Shi HM, Yu DM, Shan K, Li LX, et al. Gastrointestinal bleeding after intracerebral hemorrhage: a retrospective review of 808 cases. Am J Med Sci. (2013) 346:279–82. doi: 10.1097/MAJ.0b013e318271a621
19. Wen SW, Wong CHY. An unexplored brain-gut microbiota axis in stroke. Gut Microbes. (2017) 8:601–6. doi: 10.1080/19490976.2017.1344809
20. Stanley D, Moore RJ, Wong CHY. An insight into intestinal mucosal microbiota disruption after stroke. Sci Rep. (2018) 8:568. doi: 10.1038/s41598-017-18904-8
21. Arya AK, Hu B. Brain-Gut axis after stroke. Brain Circ. (2018) 4:165–73. doi: 10.4103/bc.bc_32_18
22. Yang Z, Wei F, Zhang B, Luo Y, Xing X, Wang M, et al. Cellular immune signal exchange from ischemic stroke to intestinal lesions through brain-gut axis. Front Immunol. (2022) 13:688619. doi: 10.3389/fimmu.2022.688619
23. Houlden A, Goldrick M, Brough D, Vizi ES, Lénárt N, Martinecz B, et al. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav Immun. (2016) 57:10–20. doi: 10.1016/j.bbi.2016.04.003
24. Holmes MV, Ala-Korpela M, Smith GD. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nat Rev Cardiol. (2017) 14:577–90. doi: 10.1038/nrcardio.2017.78
25. Cui G, Li S, Ye H, Yang Y, Huang Q, Chu Y, et al. Are neurodegenerative diseases associated with an increased risk of inflammatory bowel disease? A two-sample mendelian randomization study. Front Immunol. (2022) 13:956005. doi: 10.3389/fimmu.2022.956005
26. Wang WJ, Lu JJ, Wang YJ, Wang CX, Wang YL, Hoff K, et al. Clinical characteristics, management, and functional outcomes in chinese patients within the first year after intracerebral hemorrhage: analysis from china national stroke registry. CNS Neurosci Ther. (2012) 18:773–80. doi: 10.1111/j.1755-5949.2012.00367.x
27. Freuer D, Linseisen J, Meisinger C. Asthma and the risk of gastrointestinal disorders: a mendelian randomization study. BMC Med. (2022) 20:82. doi: 10.1186/s12916-022-02283-7
28. Wu Y, Murray GK, Byrne EM, Sidorenko J, Visscher PM, Wray NR. Gwas of peptic ulcer disease implicates helicobacter pylori infection, other gastrointestinal disorders and depression. Nat Commun. (2021) 12:1146. doi: 10.1038/s41467-021-21280-7
29. Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the strobe-Mr statement. Jama. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236
30. Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, et al. Publisher correction: multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. (2019) 51:1192–3. doi: 10.1038/s41588-019-0449-0
31. Woo D, Falcone GJ, Devan WJ, Brown WM, Biffi A, Howard TD, et al. Meta-Analysis of genome-wide association studies identifies 1q22 as a susceptibility locus for intracerebral hemorrhage. Am J Hum Genet. (2014) 94:511–21. doi: 10.1016/j.ajhg.2014.02.012
32. Kwok MK, Kawachi I, Rehkopf D, Schooling CM. The role of cortisol in ischemic heart disease, ischemic stroke, type 2 diabetes, and cardiovascular disease risk factors: a bi-directional mendelian randomization study. BMC Med. (2020) 18:363. doi: 10.1186/s12916-020-01831-3
33. Du W, Wang T, Zhang W, Xiao Y, Wang X. Genetically supported causality between benign prostate hyperplasia and urinary bladder neoplasms: a mendelian randomization study. Front Genet. (2022) 13:1016696. doi: 10.3389/fgene.2022.1016696
34. Müller R, Freitag-Wolf S, Weiner J, 3rd Chopra A, Top T, Dommisch H, et al. Case-only design identifies interactions of genetic risk variants at siglec5 and plg with the lncrna Ctd-2353f22.1 implying the importance of periodontal wound healing for disease aetiology. J Clin Periodontol. (2023) 50:90–101. doi: 10.1111/jcpe.13712
35. Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. Phenoscanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. (2019) 35:4851–3. doi: 10.1093/bioinformatics/btz469
36. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
37. Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from mendelian randomization analyses with multiple genetic variants. Epidemiology. (2017) 28:30–42. doi: 10.1097/EDE.0000000000000559
38. Bowden J, Spiller W, Del Greco MF, Sheehan N, Thompson J, Minelli C, et al. Improving the visualization, interpretation and analysis of two-sample summary data mendelian randomization via the radial plot and radial regression. Int J Epidemiol. (2018) 47:1264–78. doi: 10.1093/ije/dyy101
