
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Neurol., 07 February 2023
Sec. Experimental Therapeutics
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1125121
This article is part of the Research TopicNovel Insights into CAR T-cell Associated NeurotoxicityView all 7 articles
 Umberto Pensato1,2
Umberto Pensato1,2 Chiara de Philippis3*
Chiara de Philippis3* Flavio Pistolese1,2
Flavio Pistolese1,2 Daniele Mannina3
Daniele Mannina3 Simona Marcheselli1
Simona Marcheselli1 Letterio S. Politi1,2
Letterio S. Politi1,2 Armando Santoro2,3
Armando Santoro2,3 Stefania Bramanti3
Stefania Bramanti3Introduction: Chimeric antigen receptor T-cell therapy-related neurotoxicity is a novel cytokine-mediated neurological syndrome that may present with a broad spectrum of manifestations. Descriptions of novel distinctive features are pivotal to untangling this condition's clinical and instrumental signature in order to inform diagnosis and pathophysiology.
Case: A 27-year-old female patient received anti-CD19 CAR T cells for a refractory primary mediastinal B-cell lymphoma. At 6 days after the infusion, she developed mild ideo-motor slowing, dysgraphia, and drowsiness. Despite specific treatment with dexamethasone, her neurological status progressively worsened to a comatose state within 24 h. EEG and CSF analyses were non-specific, showing background slowing and inflammatory findings. Brain MRI revealed multiple focal punctate areas of T2-weighted hyperintensity localized in the body and isthmus of the corpus callosum. Following the administration of high-dose intravenous methylprednisolone, her neurological status resolved within 48 h. Notably, the follow-up brain MRI did not reveal any abnormalities in the corpus callosum, except for a reduction of fractional anisotropy.
Conclusion: Reversible punctate inflammatory foci of the body and isthmus of the corpus callosum may represent a novel radiological finding of CAR T-cell therapy-related neurotoxicity.
Chimeric antigen receptor (CAR) T-cell therapies are changing the treatment paradigms for B-cell malignancies by achieving durable complete remission in patients refractory to multiple lines of treatment (1). In contrast, the potent anti-lymphoma effect of anti-CD19 CAR T-cell therapy might be hampered by significant toxicities including immune effector cell-associated neurotoxicity syndrome (ICANS) and cytokine release syndrome (CRS) (2–4). ICANS is a cytokine-mediated neurological syndrome that may present with a broad spectrum of manifestations, ranging from mild tremor, dysgraphia, and aphasia to seizures, akinetic mutism, and life-threatening cerebral edema (5–8). The incidence of ICANS is variable according to multiple factors, and in particular, it is higher in the case of infusion of CD28 co-stimulated
products. Descriptions of novel distinctive features are pivotal to untangling this condition's clinical and instrumental signature in order to inform diagnosis and pathophysiology. Most patients present with unremarkable brain MRI, yet a few recurrent neuroradiological features have been recognized (4, 9, 10). Herein, we present a case of CAR T-cell therapy-related neurotoxicity associated with reversible corpus callosum abnormalities, which expand the spectrum of inflammatory neuroradiological abnormalities associated with ICANS.
An otherwise healthy 27-year-old female patient was referred to our center for anti-CD19 CAR T-cell therapy (axicabtagene ciloleucel: axi-cell) for primary mediastinal B-cell lymphoma (PMBCL), stage IIXB at diagnosis for mediastinal bulky, refractory to two lines of standard chemotherapy (Figure 1). Before CAR T-cell infusion, she underwent a 30-Gray mediastinal irradiation as a bridge therapy. After 48 h from CAR T-cell infusion, the patient developed a fever with no associated hypotension or hypoxia (CRS grade 1). In accordance with our local guidelines, for persistent grade 1 CRS, she was treated with tocilizumab (8 mg/kg), resulting in complete resolution of systemic symptoms on day +6. Nevertheless, the following day, she developed mild ideo-motor slowing, dysgraphia, and drowsiness. The clinical picture was consistent with a grade 2 ICANS. A head CT scan was unrevealing, and treatment with dexamethasone (10 mg q6h) was started. EEG revealed frontal intermittent rhythmic delta activity (FIRDA), while cerebrospinal fluid (CSF) analysis showed normal cellular counts and negative comprehensive microbiological investigations, but very high protein levels (320 mg/dL). Despite prompt administration of steroid treatment, at day +9, her neurological status progressively worsened to a comatose state within 24 h (ICANS grade 4); therefore, she was transferred to the intensive care unit (ICU). Brain MRI revealed multiple focal punctate areas of T2-weighted fluid-attenuated inversion recovery (FLAIR) hyperintensity localized in the body and isthmus of the corpus callosum, with no contrast enhancement (Figures 2A, B). No restriction on diffusion-weighted imaging (DWI) or hypointensity on susceptibility-weighted imaging (SWI) was observed within the corpus callosum. Following high-dose intravenous methylprednisolone (1 g daily) administration, her neurological status resolved within 48 h. Neurological examination revealed retrograde amnesia regarding the days spent in ICU and mild dysgraphia that persisted until day +12. Remarkably, the follow-up brain MRI (day +13) did not reveal any abnormalities in the corpus callosum, except for a reduction of fractional anisotropy in the diffusion tensor imaging (DTI) sequences (Figures 2C, D). The patient was re-transferred to the hematology ward, and the corticosteroid therapy was gradually tapered. On day +16, steroid treatment was stopped entirely, and the patient was discharged home. She did not develop any late-onset neurotoxicity, and her lymphoma was still in complete remission at the last follow-up, more than 2 years after CAR T-cell therapy.
