
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 24 May 2023
Sec. Neurological Biomarkers
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1125359
 Xia Huang1
Xia Huang1 Yuanyuan Zhang2*
Yuanyuan Zhang2*Aim: To explore the relationship between baseline bicarbonate levels and their changes with 30-day mortality in patients with acute ischemic stroke who were admitted to the intensive care unit (ICU).
Methods: This cohort study collected the data of 4,048 participants from the Medical Information Mart for Intensive Care (MIMIC)-III and MIMIC-IV databases. Univariate and multivariable Cox proportional risk models were utilized to explore the relationship between bicarbonate T0 and Δbicarbonate with 30-day mortality in patients with acute ischemic stroke. The Kaplan–Meier curves were plotted to measure the 30-day survival probability of patients with acute ischemic stroke.
Results: The median follow-up time was 30 days. At the end of the follow-up, 3,172 patients survived. Bicarbonate T0 ≤ 21 mEq/L [hazard ratio (HR) = 1.24, a 95% confidence interval (CI): 1.02–1.50] or 21 mEq/L < bicarbonate T0 ≤ 23 mEq/L (HR = 1.29, 95%CI: 1.05–1.58) were associated with an increased risk of 30-day mortality in patients with acute ischemic stroke compared with bicarbonate T0 > 26 mEq/L. −2 mEq/L < Δbicarbonate ≤ 0 mEq/L (HR = 1.40, 95%CI: 1.14–1.71), 0 mEq/L < Δbicarbonate ≤ 2 mEq/L (HR = 1.44, 95%CI: 1.17–1.76), and Δbicarbonate >2 mEq/L (HR = 1.40, 95%CI: 1.15–1.71) were correlated with an elevated risk of 30-day mortality in acute ischemic stroke patients. The 30-day survival probability of acute ischemic stroke patients with 21 mEq/L < bicarbonate T0 ≤ 23 mEq/L, 23 mEq/L < bicarbonate T0 ≤ 26 mEq/L, or bicarbonate T0 >26 mEq/L was higher than that of patients with bicarbonate T0 ≤ 21 mEq/L. The 30-day survival probability was greater for patients in the Δbicarbonate ≤-2 mEq/L group than for those in the Δbicarbonate >2 mEq/L group.
Conclusion: Low baseline bicarbonate levels and decreased bicarbonate levels during the ICU stay were associated with a high risk of 30-day mortality in acute ischemic stroke patients. Special interventions should be offered to those with low baseline and decreased bicarbonate levels during their ICU stay.
Stroke remains one of the leading causes of death and a major cause of disability worldwide (1). Ischemic stroke accounts for 87% of all stroke cases. Acute ischemic stroke is considered a medical emergency due to the decreased blood flow to the brain and is characterized by sudden-onset numbness or weakness in the arm or the leg, facial drooping, difficulty speaking or understanding speech, confusion, trouble with balance or coordination, and the loss of vision (2). Although treatments, including intravenous thrombolysis (IVT) using tissue plasminogen activator (tPA), have improved the functional outcomes of patients with acute ischemic stroke, the prognosis of these patients remains poor (3). Stroke has caused ~5.8 million deaths (4) and is a tremendous financial burden (5). Identifying more reliable biomarkers for predicting the prognosis of patients with acute ischemic stroke could improve patient management and treatment.
In a previous study, endothelial dysfunction was reported to be one of the pathological mechanisms of ischemic stroke (6). Evidence shows that lower serum bicarbonate levels may be associated with endothelial dysfunction, and bicarbonate therapy can improve endothelial function in patients with chronic kidney disease (7, 8). However, researchers have found that peripheral blood electrolyte changes occurred after stroke and that alterations in cerebrovascular acid-base balance directly affected cerebral blood flow (9). Bicarbonate measurements are well-acknowledged as a clinically useful biomarker for assessing the acid-base status to diagnose various disease conditions (10, 11). A previous study indicated that, although there was no statistical significance between the serum bicarbonate levels and the mortality of stroke patients, the serum bicarbonate levels were also considered an important factor that correlated with the prognosis of stroke patients and were included in the prediction model for predicting 30-, 180-, and 360-day survival of stroke patients (12). Other studies have revealed that higher serum bicarbonate levels in patients with hypertension are associated with a higher risk of cardiovascular disease (13, 14). The role of bicarbonate levels in patients with cardiovascular diseases was conflicting. Thus, clarifying the association between serum bicarbonate levels and the risk of death in patients with acute ischemic stroke would be highly valuable. In addition, in severe cases, serum bicarbonate levels can fluctuate with changes in the patient's condition as a result of the treatments received (15). Evaluating the influence of the change in bicarbonate levels on the prognosis of patients with ischemic stroke may be valuable.
In this study, the associations between serum bicarbonate levels and their changes with 30-day mortality in patients with acute ischemic stroke were measured based on the data from the Medical Information Mart for Intensive Care (MIMIC)-III and MIMIC-IV databases. Subgroup analyses were stratified by age, the Charlson comorbidity index (CCI), thrombolysis, antiplatelet agents, and anticoagulation agents.
In this cohort study, 4,674 participants with acute ischemic stroke were identified from the MIMIC-III and MIMIC-IV databases. MIMIC-III is a publicly available single-center critical care database that was approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center (BIDMC, Boston, MA, USA) and the Massachusetts Institute of Technology (Cambridge, MA, USA), and it contains information on 46,520 patients who were admitted to the ICUs of BIDMC in Boston from 2001 to 2012 (16). The information included demographics, vital signs, laboratory tests, fluid balance, and vital status; documents of the International Classification of Diseases and Ninth Revision (ICD-9) codes; records of hourly physiologic data from bedside monitors validated by the ICU nurses; and written evaluations of radiologic films by specialists covering the corresponding time period (17). MIMIC-IV is an updated version of MIMIC-III that features improvements, including a simplified structure, new data elements, and improved usability of previous data elements (18). The project was approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center (Boston, MA) and the Massachusetts Institute of Technology (Cambridge, MA). The use of the data provided by clinicians, data scientists, and information technology personnel, as well as unidentified health information from patients, did not require individual patient consent due to the anonymization of the health information. The diagnosis of acute ischemic stroke was based on ICD-9 (43,301, 43,311, 43,321, 43,331, 43,381, 43,391, 43,401, 43,411, or 43,491) or ICD-10 code (I63). In our study, we excluded individuals under the age of 18 years, those who did not have bicarbonate levels measured at 24-h intervals, and those who died within 2 days of being admitted to the ICU. Finally, 4,048 participants were included, with 3,172 subjects surviving at 30 days and 876 dying within 30 days.
