
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 06 February 2023
Sec. Neuro-Otology
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1124217
This article is part of the Research Topic Stroke and Balance Disorders View all 11 articles
Background: Isolated (hemi)nodular strokes as underlying cause of acute dizziness are rare, thus there are still gaps of knowledge in the clinical presentation of affected patients. Clinical and experimental evidence has suggested that lesions involving the nodulus lead to various vestibulo-ocular deficits including prolonged velocity-storage, periodic-alternating nystagmus, positional nystagmus, abolished suppression of post-rotatory nystagmus by head-tilt and impaired verticality perception. At the bedside, the angular vestibulo-ocular reflex (aVOR), as assessed by the horizontal head-impulse test (HIT), has been reported to be normal, however quantitative assessments of all six semicircular canals are lacking.
Objective: The primary aim of this case series was to characterize the spectrum of clinical presentations in isolated (hemi)nodular strokes. Furthermore, based on preliminary observations, we hypothesized that the aVOR is within normal limits in isolated nodular strokes.
Methods: We retrospectively included patients with isolated (hemi)nodular stroke on diffusion-weighted MR-imaging from a prospective stroke-registry. All patients received a standardized bedside neuro-otological assessment and quantitative, video-based HIT (vHIT) of all six semicircular canals. Overall ratings of vHIT (normal vs. abnormal function) were performed independently by two reviewers and disagreements were resolved.
Results: Between January 2015 and December 2021 six patients with isolated nodular (n = 1) or heminodular (n = 5) ischemic stroke were included. Clinical presentation met diagnostic criteria for acute vestibular syndrome (AVS) in 5/6 patients and for episodic vestibular syndrome (EVS) in 1/6 patients. Ocular motor abnormalities observed included the presence of spontaneous horizontal nystagmus (n = 2), positional nystagmus (5/6), head-shaking nystagmus (3/6), skew deviation (n = 1), and moderate or severe truncal ataxia (5/6). Bedside HIT was normal in all patients and no gaze-evoked or periodic alternating nystagmus was observed. aVOR-gains were within normal range in all patients and overall aVOR-function as assessed by vHIT was rated as normal in all six patients.
Conclusions: Using quantitative, video-based testing of the horizontal and vertical aVOR, preserved integrity of the aVOR in (hemi)nodular strokes was confirmed, extending preliminary findings at the bedside. Furthermore, widespread deficits of both ocular stability, postural control and volitional eye movements were observed in our study cohort, being consistent with findings reported in previous studies.
The nodulus, i.e., the most caudal part of the cerebellar vermis (lobule X) is essential in the processing of vestibular information (1). Together with the flocculus, the paraflocculus and the ventral uvula it constitutes the vestibulocerebellum (2). Clinical and experimental evidences have suggested that lesions involving the nodulus lead to various vestibulo-ocular deficits. Specifically, the nodulus and the uvula control the time constant of the velocity storage, particularly in interaction with gravity signals (3), reflected by a prolonged velocity storage (4) and an inability to suppress post-rotatory nystagmus by head-tilt (5–7). Other clinical findings in isolated nodular lesions include periodic alternating nystagmus (PAN) (6, 7), positional downbeat nystagmus in association with a disturbance in the integration of otolith signals and perverted head-shaking nystagmus (HSN) (7). Also the presence of direction-changing positional apogeotropic horizontal nystagmus (8–10), and a contraversive ocular tilt reaction (2, 7) have been linked to nodular strokes. Besides vestibulo-ocular motor deficits also vestibulo-perceptual impairments may be observed. Specifically, an abolished earth verticality perception has been demonstrated in a single patient with acute heminodular stroke (10).