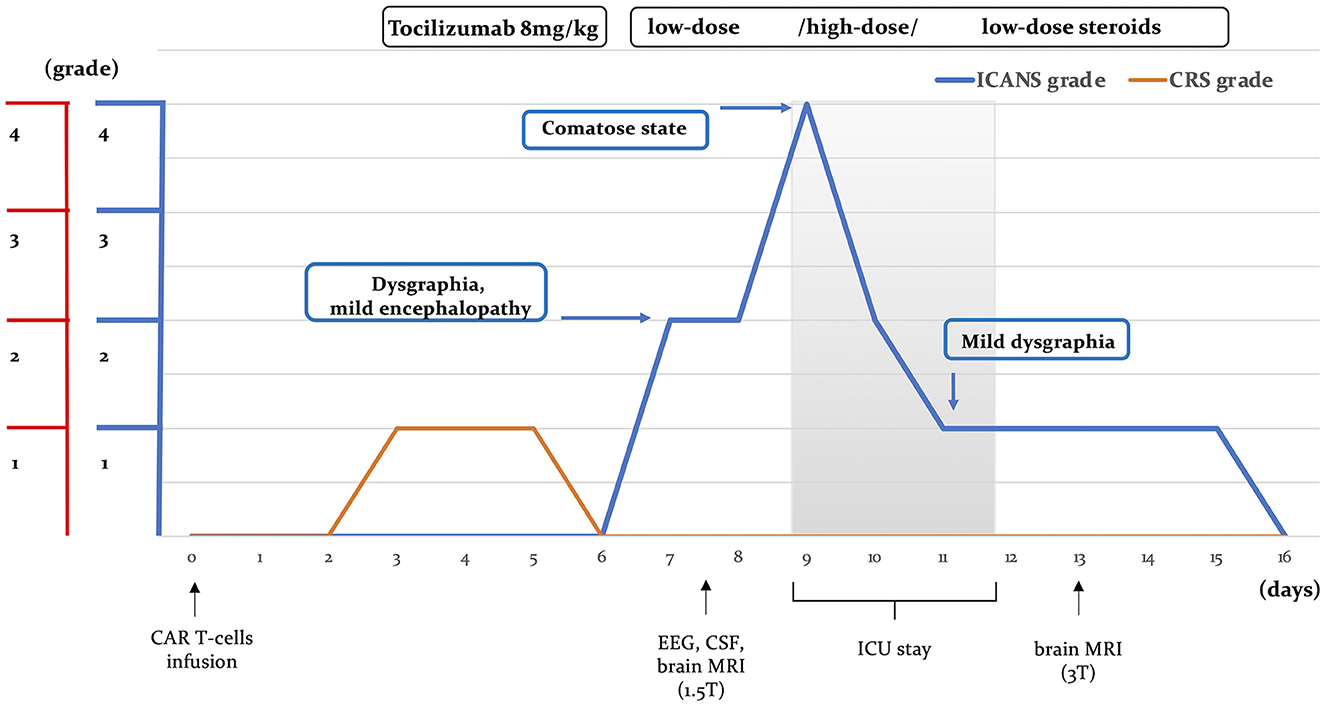
Figure 1. Disease course. Clinical manifestations of neurotoxicity, CRS, and ICANS severity, along with the timing of diagnostic investigations and treatment following CAR T-cell therapy, are illustrated. CRS, cytokine release syndrome; ICANS, immune effector-associated neurotoxicity syndrome; CSF, cerebrospinal fluid; ICU, intensive care unit.