Potential confounders analyzed in this study including demographic characteristics, such as age (years), gender (female or male), and ethnicity (Black, White, others or unknown), and clinical data such as ventilation (yes or no), vasopressor (yes or no), coronary artery bypass grafting (CABG) (yes or no), coronary artery disease (yes or no), congestive heart failure (yes or no), peripheral vascular disease (yes or no), hyperlipidemia (yes or no), hypertension (yes or no), chronic kidney disease (yes or no), atrial fibrillation (yes or no), malignant cancer (yes or no), diabetes (yes or no), systolic (mmHg), diastolic (mmHg), respiratory rate (beat/min), heart rate (beat/min), temperature (°C), oxygen saturation (SPO2, %), white blood cell (WBC, K/uL), platelet (K/uL), hemoglobin (g/dL), red cell distribution width (RDW, %), blood urea nitrogen (BUN, mg/dL), creatinine (mg/dL), glucose (mg/dL), CCI, simplified acute physiology score II (SAPSII), sequential organ failure assessment (SOFA), quick SOFA (qSOFA), systemic inflammatory response syndrome (SIRS), thrombolysis (yes or no), antiplatelet agents (yes or no), anticoagulation agents, mechanical bolt removal (yes or no), and hemorrhagic transformation (yes or no). The laboratory data were collected within 24 h after admission to the ICU, and drug use was defined as receiving respective drugs at any point during admission.
Bicarbonate T0 (mEq/L), bicarbonate T1 (mEq/L), and Δbicarbonate were the main variables. Bicarbonate T0 was the first measurement of bicarbonate from 0 to 24 h after admission to the ICU, and bicarbonate T1 was the first measurement of bicarbonate between 24 and 48 h after admission to the ICU. Δbicarbonate = bicarbonate T0—bicarbonate T1. Bicarbonate T0 was divided into ≤ Q1, Q1-Q2, Q2-Q3, and >Q3 groups according to the quartiles, and Q1 was 21 mEq/L, Q2 was 23 mEq/L, and Q3 was 26 mEq/L. Δbicarbonate was also divided into ≤ Q1, Q1-Q2, Q2-Q3, and >Q3 groups according to the quartiles, and Q1 was −2 mEq/L, Q2 was 0 mEq/L, and Q3 was 2 mEq/L.
Whether the participant survived within 30 days was regarded as the outcome of this study. In-hospital mortality was recorded by the MIMIC III and MIMIC IV databases, while out-of-hospital mortality was recorded by the Social Security Bureau. The median follow-up time was 30.00 (30.00, 30.00) days.
The mean ± standard deviation (SD) was used to describe the measurement data with a normal distribution, and a t-test was applied to compare the difference between the two groups. The median and quartile [M (Q1, Q3)] were used to display the measurement data with a non-normal distribution, and the Wilcoxon rank sum test was employed to compare the difference between the two groups. The enumeration data were shown as the number of cases and the component ratio [n (%)], and differences between groups were compared using the chi-squared test or Fisher's exact probability methods. Variables with missing values ≥20% were deleted. The Random Forest interpolation method (n_estimators = 500) was applied to variables with missing values of < 20% (Supplementary Table 1), and sensitivity analysis was performed to compare the data before and after interpolation (Supplementary Table 2). Confounders were identified using the univariate Cox proportional risk models, which were then subjected to stepwise regression analysis. Univariate and multivariable Cox proportional risk models were utilized for exploring the relationship between bicarbonate T0 and Δbicarbonate with 30-day mortality in patients with acute ischemic stroke. To explore the association between bicarbonate T0 and 30-day mortality in patients with acute ischemic stroke, a multivariable model was adjusted for age, gender, ethnicity, ventilation, vasopressor, hyperlipidemia, atrial fibrillation, heart rate, hemoglobin, RDW, CCI, SAPSII, CABG, thrombolysis, antiplatelet agents, and anticoagulation agents. To explore the association between Δbicarbonate and 30-day mortality in patients with acute ischemic stroke, a multivariable model was adjusted for age, gender, ethnicity, ventilation, vasopressor, hyperlipidemia, atrial fibrillation, diastolic, RDW, glucose, CCI, SAPSII, CABG, thrombolysis, antiplatelet agents, and anticoagulation agents. A sensitivity analysis was conducted by comparing the data before and after interpolating the missing data. A subgroup analysis was conducted by stratifying age, CCI, thrombolytic therapy, antiplatelet therapy, and anticoagulant therapy. The Kaplan–Meier curves were plotted to measure the 30-day survival probability of patients with acute ischemic stroke. The Hazard Ratio (HR) was applied to evaluate the associations between bicarbonate T0 and Δbicarbonate with 30-day mortality in patients with acute ischemic stroke. The value of alpha equal to 0.05 was set as the confidence level. Missing value interpolation was completed using Python 3.7.4 (Python Software Foundation, Delaware, USA). Sensitivity analysis, difference comparison, univariate/multivariate Cox proportional risk model modeling, and subgroup analysis were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). The Kaplan–Meier curve was drawn using R version 4.2.0 (2022-04-22 ucrt).
This study collected data from both the MIMIC-III and MIMIC-IV databases, which included information from 4,674 participants with acute ischemic stroke. Among them, people aged < 18 years (n = 2), without measurements on bicarbonate levels at 24-h intervals (n = 570), and who died within 2 days of being admitted to the ICU (n = 54) were excluded. Finally, 4,048 participants were included. The screening process is shown in Figure 1.
Compared with the survival group, the mean age (73.29 vs. 66.14 years), respiratory rate (19.41 beats vs. 18.41 beats), heart rate (86.46 beats vs. 82.67 beats), and RDW (15.05 vs. 14.39%) in the death group were high. The percentages of patients who received ventilation (83.79 vs. 61.38%) and vasopressors (43.84 vs. 28.31%) in the death group were higher than those in the percentages of the survival group. The percentages of participants who received thrombolysis (10.16 vs. 14.28%), antiplatelet agents (55.14 vs. 62.30%), and anticoagulation agents (9.70 vs. 22.35%) in the death group were lower than those in the survival group. The median length of stay (LOS) in the death group was longer than the survival group (5.02 vs. 3.07 days) (Table 1).
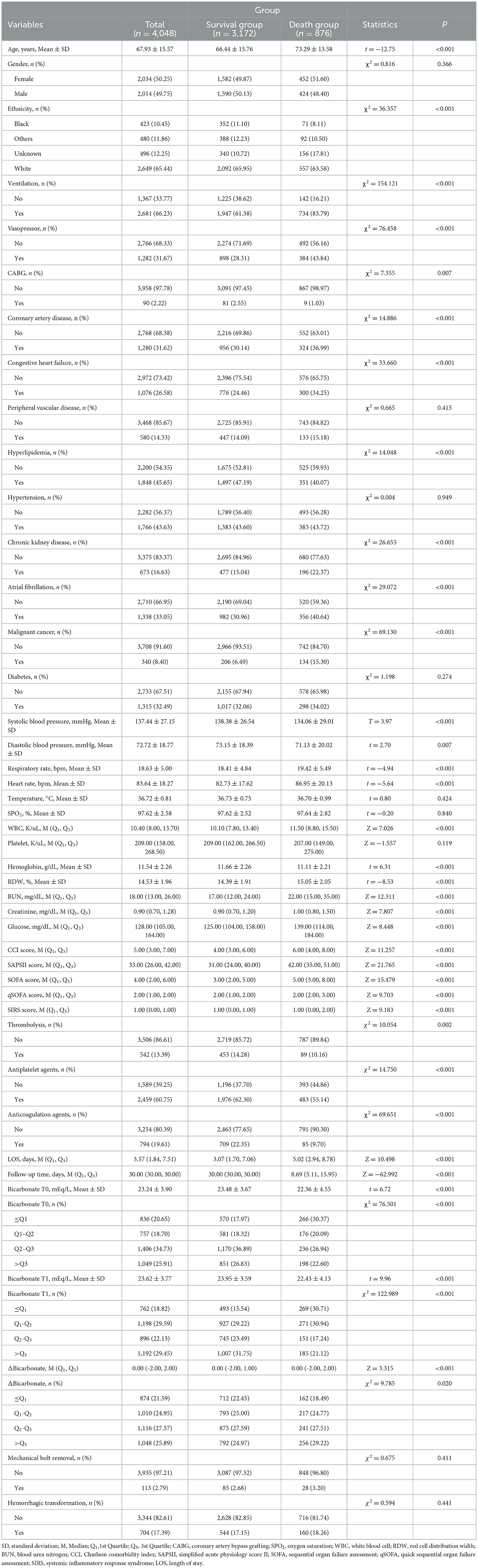
Table 1. Comparisons of characteristics of acute ischemic stroke patients the between survival and death groups.