Isolated nodular infarction, however, is very rare and acute lesions involving the nodulus with or without associated cerebellar structures supported by the medial posterior inferior cerebellar artery (mPICA) (11) may cause an acute vestibular syndrome (AVS), mimicking acute peripheral vestibulopathy (so-called pseudo-vestibular neuritis) (12, 13). Misdiagnosis of AVS may lead to a disastrous result, and many efforts have been made to increase the bedside diagnostic accuracy in distinguishing peripheral from central AVS cases, including the application of the “HINTS plus: Head-Impulse, Nystagmus, Test-of-Skew and new-onset hearing loss” (14, 15) and other exams (16). Of note, head impulse testing (HIT) has been a key examination to differentiate a dangerous stroke from a benign, usually self-limited condition such as an acute peripheral vestibulopathy. In isolated nodular infarction, normal HIT is predicted based on former studies and neuro-anatomical knowledge (7, 13, 17). Likewise, in PICA territory strokes the bedside HIT is usually preserved (2, 17). Mild angular vestibulo-ocular reflex (aVOR)-gain reductions with small corrective saccades (which may go undetected by the naked eye alone) have been reported in a single study on PICA stroke patients using magnetic search coils for quantifying the head-impulse test (18). While in some of these patients the nodulus was affected as well, no isolated nodular stroke cases were included. Noteworthy, reduced horizontal head-impulse gains on the contralesional side have been reported in two patients with ischemic strokes involving the right flocculus (which belongs also to the vestibulo-cerebellum) (19, 20).
With regards to isolated nodular infarctions, two cases series have reported on head impulse testing (7, 13). Specifically, bedside HIT was rated as normal in all patients included, however, these studies did not assess the aVOR quantitatively by using either video head-impulse testing (vHIT) or search-coil techniques and also did not test the vertical canals. Thus, in order to address this area of uncertainty (17), we analyzed the aVOR and associated ocular motor findings in a series of patients with isolated nodular infarction using a vHIT-device. We predicted preserved aVOR of both horizontal and vertical semicircular canals when quantitatively assessed in patients with isolated (hemi)nodular stroke.
This study was carried out in accordance with the recommendations of the Institutional Review Board of the Chonnam National University Hospital (Gwangju, South Korea) with written informed consent from all subjects in accordance with the Declaration of Helsinki. The protocol was approved by the Institutional Review Board of the Chonnam National University Hospital (Gwangju, South Korea). The research project was conducted in accordance with university policies, the Personal Information Protection Act of Korea, the Declaration of Helsinki (except for registration in a database), the principles of Good Clinical Practice, the Human Research Act (HRA) and the Human Research Ordinance (HRO). Data will be made available on request from the authors.
We searched the prospective stroke registry of the Chonnam National University Hospital, Gwangju, South Korea, for patients presenting to the emergency department or the outpatient clinic that received a diagnosis of isolated nodular infarction on magnetic resonance imaging (MRI) including diffusion-weighted imaging (DWI) (see Figure 1). Between January 2015 and December 2021, we retrospectively identified 8 consecutive patients who received quantitative vestibular testing. Two patients were excluded. Reasons for exclusion were pre-existing ophthalmoplegia (right abducens nerve palsy) affecting the results of vHIT (n = 1) and previous strokes in the inferior-medial cerebellum and the pons (potentially affecting the ocular motor findings) (n = 1). Eventually, six patients with isolated nodular infarction with compatible radiologic findings were enrolled for this study. None of these patients have been previously published.