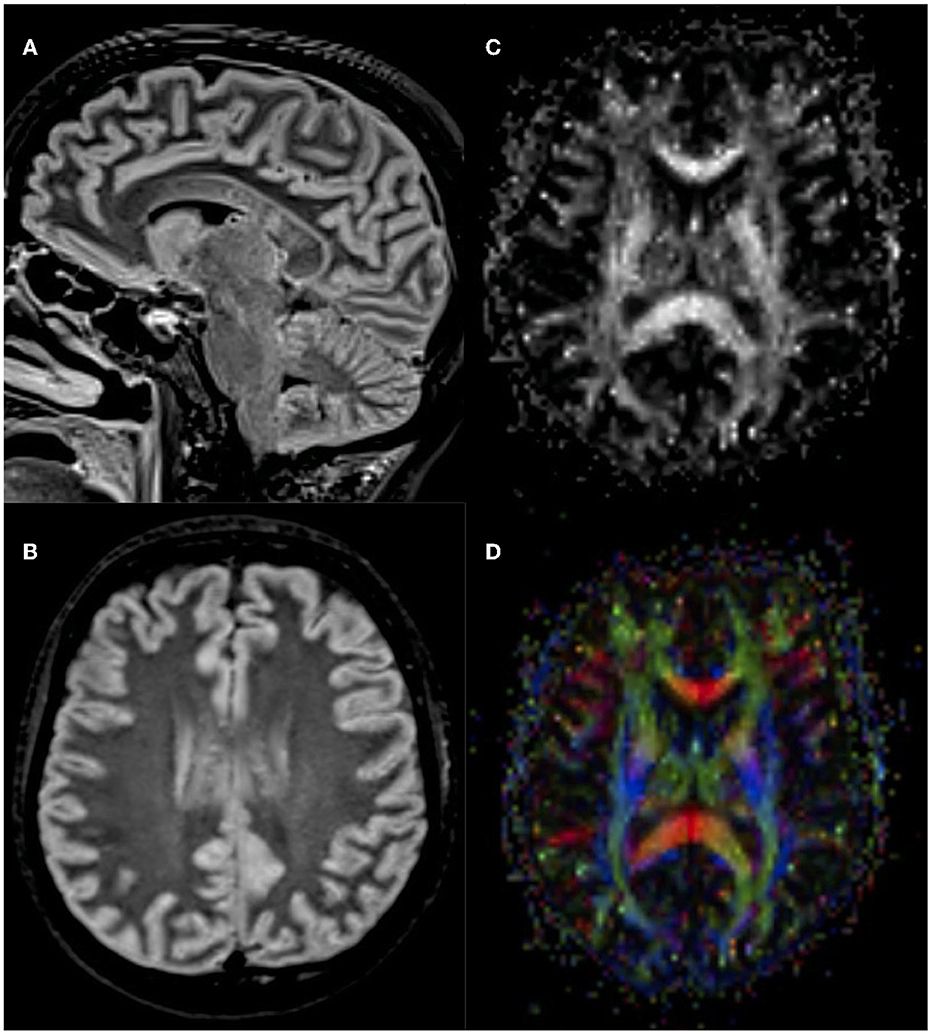
Figure 2. Brain MRI findings. Axial (A) and sagittal (B) 1.5 Tesla T2-weighted fluid-attenuated inversion recovery (FLAIR) showed multiple focal punctate areas of hyperintensities localized in the splenium and isthmus of the corpus callosum, with no contrast enhancement or diffusion restriction. Follow-up 3 Tesla brain MRI revealed no T2-weighted abnormalities (images not shown). Yet, diffusion tensor imaging (DTI) showed fractional anisotropy reduction within the corpus callosum's body and splenium (C, D).