To identify the associations between bicarbonate T0 and Δbicarbonate and 30-day mortality in patients with acute ischemic stroke, potential confounders were found using univariate Cox proportional hazard model analysis. The data revealed that several factors, including age, being Black, ventilation, vasopressor use, coronary artery disease, hyperlipidemia, chronic kidney disease, atrial fibrillation, systolic blood pressure, diastolic blood pressure, respiratory rate, heart rate, WBC count, hemoglobin levels, RDW, BUN, creatinine, glucose levels, CCI, SAPS II, SOFA, qSOFA, SIRS, CABG, thrombolysis, antiplatelet agents, and anticoagulation agents, might be potential confounding variables in the associations between bicarbonate T0 and Δbicarbonate levels and 30-day mortality risk in patients with acute ischemic stroke (Table 2).
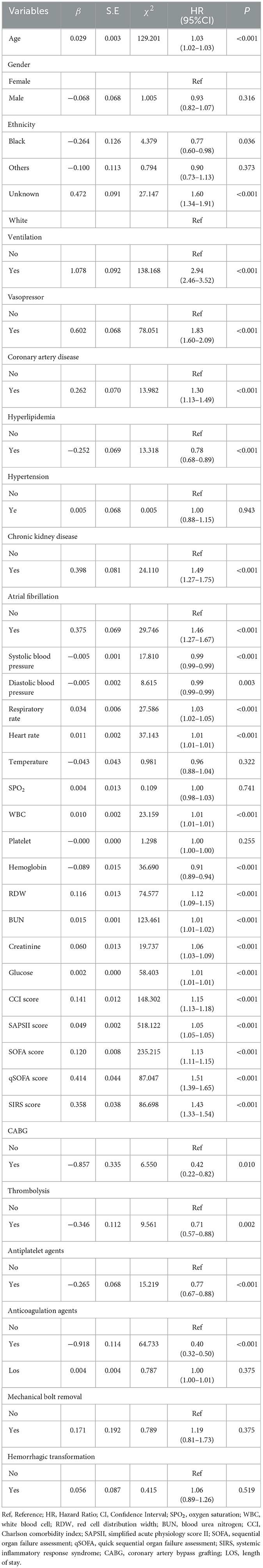
Table 2. Potential confounding factors associated with 30-day mortality in patients with acute ischemic stroke.
Variables with a statistically significant difference were involved in the multivariable Cox proportional hazard model through stepwise regression. The results are displayed in Table 3, with age (HR = 1.02, 95%CI: 1.02–1.03), being Black (HR = 0.75, 95%CI: 0.59–0.97), ventilation (HR = 2.16, 95%CI: 1.78–2.62), vasopressor use (HR = 1.27, 95%CI: 1.09–1.48), hyperlipidemia (HR = 0.80, 95%CI: 0.70–0.92), atrial fibrillation (HR = 1.22, 95%CI: 1.05–1.41), diastolic blood pressure (HR = 1.01, 95%CI: 1.01–1.01), heart rate (HR = 1.01, 95%CI: 1.01–1.01), RDW (HR = 1.05, 95%CI: 1.01–1.08), CCI (HR = 1.09, 95%CI: 1.06–1.12), SAPS II (HR = 1.03, 95%CI: 1.02–1.04), CABG (HR = 0.22, 95%CI: 0.11–0.43), thrombolysis (HR = 0.72, 95%CI: 0.58–0.90), antiplatelet agents (HR = 0.82, 95%CI: 0.72–0.95), and anticoagulation agents (HR = 0.35, 95%CI: 0.28–0.44) considered as confounders associated with 30-day mortality in patients with acute ischemic stroke. Gender was an important variable associated with mortality in patients with acute ischemic stroke, which was also adjusted for in the multivariable Cox proportional hazard model. The data revealed that after adjusting for these confounding factors, bicarbonate T0 ≤ Q1 (HR = 1.22, 95%CI: 1.01–1.48) or bicarbonate T0 of Q1-Q2 (HR = 1.26, 95%CI: 1.03–1.55) was associated with an increased risk of 30-day mortality in patients with acute ischemic stroke compared with bicarbonate T0 >Q3 (Table 3). The 30-day survival probability of acute ischemic stroke patients with bicarbonate T0 of Q2-Q3, bicarbonate T0 of Q2-Q3, or bicarbonate T0 >Q3 was higher than that of the participants with bicarbonate T0 ≤ Q1 (Figure 2).
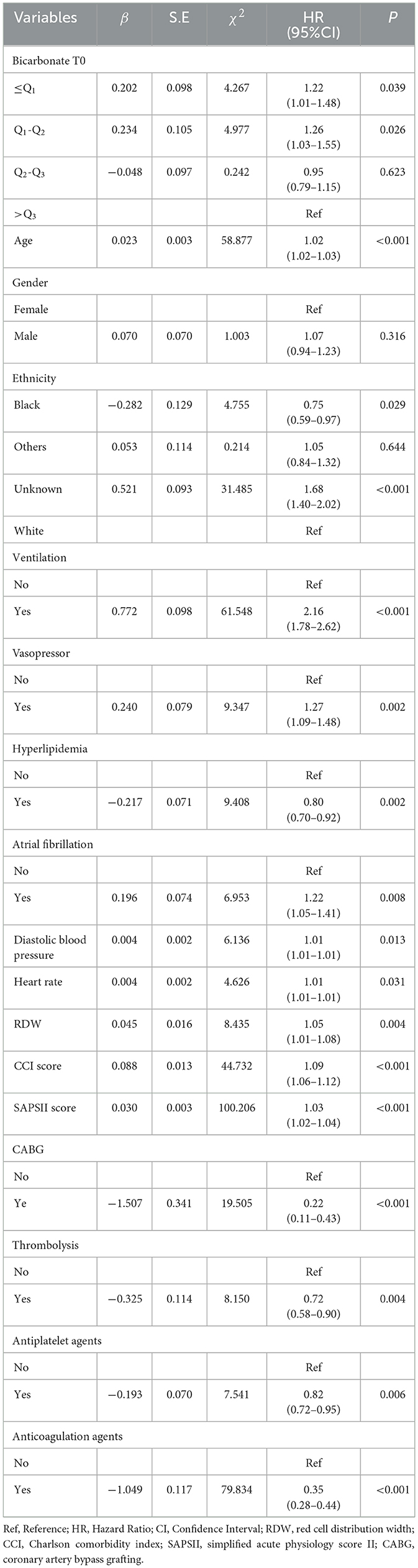
Table 3. Association between bicarbonate T0 and 30-day mortality in patients with acute ischemic stroke.