39. Bilski J, Mazur-Bialy A, Wojcik D, Surmiak M, Magierowski M, Sliwowski Z, et al. Role of obesity, mesenteric adipose tissue, and adipokines in inflammatory bowel diseases. Biomolecules. (2019) 9:780. doi: 10.3390/biom9120780
40. Korman MG, Hansky J, Eaves ER, Schmidt GT. Influence of cigarette smoking on healing and relapse in duodenal ulcer disease. Gastroenterology. (1983) 85:871–4. doi: 10.1016/0016-5085(83)90438-9
41. Kawamura N, Ito Y, Sasaki M, Iida A, Mizuno M, Ogasawara N, et al. Low-Dose aspirin-associated upper gastric and duodenal ulcers in japanese patients with no previous history of peptic ulcers. BMC Res Notes. (2013) 6:455. doi: 10.1186/1756-0500-6-455
42. Ong JS, An J, Han X, Law MH, Nandakumar P, Schumacher J, et al. Multitrait genetic association analysis identifies 50 new risk loci for gastro-oesophageal reflux, seven new loci for barrett's oesophagus and provides insights into clinical heterogeneity in reflux diagnosis. Gut. (2022) 71:1053–61. doi: 10.1136/gutjnl-2020-323906
43. Li Z, Chen H, Chen T. Genetic liability to obesity and peptic ulcer disease: a mendelian randomization study. BMC Med Genomics. (2022) 15:209. doi: 10.1186/s12920-022-01366-x
44. Kitamura T, Ito K. Acute gastric changes in patients with acute stroke. Part 1: with reference to gastroendoscopic findings. Stroke. (1976) 7:460–3. doi: 10.1161/01.STR.7.5.460
45. Liu S, Wang Y, Gao B, Peng J. A nomogram for individualized prediction of stress-related gastrointestinal bleeding in critically ill patients with primary intracerebral hemorrhage. Neuropsychiatr Dis Treat. (2022) 18:221–9. doi: 10.2147/NDT.S342861
46. Alhazzani W, Jaeschke R. Stress ulcer prophylaxis in critical care: a 2016 perspective Dr. Waleed Alhazzani in an interview with Dr. Roman Jaeschke: part 2. Pol Arch Med Wewn. (2016) 126:796–7. doi: 10.20452/pamw.3606
47. Liu BL, Li B, Zhang X, Fei Z, Hu SJ, Lin W, et al. A randomized controlled study comparing omeprazole and cimetidine for the prophylaxis of stress-related upper gastrointestinal bleeding in patients with intracerebral hemorrhage. J Neurosurg. (2013) 118:115–20. doi: 10.3171/2012.9.JNS12170
48. Misra UK, Kalita J, Pandey S, Mandal SK. Predictors of gastrointestinal bleeding in acute intracerebral haemorrhage. J Neurol Sci. (2003) 208:25–9. doi: 10.1016/S0022-510X(02)00415-X
49. Pastores SM, Katz DP, Kvetan V. Splanchnic ischemia and gut mucosal injury in sepsis and the multiple organ dysfunction syndrome. Am J Gastroenterol. (1996) 91:1697–710.
50. Overhaus M, Tögel S, Pezzone MA, Bauer AJ. Mechanisms of polymicrobial sepsis-induced ileus. Am J Physiol Gastrointest Liver Physiol. (2004) 287:G685–94. doi: 10.1152/ajpgi.00359.2003
51. Hsu HL, Lin YH, Huang YC, Weng HH, Lee M, Huang WY, et al. Gastrointestinal hemorrhage after acute ischemic stroke and its risk factors in Asians. Eur Neurol. (2009) 62:212–8. doi: 10.1159/000229018
52. Schaller BJ, Graf R, Jacobs AH. Pathophysiological changes of the gastrointestinal tract in ischemic stroke. Am J Gastroenterol. (2006) 101:1655–65. doi: 10.1111/j.1572-0241.2006.00540.x
53. Wijdicks EF, Fulgham JR, Batts KP. Gastrointestinal bleeding in stroke. Stroke. (1994) 25:2146–8. doi: 10.1161/01.STR.25.11.2146
54. Tsai CF, Jeng JS, Anderson N, Sudlow CLM. Comparisons of risk factors for intracerebral hemorrhage versus ischemic stroke in chinese patients. Neuroepidemiology. (2017) 48:72–8. doi: 10.1159/000475667
55. Haak BW, Westendorp WF, van Engelen TSR, Brands X, Brouwer MC, Vermeij JD, et al. Disruptions of anaerobic gut bacteria are associated with stroke and post-stroke infection: a prospective case-control study. Transl Stroke Res. (2021) 12:581–92. doi: 10.1007/s12975-020-00863-4
56. Kamada T, Fusamoto H, Kawano S, Noguchi M, Hiramatsu K. Gastrointestinal bleeding following head injury: a clinical study of 433 cases. J Trauma. (1977) 17:44–7. doi: 10.1097/00005373-197701000-00006
57. Matsukawa H, Shinoda M, Fujii M, Takahashi O, Yamamoto D, Murakata A, et al. Factors associated with lobar vs. non-lobar intracerebral hemorrhage. Acta Neurol Scand. (2012) 126:116–21. doi: 10.1111/j.1600-0404.2011.01615.x