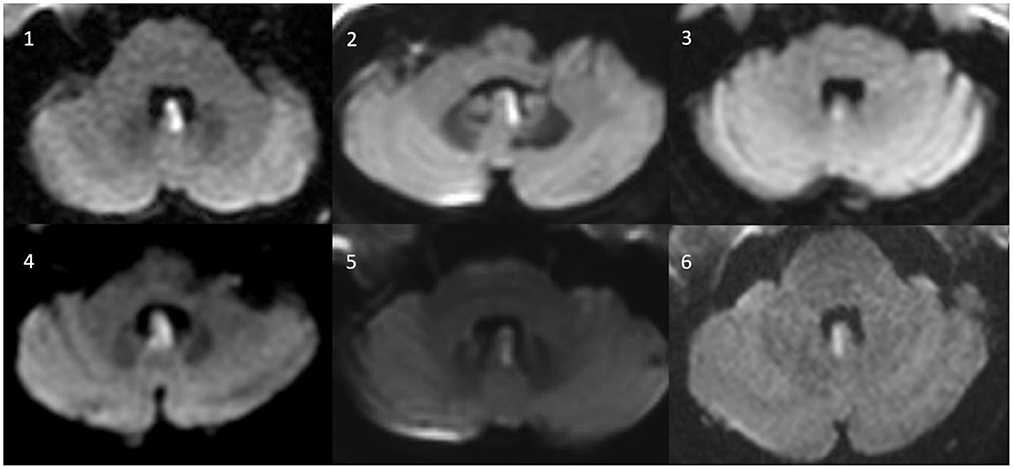
Figure 1. Axial MR-images (diffusion weighted images) of all six patients with isolated (hemi)nodular infarction.
A structured bedside neuro-otologic examination was obtained in all patients. Video-oculography (VOG; SLMed, Seoul, South Korea, recording frequency = 60 Hz) was performed in a sitting position with fixation removed for the detection of spontaneous (horizontal, vertical, or torsional) nystagmus, positional nystagmus, head-shaking nystagmus and periodic alternating nystagmus for at least 2 min in each patient. For testing of pursuit eye movements, saccades and eccentric gaze holding [looking for gaze-evoked nystagmus (GEN)] visual targets were used. All subjects received a detailed neurologic examination and vHIT after a median of 5 days (range: 1–20 days) after symptom onset.
Imbalance was graded from 0 to III as follows (7): Grade 0 (normal), able to stand on tandem Romberg with the eyes open for 3 s; grade I (mild), unable to stand on tandem Romberg with the eyes open at least for 3 s; grade II (moderate), unable to stand on Romberg with the eyes open at least for 3 s; grade III (severe), unable to stand or sit without support.
Details on vHIT have been described in detail before (21). In brief, vHIT of both the horizontal and vertical canals was obtained using a lightweight, portable VOG device (ICS Impulse; Otometrics, Taastrup, Denmark) (22). We aimed for head velocities between 150 and 200°/s and head displacements of 10–20°. For each canal, 20 valid head-impulses were required.
Both clinical information (presenting symptoms, symptom duration, findings from bedside examination) and results from quantitative testing were retrieved. Gains of the vHIT recordings were analyzed using OtosuiteV 4.0 (Otometrics). This software visualizes all compensatory saccades to ensure accurate characterization. Overt saccades were defined as saccades that occurred in the opposite direction of the head rotation and that reached peak acceleration after the head had stopped moving. Covert saccades, on the other hand, reached peak acceleration before the head had stopped moving (23). Noteworthy, a distinction from early (covert) catch-up saccades (CS) is usually readily possible. Therefore, visual inspection as done for all traces as part of the overall rating of vHIT will ensure that inappropriate traces may still be removed. All vHIT traces were independently reviewed by two experienced neuro-otologists (SHL and AAT). Reviewers were blinded to the clinical findings and the results from MR imaging.
For this study, we relied on the standard aVOR gain calculations from the Otometrics vHIT goggles (OtosuiteV 4.0). This algorithm calculates gain as the ratio of the area under the desaccaded eye-velocity curve (AUC) to the area under the head-velocity curve, corresponding to a desaccaded position gain (22). Thus, the gain of the aVOR was calculated as the ratio of the cumulative slow-phase eye velocity over the cumulative head velocity from the onset of the head impulse to the moment when head velocity crossed zero again (22). We used the cutoff values in aVOR gains as proposed by the manufacturer of the video-goggles (Otometrics), i.e., 0.8 for the horizontal canals and 0.7 for the vertical canals. These values were also in agreement with normative values for a wide range of ages reported (24). The video-head impulse traces were evaluated by the reviewers for reduced aVOR-gain, increased corrective saccades (overt or covert), or a combination of both (25) and an overall rating was provided (“normal” or “abnormal”). Disagreements between the two raters were resolved by discussion and–if needed–by a judgment call of a third rater.