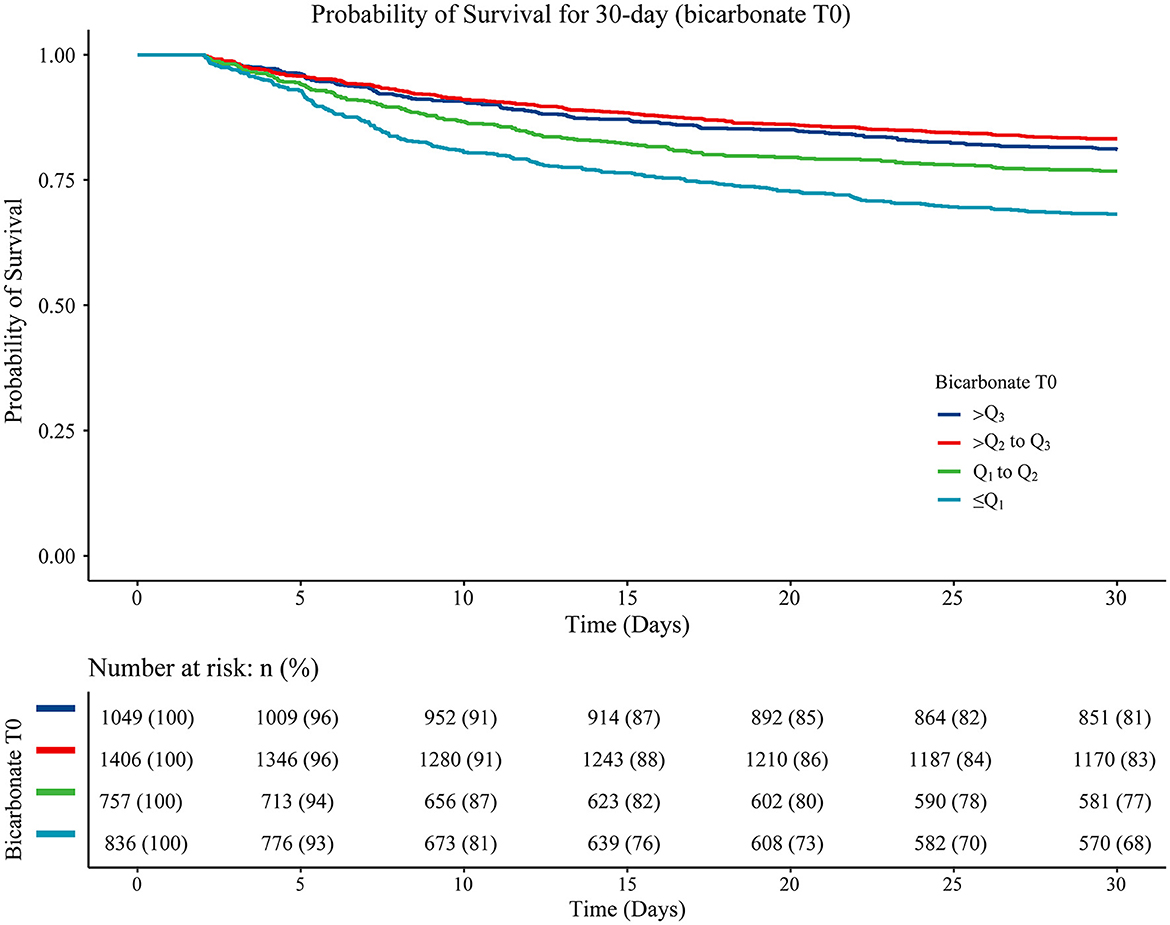
Figure 2. The Kaplan–Meier curve showing the 30-day survival probability in patients from different bicarbonate T0 group. Log-rank ≤Q1 vs. Q1-Q2 (P < 0.001), ≤Q1 vs. Q2-Q3 (P < 0.001), ≤Q1 vs. >Q3 (P < 0.001), Q1-Q2 vs. Q2-Q3 (P = 0.022), Q1-Q2 vs. >Q3 (P < 0.001), and Q2-Q3 vs. >Q3 (P = 0.185).
When bicarbonate T0 was dealt with as a continuous variable, we found that age (HR = 1.02, 95%CI: 1.02–1.03), Black (HR = 0.75, 95%CI: 0.58–0.97), ventilation (HR = 2.19, 95%CI: 1.80–2.65), vasopressor (HR = 1.27, 95%CI: 1.09–1.49), hyperlipidemia (HR = 0.80, 95%CI: 0.70–0.92), atrial fibrillation (HR = 1.22, 95%CI: 1.05–1.41), diastolic blood pressure (HR = 1.01, 95%CI: 1.01–1.01), heart rate (HR = 1.01, 95%CI: 1.01–1.01), RDW (HR = 1.05, 95%CI: 1.02–1.08), CCI (HR = 1.09, 95%CI: 1.06–1.12), SAPS II (HR = 1.03, 95%CI: 1.02–1.04), CABG (HR = 0.23, 95%CI: 0.12–0.44), thrombolysis (HR = 0.72, 95%CI: 0.57–0.90), antiplatelet agents (HR = 0.83, 95%CI: 0.72–0.95), and anticoagulation agents (HR = 0.35, 95%CI: 0.28–0.45) were confounding factors. After adjusting for these variables and gender, increased bicarbonate T0 was related to a decreased risk of 30-day mortality in patients with acute ischemic stroke (HR = 0.98, 95%CI: 0.97–0.99) (Supplementary Table 3).
As for the association between Δbicarbonate and 30-day mortality in acute ischemic stroke patients, the results of the multivariable Cox proportional hazard model revealed that age (HR = 1.02, 95%CI: 1.02–1.03), being Black (HR = 0.75, 95%CI: 0.58–0.97), ventilation (HR = 2.20, 95%CI: 1.81–2.66), vasopressor use (HR = 1.33, 95%CI: 1.14–1.55), hyperlipidemia (HR = 0.81, 95%CI: 0.70–0.93), atrial fibrillation (HR = 1.21, 95%CI: 1.05–1.40), diastolic blood pressure (HR = 1.01, 95%CI: 1.01–1.01), heart rate (HR = 1.01, 95%CI: 1.01–1.01), RDW (HR = 1.05, 95%CI: 1.02–1.08), CCI (HR = 1.09, 95%CI: 1.06–1.12), SAPSII (HR = 1.03, 95%CI: 1.03–1.04), CABG (HR = 0.23, 95%CI: 0.12–0.45), thrombolysis (HR = 0.70, 95%CI: 0.56–0.88), antiplatelet agents (HR = 0.82, 95%CI: 0.72–0.94), and anticoagulation agents (HR = 0.35, 95%CI: 0.28–0.44) were confounding factors associated with the mortality in acute ischemic stroke patients. In the multivariable Cox proportional hazard model, Δbicarbonate of Q1-Q2 (HR = 1.36, 95%CI: 1.11–1.67), Δbicarbonate of Q2-Q3 (HR = 1.40, 95%CI: 1.14–1.71), and Δbicarbonate >Q3 (HR = 1.37, 95%CI: 1.12–1.03) were correlated with an elevated risk of 30-day mortality in acute ischemic stroke patients (Table 4). The Kaplan–Meier curve showed that the 30-day survival probability in patients with Δbicarbonate ≤ Q1 group was higher than that in patients with Δbicarbonate >Q3 group (Figure 3).