58. Tveiten A, Ljøstad U, Mygland Å, Naess H. Functioning of long-term survivors of first-ever intracerebral hemorrhage. Acta Neurol Scand. (2014) 129:269–75. doi: 10.1111/ane.12185
59. Ercelep OB, Caglar E, Dobrucali A. The prevalence of gastroesophageal reflux disease among hospital employees. Dis Esophagus. (2014) 27:403–8. doi: 10.1111/j.1442-2050.2012.01402.x
60. Cunningham KM, Horowitz M, Riddell PS, Maddern GJ, Myers JC, Holloway RH, et al. Relations among autonomic nerve dysfunction, oesophageal motility, and gastric emptying in gastro-oesophageal reflux disease. Gut. (1991) 32:1436–40. doi: 10.1136/gut.32.12.1436
61. Orlando RC. Pathophysiology of gastroesophageal reflux disease. J Clin Gastroenterol. (2008) 42:584–8. doi: 10.1097/MCG.0b013e31815d0628
62. Schrader J, Lüders S, Kulschewski A, Hammersen F, Plate K, Berger J, et al. Morbidity and mortality after stroke, eprosartan compared with nitrendipine for secondary prevention: principal results of a prospective randomized controlled study (moses). Stroke. (2005) 36:1218–26. doi: 10.1161/01.STR.0000166048.35740.a9
63. Mohammed I, Nightingale P, Trudgill NJ. Risk factors for gastro-oesophageal reflux disease symptoms: a community study. Aliment Pharmacol Ther. (2005) 21:821–7. doi: 10.1111/j.1365-2036.2005.02426.x
64. Labovitz DL, Sacco RL. Intracerebral hemorrhage: update. Curr Opin Neurol. (2001) 14:103–8. doi: 10.1097/00019052-200102000-00016
65. Osadchuk AM, Davydkin IL, Gricenko TA, Osadchuk MA. [Gastroesophageal reflux disease and esophagitis associated with the use of drugs: the modern state of the problem]. Ter Arkh. (2019) 91:135–40. doi: 10.26442/00403660.2019.08.000228
66. Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Intracerebral hemorrhage versus infarction: stroke severity, risk factors, and prognosis. Ann Neurol. (1995) 38:45–50. doi: 10.1002/ana.410380110
67. Ratha Krishnan R, Yeo EQY, Lim CJ, Chua KSG. The impact of stroke subtype on recovery and functional outcome after inpatient rehabilitation: a retrospective analysis of factors. Life. (2022) 12:1295. doi: 10.3390/life12091295
68. Zou X, Wang L, Xiao L, Wang S, Zhang L. Gut microbes in cerebrovascular diseases: gut flora imbalance, potential impact mechanisms and promising treatment strategies. Front Immunol. (2022) 13:975921. doi: 10.3389/fimmu.2022.975921
69. Zhang J, Zhang CH, Lin XL, Zhang Q, Wang J, Shi SL. Serum glial fibrillary acidic protein as a biomarker for differentiating intracerebral hemorrhage and ischemic stroke in patients with symptoms of acute stroke: a systematic review and meta-analysis. Neurol Sci. (2013) 34:1887–92. doi: 10.1007/s10072-013-1541-3
70. Zhang H, Huang Y, Li X, Han X, Hu J, Wang B, et al. Dynamic process of secondary pulmonary infection in mice with intracerebral hemorrhage. Front Immunol. (2021) 12:767155. doi: 10.3389/fimmu.2021.767155
71. Rost NS. Clinical neurogenetics: stroke. Neurol Clin. (2013) 31:915–28. doi: 10.1016/j.ncl.2013.05.001
72. Ekkert A, Šliachtenko A, Utkus A, JatuŽis D. Intracerebral hemorrhage genetics. Genes. (2022) 13:1250. doi: 10.3390/genes13071250
73. Hostettler IC, Seiffge DJ, Werring DJ. Intracerebral hemorrhage: an update on diagnosis and treatment. Expert Rev Neurother. (2019) 19:679–94. doi: 10.1080/14737175.2019.1623671
74. Camilleri M. Gastrointestinal motility disorders in neurologic disease. J Clin Invest. (2021) 131:e143771. doi: 10.1172/JCI143771
75. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. (2005) 36:2756–63. doi: 10.1161/01.STR.0000190056.76543.eb
Keywords: stroke, gastrointestinal disorders, Mendelian randomization, causality, risk
Citation: Song J, Chen W and Ye W (2023) Stroke and the risk of gastrointestinal disorders: A Mendelian randomization study. Front. Neurol. 14:1131250. doi: 10.3389/fneur.2023.1131250
Received: 24 December 2022; Accepted: 30 January 2023;
Published: 21 February 2023.
Edited by:
Yinong Huang, Sun Yat-sen University, ChinaReviewed by:
HaoRan Tao, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, ChinaCopyright © 2023 Song, Chen and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Ye,  eWV3ZWk3NzUyQDE2My5jb20=
eWV3ZWk3NzUyQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.