A total of six patients (4 women, mean age=59.3 ± 9) with isolated nodular infarction on DWI were consecutively enrolled for this study. Five out of six patients (5/6, 83.3%) met the diagnostic criteria for acute vestibular syndrome (AVS) as proposed by the classification committee of the Bárány Society (acute onset of vertigo or dizziness with nausea or vomiting, head-motion intolerance, and unsteadiness) (26), and the remaining sixth patient had an triggered episodic vestibular syndrome (tEVS) (see Table 1 for details). All six patients had normal bedside HIT and did not show GEN. One patient (case 2) showed a skew deviation (with the ipsilesional left eye demonstrating hypertropia). Applying the HINTS battery to those five patients presenting with an AVS, they were found to be central in all subjects. All five patients with AVS had truncal ataxia; three patients had grade II imbalance and two were rated as grade III.
Two out of six patients presented with horizontal spontaneous nystagmus (SN) that was beating to the ipsilesional side. Horizontal head-shaking triggered a nystagmus in two patients (horizontally beating to the ipsilesional side in one patient and right-beating/down-beating in the other patient), and augmented a horizontal SN (with beating direction being unchanged) in a third patient. Positional testing was performed in a sequential manner (pitch-plane testing, straight head-hanging (SHH), Dix-Hallpike maneuvers, and supine roll tests). In one patient, persistent positional downbeat nystagmus (pDBN) with a horizontal component as well was evoked by SHH. In three patients (3/6, 50%), direction-changing positional nystagmus (DCPN) with an apogeotropic pattern was observed during supine roll testing (see Figure 2 for an illustrative case). Ocular motor testing for saccades and pursuit eye movements was performed in five patients. Saccades were rated as normal in all tested subjects, but pursuit was abnormal because of either decreased pursuit gain or interleaved saccadic eye movements.
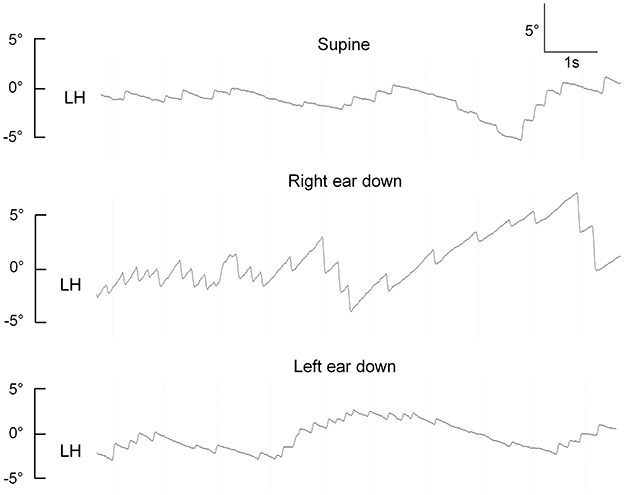
Figure 2. Apogeotropic positional nystagmus in a patient with an isolated left-sided heminodular infarction (case 1), demonstrating horizontal eye position from the left eye (LH). Upward excursions reflect eye movements toward the right side, downward excursions are consistent with eye movements to the left.
vHIT showed normal aVOR-gains and no catch-up saccades except for one patient (case 2). In case 2, there were mildly decreased gains in both horizontal canals (right 0.66, left 0.67; Table 2). However, catch-up saccades were considered as not clinically relevant and the overall patterns of the aVOR-response was rated as normal by both reviewers. Illustrative cases (case 2 and case 6) are shown in Figure 3.