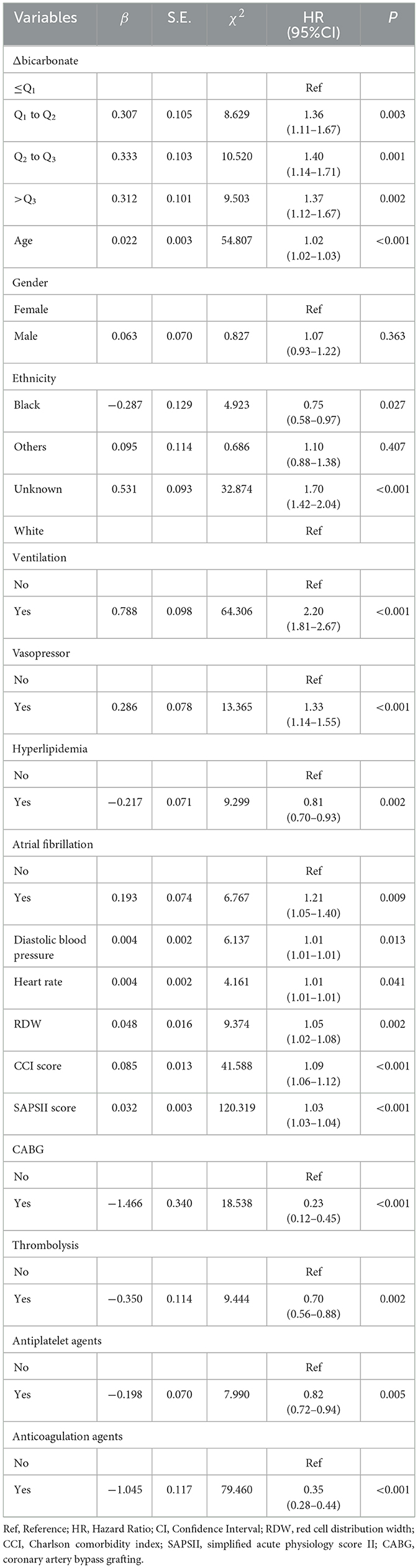
Table 4. Association between Δbicarbonate and 30-day mortality in patients with acute ischemic stroke.
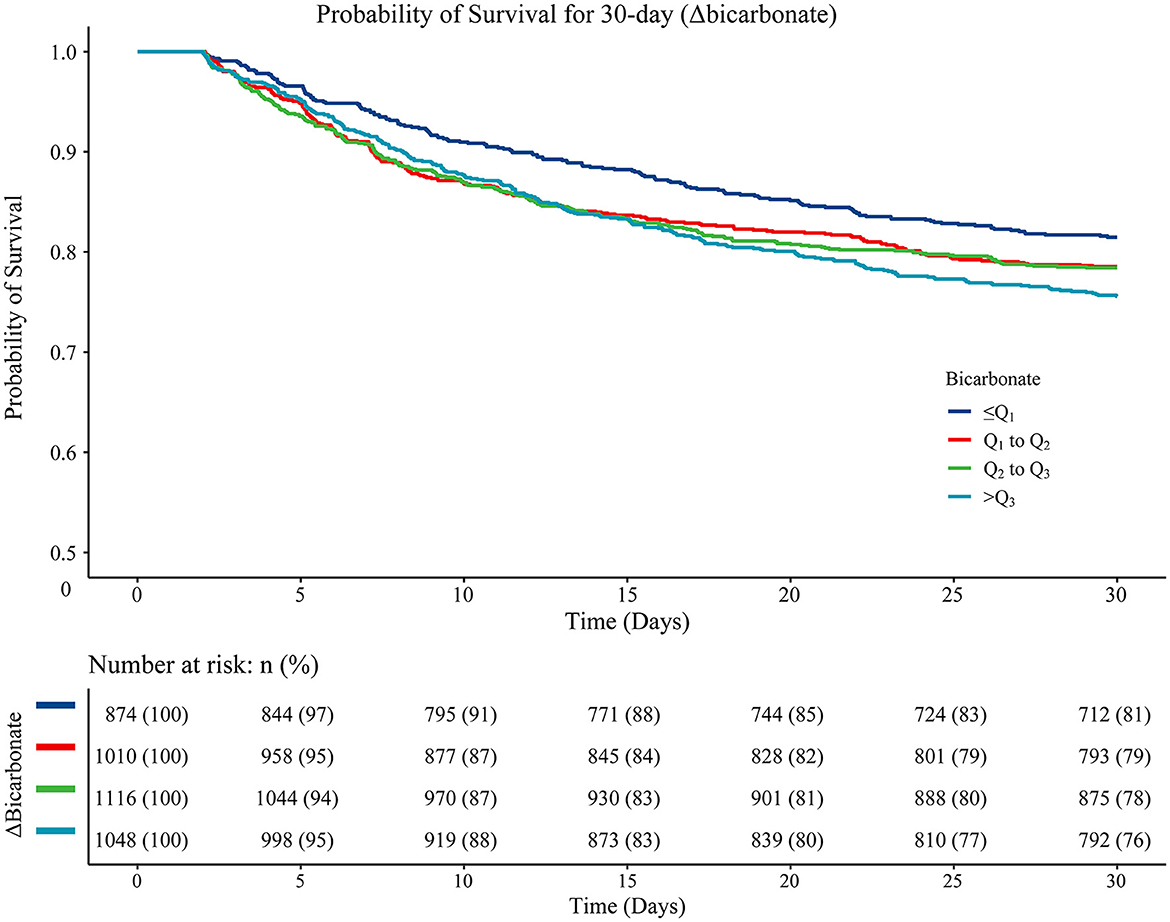
Figure 3. The Kaplan–Meier curve presenting the 30-day survival probability in patients from different Δbicarbonate group. Log-rank ≤Q1 vs. Q1-Q2 (P = 0.160), ≤Q1 vs. Q2-Q3 (P = 0.160), ≤Q1 vs. >Q3 (P = 0.010), Q1-Q2 vs. Q2-Q3 (P = 0.930), Q1-Q2 vs. >Q3 (P = 0.210), and Q2-Q3 vs. >Q3 (P = 0.210).