We described the case of a young woman affected by refractory PMBCL who displayed undescribed transitory inflammatory abnormalities of the corpus callosum during neurotoxicity related to CAR T-cell therapy. Our patient developed neurological manifestations on the seventh day from axi-cel infusion, subsequently to CRS resolution, and progressed to severe neurotoxicity within 48 h. Accordingly, ICANS median onset time is 4–6 days after CAR T-cell infusion, depending on CRS timing, and a rapid progression within a few h/days has been reported as the typical evolution (7). She presented with dysgraphia, one of the most common early neurological presentations of ICANS, and showed complete resolution following steroid therapy, as often observed in previous cases (6, 7, 11, 12). In terms of instrumental findings, the mild inflammatory CSF findings and EEG frontal abnormalities detected in our patient have been recurrently described (7, 12–14), but her neuroradiological picture has never been described. Brain MRI is usually unremarkable in ICANS, but some neuroradiological abnormalities have been reported, including white matter changes, diffuse cerebral edema, leptomeningeal enhancement, lesions localized in the bilateral thalami, claustrum, and the splenium of the corpus callosum (7, 10, 12). Notably, several of these radiological abnormalities have been consistently observed in cytokine storm-associated encephalopathies beyond ICANS (4). Indeed, a single transitory lesion of the splenium of the corpus callosum is a radiological hallmark of febrile infection-associated encephalopathy disorders, whose underpinning pathophysiology is cytokine-mediated neuroinflammation (4, 15, 16). Nonetheless, the body of the corpus callosum is usually spared in these conditions. Conversely, acquiring acute inflammatory patchy lesions involving the body of the corpus callosum may be observed in other neuroinflammatory disorders, such as primary central nervous system vasculitis, Susac syndrome, and multiple sclerosis (17, 18). Interestingly, a case of Susac syndrome following HIV-associated immune reconstitution syndrome (IRIS) has been observed (19). IRIS is characterized by a paradoxical worsening of clinical conditions resulting from a re-activated immune system, usually following antiretroviral treatment in patients with HIV (19). Therefore, similarities in the underlying pathophysiological mechanisms and clinical findings between the neurological form of IRIS and ICANS may exist.
Notably, at the follow-up brain MRI, no T2-weighted hyperintensity was observable, yet DTI sequences detected microstructural abnormalities related to altered integrity of the white matte fibers of the corpus callosum (20). Collectively, the corpus callosum lesions' localization, morphology, and temporal evolution, along with the persistent microstructural alterations, support an underlying inflammatory nature. The blood–brain barrier disruption and inflammatory demyelination, related to the overarching cytokine-mediate neuroinflammation, are arguably responsible for a spectrum of corpus callosum abnormalities shared by ICANS and other neuroinflammatory conditions.
Reversible punctate inflammation of the body and isthmus of the corpus callosum may represent a novel radiological finding of CAR T-cell therapy-related neurotoxicity. Descriptions of novel distinctive investigative features are pivotal to defining a neuroradiological signature that would inform diagnosis and pathophysiology.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
UP and FP: drafting of the manuscript for content. FP, CP, DM, SM, LP, AS, and SB: revision of the manuscript for content, major role in the acquisition of data, and analysis or interpretation of data. UP, CP, and SB: study concept or design. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Singh AK, McGuirk JP. CAR T cells: continuation in a revolution of immunotherapy. Lancet Oncol. (2020) 21:e168–78. doi: 10.1016/S1470-2045(19)30823-X
2. Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. (2019) 25:625–38. doi: 10.1016/j.bbmt.2018.12.758
3. Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. (2018) 15:47–62. doi: 10.1038/nrclinonc.2017.148