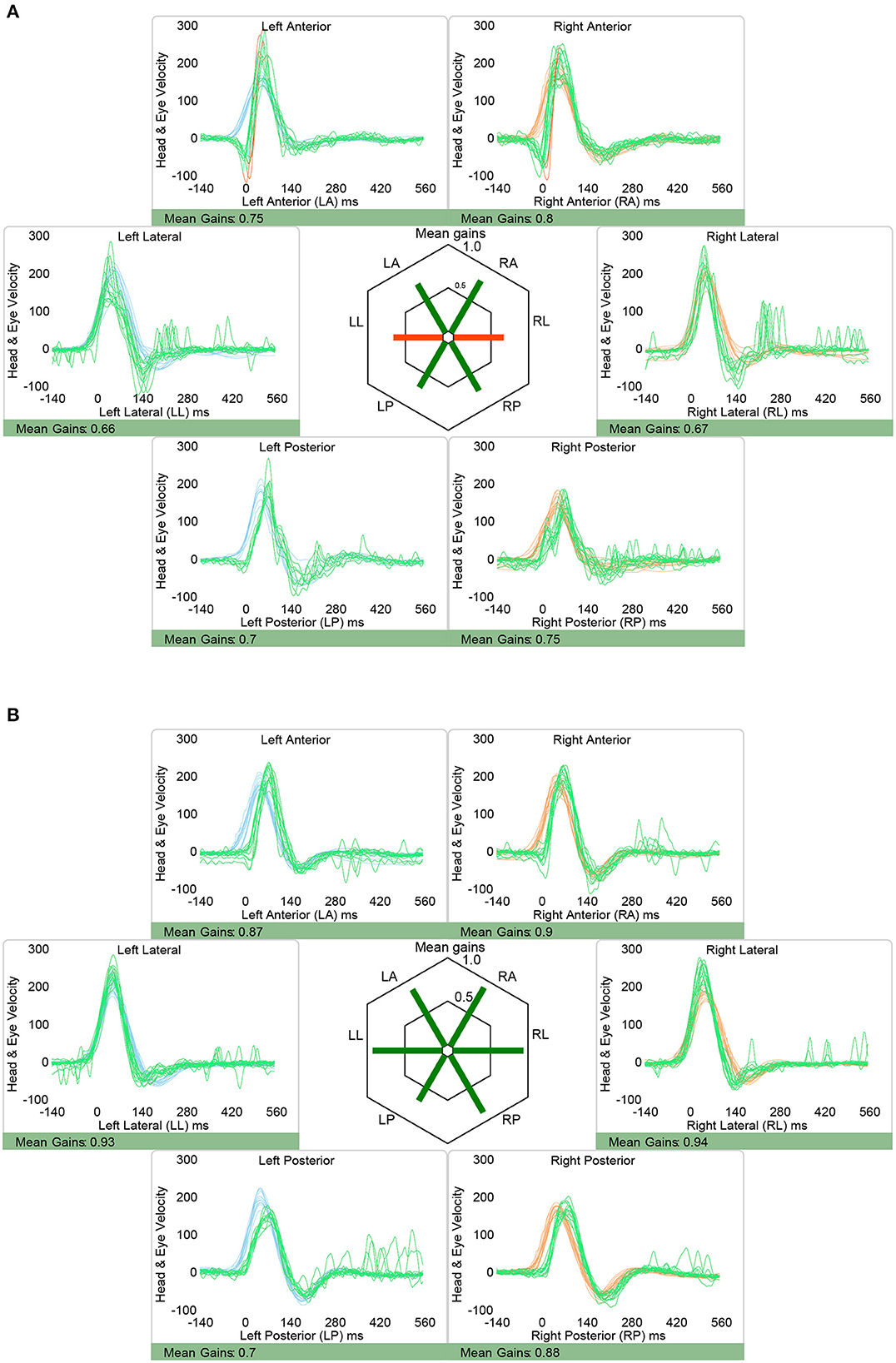
Figure 3. Representative video head impulse testing from two patients with isolated nodular infarction, demonstrating slightly reduced aVOR-response in the horizontal canals in one patient [(A), case 2] and preserved aVOR-response in all six semicircular canals in the other illustrative patient [(B), case 6]. Note that in both patients vHIT responses were judged as overall normal, however, it is acknowledged that the saccades observed when testing the right horizontal canal in case 2 may be considered clinically relevant also, reflecting minor aVOR impairment for this canal. Eye velocity traces (in green) and head velocity traces (in red for testing the right vestibular organ and in blue for assessing the left vestibular organ) are plotted against time. Note that eye velocity traces were inverted for better visualization and comparison with the head velocity traces and that gain was calculated as the ratio of the area under the desaccaded eye-velocity curve to the area under the head-velocity curve, corresponding to a desaccaded position gain. Summary plots in the center illustrate average individual vestibulo-ocular reflex (VOR)-gains ± 1SD for all six canals.