When Δbicarbonate was considered a continuous variable, an increased risk of 30-day mortality in patients with acute ischemic stroke was identified in those with higher Δbicarbonate (HR = 1.03, 95%CI: 1.01–1.05) after adjusting for confounding factors, including age (HR = 1.02, 95%CI: 1.02–1.03), gender, ethnicity (HR = 0.75, 95%CI: 0.59–0.97), ventilation (HR = 2.20, 95%CI: 1.82–2.67), vasopressor (HR = 1.31, 95%CI: 1.13–1.53), hyperlipidemia (HR = 0.81, 95%CI: 0.71–0.93), atrial fibrillation (HR = 1.22, 95%CI: 1.05–1.41), diastolic blood pressure (HR = 1.01, 95%CI: 1.01–1.01), heart rate (HR = 1.01, 95%CI: 1.01–1.01), RDW (HR = 1.05, 95%CI: 1.02–1.08), CCI (HR = 1.09, 95%CI: 1.06–1.12), SAPSII (HR = 1.03, 95%CI: 1.03–1.04), CABG (HR = 0.23, 95%CI: 0.12–0.44), thrombolysis (HR = 0.70, 95%CI: 0.56–0.88), antiplatelet agents (HR = 0.82, 95%CI: 0.71–0.94), and anticoagulation agents (HR = 0.35, 95%CI: 0.28–0.44) (Supplementary Table 4).
In patients aged < 70 years, bicarbonate T0 of Q2-Q3 was associated with an increased risk of 30-day mortality in patients with acute ischemic stroke in the adjusted model (HR = 1.67, 95%CI: 1.18–2.37). In people aged ≥70 years, bicarbonate T0 ≤ Q1 was linked with an elevated risk of 30-day mortality in patients with acute ischemic stroke (HR = 1.28, 95%CI: 1.01–1.61). Higher bicarbonate T0 was related to an increased risk of 30-day mortality in patients with acute ischemic stroke, which was observed in those aged ≥70 years (HR = 1.05, 95%CI: 1.02–1.08). After adjusting for potential confounders, we found that subjects withΔbicarbonate of Q1-Q2 (HR =1.56, 95%CI: 1.19–2.04), Δbicarbonate of Q2-Q3 (HR = 1.58, 95%CI: 1.21–2.06), and Δbicarbonate >Q3 (HR = 1.65, 95%CI: 1.28–2.14) were associated with an increased risk of 30-day mortality in patients with acute ischemic stroke in patients aged ≥70 years. In those with CCI≥5, Δbicarbonate of Q1-Q2 (HR = 1.57, 95%CI: 1.20–2.06), Δbicarbonate of Q2-Q3 (HR = 1.63, 95%CI: 1.25–2.12), or Δbicarbonate>Q3 (HR = 1.66, 95%CI: 1.29–2.15) were correlated with an increased risk of 30-day mortality in patients with acute ischemic stroke. An increased risk of 30-day mortality in patients with acute ischemic stroke was elevated with the increase of Δbicarbonate (HR = 1.05, 95%CI: 1.02–1.07) in people with CCI ≥ 5 (Table 5).
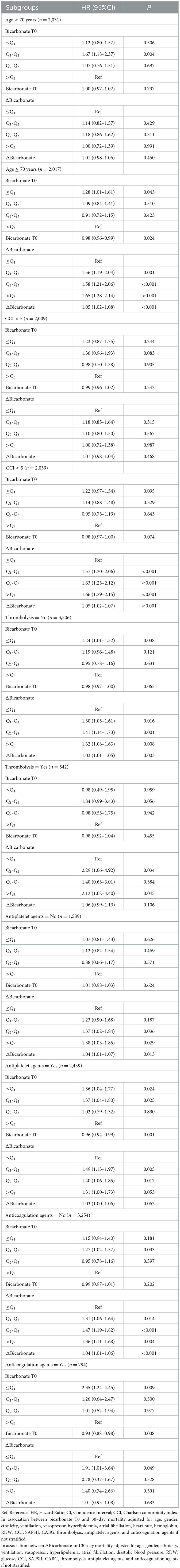
Table 5. Subgroup analysis of associations of bicarbonate T0 and Δbicarbonate with 30-day mortality in patients with acute ischemic stroke.
Among participants who did not receive thrombolysis, those with bicarbonate levels at or below the first quartile T0 ≤ Q1 had a 24% higher risk of 30-day mortality (HR = 1.24, 95%CI: 1.01–1.52). Similarly, those with changes in bicarbonate levels between the first and second quartiles (Δbicarbonate of Q1–Q2), second and third quartiles (Δbicarbonate of Q2–Q3), or above the third quartile (Δbicarbonate >Q3) had elevated risks of 30-day mortality, with hazard ratios (HRs) of 1.30 (95% CI: 1.05–1.61), 1.41 (95%CI: 1.14–1.73), and 1.32 (95%CI: 1.08–1.63), respectively. An increased risk of 30-day mortality in patients with acute ischemic stroke was elevated with the increase of Δbicarbonate in participants who did not receive thrombolysis (HR = 1.03, 95%CI: 1.01–1.05). Among patients who received thrombolysis, those with Δbicarbonate of Q1-Q2 (HR = 2.29, 95%CI: 1.06–4.92) or Δbicarbonate >Q3 (HR = 2.12, 95%CI: 1.02–4.40) had an increased risk of 30-day mortality in patients with acute ischemic stroke. Among people who did not receive antiplatelet agents, Δbicarbonate of Q2-Q3 (HR = 1.37, 95%CI: 1.02–1.84) or Δbicarbonate >Q3 (HR = 1.38, 95%CI: 1.03–1.85) were associated with an increased risk of 30-day mortality in patients with acute ischemic stroke; and patients with higher Δbicarbonate were correlated with an increased risk of 30-day mortality (HR = 1.04, 95%CI: 1.01–1.07). In people who received antiplatelet agents, bicarbonate T0 ≤ Q1 (HR = 1.36, 95%CI: 1.04–1.77), bicarbonate T0 of Q2-Q3 (HR = 1.37, 95%CI: 1.04–1.80), Δbicarbonate of Q1-Q2 (HR = 1.49, 95%CI: 1.13–1.97), and Δbicarbonate of Q2-Q3 (HR = 1.40, 95%CI: 1.06–1.85) were correlated with an increased risk of 30-day mortality in patients with acute ischemic stroke. We also observed that bicarbonate T0 of Q2-Q3 (HR = 1.27, 95%CI: 1.042–1.57), Δbicarbonate of Q1-Q2 (HR = 1.31, 95%CI: 1.06–1.64), Δbicarbonate of Q2-Q3 (HR = 1.47, 95%CI: 1.19–1.82), or Δbicarbonate >Q3 (HR = 1.36, 95%CI: 1.11–1.68) were linked with an elevated risk of 30-day mortality in acute ischemic stroke patients who did not receive anticoagulation agents. An elevated level of Δbicarbonate was linked with an increased risk of 30-day mortality in acute ischemic stroke patients who did not receive anticoagulation agents (HR = 1.04, 95%CI: 1.01–1.06). Bicarbonate T0 ≤ Q1 (HR = 2.35, 95%CI: 1.24–4.45) and Δbicarbonate of Q1-Q2 (HR = 1.91, 95%CI: 1.01–3.64) were linked with an elevated risk of 30-day mortality in acute ischemic stroke patients receiving anticoagulation agents. A decreased risk of 30-day mortality in acute ischemic stroke patients who received anticoagulation agents was found in those with higher bicarbonate T0 (HR = 0.93, 95%CI: 0.88–0.98) (Table 5).