4. Pensato U, Muccioli L, Cani I, Janigro D, Zinzani PL, Guarino M, et al. Brain dysfunction in COVID-19 and CAR-T therapy: cytokine storm-associated encephalopathy. Ann Clin Transl Neurol. (2021) 8:968–79. doi: 10.1002/acn3.51348
5. Gust J, Ponce R, Liles WC, Garden GA, Turtle CJ. Cytokines in CAR T cell-associated neurotoxicity. Front Immunol. (2020) 11:577027. doi: 10.3389/fimmu.2020.577027
6. Pensato U, Amore G, D'Angelo R, Rinaldi R, Nicodemo M, Rondelli F, et al. Frontal predominant encephalopathy with early paligraphia as a distinctive signature of CAR T-cell therapy-related neurotoxicity. J Neurol. (2022) 269:609–15. doi: 10.1007/s00415-021-10766-5
7. Rubin DB, Danish HH, Ali AB, Li K, LaRose S, Monk AD, et al. Neurological toxicities associated with chimeric antigen receptor T-cell therapy. Brain. (2019) 142:1334–48. doi: 10.1093/brain/awz053
8. Pensato U, Muccioli L, Zinzani P, D'Angelo R, Pierucci E, Casadei B, et al. Fulminant cerebral edema following CAR T-cell therapy: case report and pathophysiological insights from literature review. J Neurol. (2022) 269:4560–3. doi: 10.1007/s00415-022-11117-8
9. Lapidus AH, Anderson MA, Harrison SJ, Dickinson M, Kalincik T, Lasocki A. Neuroimaging findings in immune effector cell associated neurotoxicity syndrome after chimeric antigen receptor T-cell therapy. Leuk Lymphoma. (2022) 63:2364–74. doi: 10.1080/10428194.2022.2074990
10. Tan AP. CAR-T cell therapy-related neurotoxicity in pediatric acute lymphoblastic leukemia: spectrum of imaging findings. Pediatr Neurol. (2020) 111:51–8. doi: 10.1016/j.pediatrneurol.2020.06.014
11. Neelapu SS. Managing the toxicities of CAR T-cell therapy. Hematol Oncol. (2019) 37(Suppl. 1):48–52. doi: 10.1002/hon.2595
12. Santomasso BD, Park JH, Salloum D, Riviere I, Flynn J, Mead E, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. (2018) 8:958–71. doi: 10.1158/2159-8290.CD-17-1319
13. Gust J, Hay KA, Hanafi L-A, Li D, Myerson D, Gonzalez-Cuyar LF, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. (2017) 7:1404–19. doi: 10.1158/2159-8290.CD-17-0698
14. Beuchat I, Danish HH, Rubin DB, Jacobson C, Robertson M, Vaitkevicius H, et al. EEG FINDINGS in CAR T-cell-associated neurotoxicity: clinical and radiological correlations. Neuro Oncol. (2022) 24:313–25. doi: 10.1093/neuonc/noab174
15. Lin YY, Lee KY, Ro LS, Lo YS, Huang CC, Chang KH. Clinical and cytokine profile of adult acute necrotizing encephalopathy. Biomed J. (2019) 42:178–86. doi: 10.1016/j.bj.2019.01.008
16. Yuan J, Yang S, Wang S, Qin W, Yang L, Hu W. Mild encephalitis/encephalopathy with reversible splenial lesion (MERS) in adults-a case report and literature review. BMC Neurol. (2017) 17:103. doi: 10.1186/s12883-017-0875-5
17. David C, Sacré K, Henri-Feugeas MC, Klein I, Doan S, Cohen FA, Jouvent E, Papo T. Susac syndrome: a scoping review. Autoimmun Rev. (2022) 21:103097. doi: 10.1016/j.autrev.2022.103097
18. Uchino A, Takase Y, Nomiyama K, Egashira R, Kudo S. Acquired lesions of the corpus callosum: MR imaging. Eur Radiol. (2006) 16:905–14. doi: 10.1007/s00330-005-0037-9
19. Ferretti F, Gerevini S, Colombo B, Testa M, Guffanti M, Franciotta D, et al. Susac's syndrome as HIV-associated immune reconstitution inflammatory syndrome. AIDS Res Ther. (2013) 10:22. doi: 10.1186/1742-6405-10-22
Keywords: cancer immunotherapy, neuroimaging, brain MRI, cytokine storm-associated encephalopathy (CySE), neuroinflammation, B-cell lymphoma, neuroradiology, hematological cancer
Citation: Pensato U, de Philippis C, Pistolese F, Mannina D, Marcheselli S, Politi LS, Santoro A and Bramanti S (2023) Case report: Reversible punctate inflammatory foci in the corpus callosum: A novel radiological finding of CAR T-cell therapy-related neurotoxicity. Front. Neurol. 14:1125121. doi: 10.3389/fneur.2023.1125121
Received: 15 December 2022; Accepted: 13 January 2023;
Published: 07 February 2023.
Edited by:
Stella Bouziana, King's College Hospital NHS Foundation Trust, United KingdomReviewed by:
Jasmina Boban, University of Novi Sad, SerbiaCopyright © 2023 Pensato, de Philippis, Pistolese, Mannina, Marcheselli, Politi, Santoro and Bramanti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chiara de Philippis,  Y2hpYXJhLmRlX3BoaWxpcHBpc0BodW1hbml0YXMuaXQ=
Y2hpYXJhLmRlX3BoaWxpcHBpc0BodW1hbml0YXMuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.