Isolated (hemi)nodular strokes as underlying cause of acute dizziness are rare, thus there are still gaps of knowledge in the clinical presentation of affected patients. This includes the integrity of the horizontal and vertical angular vestibulo-ocular reflex (aVOR), with previous publications being restricted to bedside testing of the horizontal aVOR. Thus, the primary aim of this case series was to characterize the spectrum of clinical presentations in isolated (hemi)nodular strokes. In summary, we demonstrated deficits in both postural control, volitional eye movements (pursuit, saccades), ocular stability holding in primary position and during positional testing and after head-shaking. Detailed and quantitative, video-based testing of the horizontal and vertical aVOR confirmed preliminary findings at the bedside, emphasizing a preserved integrity of the aVOR in (hemi)nodular strokes. Likewise, eccentric gaze holding remained intact in these patients. In the following, we will discuss the main findings in more detail and review the literature.
The PICA supplies the lateral medulla, the inferior cerebellar peduncle, and the cerebellar nodulus and uvula (11). Infarction in the distribution of the distal PICA may cause acute vertigo and nystagmus that mimics an acute peripheral vestibular lesion. These symptoms and signs are probably due to a central vestibular imbalance created by asymmetric infarction in the vestibulo-cerebellum, which normally has a tonic inhibitory effect on the vestibular nuclei (2).
As suspected, acute infarction restricted to the cerebellar nodulus mostly resulted in a clinical presentation meeting criteria for an acute vestibular syndrome (AVS) (26). Since other obvious focal neurologic signs were not observed (excluding truncal ataxia with imbalance and subtle ocular motor abnormalities), all our six patients presented with an isolated vestibular syndrome (acute or episodic), making the differential diagnosis from vestibular neuritis, an AVS with benign course due to peripheral origin, even more challenging. Applying the HINTS battery, however, a three-step bedside diagnostic algorithm to differentiate dangerous central AVS (pseudo-VN) from benign peripheral AVS [e.g., vestibular neuritis (VN)] (14), it pointed to a central cause of AVS (normal bedside HIT in 5/5 and skew deviation in 1/5) in all five patients with AVS in our case series. Also, there was an imbalance of at least moderate severity, which suggested more frequently a central pathology than a peripheral one (27, 28).
Of interest, one patient presented with a triggered episodic vestibular syndrome (tEVS), with vertigo being evoked by positional changes, resembling rather benign paroxysmal positional vertigo (BPPV) than vestibular neuritis. This patient (case 6) showed apogeotropic positional nystagmus mimicking horizontal canal BPPV with cupulolithiasis or short-arm canalolithiasis (29, 30). However, the patient did not improve after repetitive canalith repositioning maneuvers and she had abnormal pursuit eye movements. Both findings suggested a central pathology rather than BPPV having a benign course. Noteworthy, apogeotropic horizontal positional nystagmus in supine-roll testing is much more likely to be of central origin than classic upbeat-torsional nystagmus seen in Dix-Hallpike maneuver (31).
Using quantitative, video-based head-impulse testing of both the horizontal and vertical semicircular canals, we confirmed and extended findings previously reported at the bedside (7), emphasizing a preserved aVOR in isolated (hemi)nodular stroke. This was reflected by both normal vHIT gains and absence of clinically relevant compensatory overt / covert catch-up saccades.