The current study assessed the relationship between the bicarbonate levels measured from 0 to 24 h after admission to the ICU and the bicarbonate level changes with 30-day mortality in patients with acute ischemic stroke. The data revealed that bicarbonate T0 ≤ Q1 or bicarbonate T0 of Q2-Q3 were associated with an increased risk of 30-day mortality in patients with acute ischemic stroke. Δbicarbonate of Q1-Q2, Δbicarbonate of Q2-Q3, and Δbicarbonate >Q3 were correlated with an elevated risk of 30-day mortality in acute ischemic stroke patients. The findings of our study might provide a reference for the management of the prognosis of acute ischemic stroke patients in the ICU.
Bicarbonate is an essential marker that plays an important role in regulating body fluids, acid-base balance, and participation in life activities (19). A low bicarbonate concentration usually represents metabolic acidosis, and low bicarbonate levels may cause astrocyte dysfunction, which was negatively associated with the outcome of stroke patients (20). Another study indicated that lower baseline bicarbonate concentrations were associated with a higher mortality risk among critically ill patients with ischemic cardiogenic shock (21). Lower serum bicarbonate concentrations were found to be significantly associated with higher cardiovascular disease mortality in type 2 diabetes (22). Evidence showed that a low serum bicarbonate level was an independent risk factor for kidney disease progression and mortality in heart failure patients (23). Our study found low baseline bicarbonate levels correlated with an elevated risk of 30-day mortality in patients with acute ischemic stroke. Low serum bicarbonate levels indicated metabolic acidosis, which is a kind of disorder associated with increased mortality, as it is implicated in multiple complications, including cardiac dysfunction, hypotension, and an increased risk of infection (24–26). Bicarbonate is involved in endothelial function, which is one of the pathological mechanisms involved in the development of ischemic stroke (6, 7, 27). It was produced from carbonic anhydrases, which regulated the Neurovascular Unit cells in vitro and in vivo models of stroke pathology (28). The patients with increased Δbicarbonate had a poor prognosis. The possible reason might be that increased Δbicarbonate indicated the bicarbonate concentration showed a decreasing trend during ICU admission, which usually reflects underlying metabolic acidosis (14), and this might lead to the severity of acute ischemic stroke. Acidosis modulates a wide range of inflammatory gene expression in endothelial cells and regulates endothelial cell adhesion (29), which, in turn, contributes to leukocyte infiltration and plasma leakage with subsequent tissue damage.
In addition, the change in bicarbonate levels was associated with an increased risk of 30-day mortality in acute ischemic stroke patients. These results underscore the importance of monitoring not only the baseline bicarbonate levels but also changes in bicarbonate levels over time. Clinicians should provide special interventions for patients with a decreased trend in the bicarbonate levels. The subgroup analysis revealed that, for patients under 70 years of age, attention should be given to the baseline bicarbonate level, while for patients 70 years of age and older, both the baseline bicarbonate level and changes in bicarbonate levels should be monitored. The Charlson comorbidity index (CCI) is a validated and straightforward method for evaluating the risk of death from comorbid diseases. It has been widely used to predict the prognosis and survival of patients with different diseases (30). In our study, the association between changes in the bicarbonate level and 30-day mortality in acute ischemic stroke patients was identified in those with CCI ≥ 5, suggesting that patients with more comorbid diseases should pay attention to the change in the bicarbonate levels. For patients who received thrombolysis or did not receive antiplatelet agent treatments, dynamic bicarbonate levels should be detected, and those with decreased bicarbonate levels require special care and treatments.
The present study involved a large sample size and found an association between the change in bicarbonate level and the prognosis of patients with acute ischemic stroke. The findings of our study have potential implications for the clinical management of acute ischemic stroke patients. Our findings highlight that the association between bicarbonate levels and the prognosis of patients with acute ischemic stroke may influence clinical decision-making regarding the dose and level of acidosis correction. Sequential monitoring of bicarbonate concentrations may be useful in predicting the prognosis of patients with acute ischemic stroke. In addition, low bicarbonate levels might be associated with a poor prognosis for patients with acute ischemic stroke, which reminds clinicians to be careful with other modifiable factors associated with the prognosis of patients with acute ischemic stroke and provide timely interventions for these patients.
There were several limitations to this study. First, the data were collected from a single-center database, which may limit the generalizability of the results to other populations. Therefore, caution should be exercised when interpreting and applying our findings to other settings. Second, the data on liquid input during the ICU stay, the location and size of the infarction, and stroke etiology were missing or not reported, which might influence the results. Third, the last known well time and the delayed presentation of patients with medical attention were not reported. Fourth, the deep mechanisms underlying the results were not explored. Moreover, the data of the study population were collected from the MIMIC III and MIMIC IV databases and consisted of patients with acute ischemic stroke who were admitted to the ICU. Therefore, caution should be exercised when generalizing our findings to the general population of patients with acute ischemic stroke, as the characteristics of ICUS patients may differ from those of the general population of patients. Finally, blood samples were not obtained at the same time point for all patients, and the difference in the time interval between T0 and T1 may have varied between the patients, which might affect the results. Therefore, further studies are needed to confirm the findings of our study and to determine the optimal timing of bicarbonate level measurements in patients with acute ischemic stroke.
Our study assessed the relationship between the serum bicarbonate levels and their changes with 30-day mortality in patients with acute ischemic stroke. The results indicated that low baseline bicarbonate levels and decreased bicarbonate levels during ICU stay were associated with a high risk of 30-day mortality in acute ischemic stroke. The findings highlighted the importance of detecting bicarbonate levels and monitoring changes in acute ischemic stroke patients. Special interventions should be provided for those with low baseline bicarbonate levels or/and decreased bicarbonate levels.
Publicly available datasets were analyzed in this study. This data can be found here: MIMIC-III: https://www.physionet.org/content/mimiciii/1.4/ and MIMIC-IV: https://www.physionet.org/content/mimiciv/2.2/.
The studies involving human participants were reviewed and approved by Beth Israel Deaconess Medical Center (Boston, MA) and the Massachusetts Institute of Technology (Cambridge, MA). This study did not require individual patient consent due to the anonymization of the health information.
XH and YZ designed the study, collected, analyzed, and interpreted the data. XH wrote the manuscript. YZ critically reviewed, edited, and approved the manuscript. Both authors read and approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1125359/full#supplementary-material
1. Saini V, Guada L, Yavagal DR. Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology. (2021) 97(20 Suppl. 2):S6–16. doi: 10.1212/WNL.0000000000012781
3. Heo NH, Lee MR, Yang KH, Hong OR, Shin JH, Lee BY, et al. Short- and long-term mortality after intravenous thrombolysis for acute ischemic stroke: a propensity score-matched cohort with 5-year follow-up. Medicine. (2021) 100:e27652. doi: 10.1097/MD.0000000000027652
4. Phipps MS, Cronin CA. Management of acute ischemic stroke. BMJ. (2020) 368:l6983. doi: 10.1136/bmj.l6983
5. Ho JP. Acute ischemic stroke: emergency department management after the 3-hour window. Emerg Med Pract. (2021) 23:1–33.
6. Tuttolomondo A, Daidone M, Pinto A. Endothelial dysfunction and inflammation in ischemic stroke pathogenesis. Curr Pharm Des. (2020) 26:4209–19. doi: 10.2174/1381612826666200417154126
7. Kendrick J, Shah P, Andrews E, You Z, Nowak K, Pasch A, et al. Effect of treatment of metabolic acidosis on vascular endothelial function in patients with CKD: a pilot randomized cross-over study. Clin J Am Soc Nephrol. (2018) 13:1463–70. doi: 10.2215/CJN.00380118
8. Cheng F, Li Q, Wang J, Wang Z, Zeng F, Zhang Y. The effects of oral sodium bicarbonate on renal function and cardiovascular risk in patients with chronic kidney disease: a systematic review and meta-analysis. Ther Clin Risk Manag. (2021) 17:1321–31. doi: 10.2147/TCRM.S344592
9. Caldwell HG, Carr J, Minhas JS, Swenson ER, Ainslie PN. Acid-base balance and cerebrovascular regulation. J Physiol. (2021) 599:5337–59. doi: 10.1113/JP281517
10. Martha SR, Fraser JF, Pennypacker KR. Acid-base and electrolyte changes drive early pathology in ischemic stroke. Neuromolecular Med. (2019) 21:540–5. doi: 10.1007/s12017-019-08555-5
11. Spears RC, McLouth CJ, Pennypacker KR, Frank JA, Maglinger B, Martha S, et al. Alterations in local peri-infarct blood gases in stroke patients undergoing thrombectomy. World Neurosurg. (2022) 158:e317–22. doi: 10.1016/j.wneu.2021.10.171
12. Li XD, Li MM. A novel nomogram to predict mortality in patients with stroke: a survival analysis based on the MIMIC-III clinical database. BMC Med Inform Decis Mak. (2022) 22:92. doi: 10.1186/s12911-022-01836-3
13. Kendrick JB, Zelnick L, Chonchol MB, Siscovick D, Hoofnagle AN, Ix JH, et al. Serum bicarbonate is associated with heart failure in the multi-ethnic study of atherosclerosis. Am J Nephrol. (2017) 45:118–26. doi: 10.1159/000454783
14. Dobre M, Pajewski NM, Beddhu S, Chonchol M, Hostetter TH, Li P, et al. Serum bicarbonate and cardiovascular events in hypertensive adults: results from the Systolic Blood Pressure Intervention Trial. Nephrol Dial Transplant. (2020) 35:1377–84. doi: 10.1093/ndt/gfz149
15. Semenenko AI, Hrebtiy GI, Semenenko NA, Malyk SL, Dmytriiev DV, Bodnar RY, et al. Evaluation of influence of infusion therapy on the dynamics of acid alkaline balance indices in patients with acute ischemic stroke. Wiad Lek. (2018) 71:1316–9.
16. Johnson AE, Pollard TJ, Shen L, Lehman LW, Feng M, Ghassemi M, et al. MIMIC-III, a freely accessible critical care database. Sci Data. (2016) 3:160035. doi: 10.1038/sdata.2016.35
17. Hou N, Li M, He L, Xie B, Wang L, Zhang R, et al. Predicting 30-days mortality for MIMIC-III patients with sepsis-3: a machine learning approach using XGboost. J Transl Med. (2020) 18:462. doi: 10.1186/s12967-020-02620-5
18. Liu T, Zhao Q, Du B. Effects of high-flow oxygen therapy on patients with hypoxemia after extubation and predictors of reintubation: a retrospective study based on the MIMIC-IV database. BMC Pulm Med. (2021) 21:160. doi: 10.1186/s12890-021-01526-2
19. Nagami GT, Kraut JA. Regulation of acid-base balance in patients with chronic kidney disease. Adv Chronic Kidney Dis. (2022) 29:337–42. doi: 10.1053/j.ackd.2022.05.004
20. Yao H, Azad P, Zhao HW, Wang J, Poulsen O, Freitas BC, et al. The Na(+)/HCO(-) co-transporter is protective during ischemia in astrocytes. Neuroscience. (2016) 339:329–37. doi: 10.1016/j.neuroscience.2016.09.050
21. Wigger O, Bloechlinger S, Berger D, Häner J, Zanchin T, Windecker S, et al. Baseline serum bicarbonate levels independently predict short-term mortality in critically ill patients with ischaemic cardiogenic shock. Eur Heart J Acute Cardiovasc Care. (2018) 7:45–52. doi: 10.1177/2048872616683526
22. Li Y, Gao R, Zhao B, Zhang Y. Low serum bicarbonate levels increased the risk of all-cause, cardiovascular and cancer Mortality in type 2 diabetes. J Clin Endocrinol Metab. (2022) 105:290–304. doi: 10.1210/clinem/dgac504
23. Dobre M, Yang W, Chen J, Drawz P, Hamm LL, Horwitz E, et al. Association of serum bicarbonate with risk of renal and cardiovascular outcomes in CKD: a report from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. (2013) 62:670–8. doi: 10.1053/j.ajkd.2013.01.017
24. Gunnerson KJ, Saul M, He S, Kellum JA. Lactate versus non-lactate metabolic acidosis: a retrospective outcome evaluation of critically ill patients. Crit Care. (2006) 10:R22. doi: 10.1186/cc3987
25. Masevicius FD, Rubatto Birri PN, Risso Vazquez A, Zechner FE, Motta MF, Valenzuela Espinoza ED, et al. Relationship of at admission lactate, unmeasured anions, and chloride to the outcome of critically ill patients. Crit Care Med. (2017) 45:e1233–9. doi: 10.1097/CCM.0000000000002730
26. Lim SY, Park Y, Chin HJ, Na KY, Chae DW, Kim S. Short-term and long-term effects of low serum bicarbonate level at admission in hospitalised patients. Sci Rep. (2019) 9:2798. doi: 10.1038/s41598-019-38892-1
27. Crimi E, Taccone FS, Infante T, Scolletta S, Crudele V, Napoli C. Effects of intracellular acidosis on endothelial function: an overview. J Crit Care. (2012) 27:108–18. doi: 10.1016/j.jcrc.2011.06.001
28. Lemon N, Canepa E, Ilies MA, Fossati S. Carbonic anhydrases as potential targets against neurovascular unit dysfunction in Alzheimer's disease and stroke. Front Aging Neurosci. (2021) 13:772278. doi: 10.3389/fnagi.2021.772278
29. Dong L, Li Z, Leffler NR, Asch AS, Chi JT, Yang LV. Acidosis activation of the proton-sensing GPR4 receptor stimulates vascular endothelial cell inflammatory responses revealed by transcriptome analysis. PLoS ONE. (2013) 8:e61991. doi: 10.1371/journal.pone.0061991
Keywords: bicarbonate T0, Δbicarbonate, mortality, acute ischemic stroke, MIMIC
Citation: Huang X and Zhang Y (2023) Relationship between serum bicarbonate levels and the risk of death within 30 days in ICU patients with acute ischemic stroke. Front. Neurol. 14:1125359. doi: 10.3389/fneur.2023.1125359
Received: 22 December 2022; Accepted: 17 April 2023;
Published: 24 May 2023.
Edited by:
Keith Pennypacker, University of Kentucky, United StatesReviewed by:
Nikolaos Papagiannakis, National and Kapodistrian University of Athens, GreeceCopyright © 2023 Huang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanyuan Zhang, eXVhbnl1YW56aGFuZ2RjdEBvdXRsb29rLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.