Noteworthy, the nodulus/uvula may also enhance aVOR-gains during HIT (17). All nodulus-target neurons are tuned to vestibular stimuli, and most are insensitive to eye movements. Such non-eye-movement neurons are thought to project to vestibulo-spinal and/or thalamo-cortical pathways. Less than 20% of the nodulus/uvular target neurons respond to both vestibular and eye movement signals, suggesting that the nodulus/uvula can also directly influence vestibulo-ocular pathways (32).
The nodulus and the ventral uvula govern the velocity-storage mechanism of the aVOR to optimize its properties for low-frequency (sustained) rotations (4, 8, 33). In contrast, the HIT reflects a high-frequency stimulus, assessing the aVOR-response in a different frequency range. Therefore, according to our results, (hemi)nodular lesions seem to have little effect on the aVOR as assessed by the bedside HIT / vHIT.
The nodulus and the ventral uvula are also important for the generation of normal vestibulo-ocular responses to linear movements (translations), probably acting as the integrator of otolithic inputs (34). Clinical lesions involving the nodulus and uvula may also cause downbeat nystagmus (especially during positional testing), horizontal central positional nystagmus, and produce variants of skew deviation and abnormal ocular counterroll, which implies a central otolithic imbalance. We observed an apogeotropic central positional nystagmus (CPN) in 3/6 patients, being consistent with previous reports on positional nystagmus in patients with isolated nodular stroke (29). Apogeotropic CPN can result from the summation of incorrectly interpreted canal-induced nystagmus and gravity-induced nystagmus in both ear-down positions. A lesion disrupts the pathway providing the estimated direction of gravity to the rotational feedback loop and also produces a positive bias toward the nose along with a naso-occipital axis, the compensatory rotational feedback would generate a constant horizontal, apogeotropic gravity-induced nystagmus (29). Noteworthy, with an apogeotropic CPN being observed in 2/5 patients with heminodular stroke in our case series, this confirms previous observations, that partial loss of the nodulus is sufficient to cause apogeotropic CPN (7, 10).
Various other ocular motor and vestibular signs were observed in our case series, including ispilesional beating HSN (3/6 patients), misdirected HSN (cross-coupled HSN) with a DBN component (1/6 patients), positional DBN during SHH (1/6 patients), and contraversive skew deviation (1/6 patients). Impaired pursuit eye movements (decreased gains and/or saccadic pursuit) were observed in all patients.
But several vestibulo-oculomotor signs such as PAN were not observed as expected in our case series. Ischemic stroke lesions may be different from targeted, iatrogenic experimental lesions in monkeys. Furthermore, in our case series 5/6 patients had heminodular strokes only and a single patient demonstrated a bilateral ischemic stroke of the cerebellar nodulus. Previously, PAN has been reported only in patients with bilateral isolated nodular infarction (6, 7), whereas it was not seen in patients with heminodular stroke (7, 10).
In one patient (#2) gathered saccades could be depicted about 100 ms after the head-impulse was applied for testing of the right horizontal canal. While both the built-in algorithm (OtoSuite V4, Otometrics) and the two reviewers judged these saccades as not clinically relevant, their clinical significance remains debated especially in combination with a slightly reduced aVOR gain. Thus, a mildly impaired aVOR-response for this canal cannot be fully excluded, however, would be on the side opposite to the heminodular stroke in this patient. Therefore, we consider it unlikely, that these mild changes reflect a true aVOR impairment.
Providing both standardized bedside and quantitative ocular motor and vestibular testing in patients with isolated (hemi)nodular stroke, we confirmed and extended previously reported deficits in this rare condition. Specifically, at the bedside impairments in both postural control, pursuit eye movements, ocular stability (in primary position and during positional testing) and after head-shaking were identified. Detailed and quantitative, video-based testing of the horizontal and vertical aVOR confirmed preliminary findings at the bedside, emphasizing preserved integrity of the aVOR in (hemi)nodular strokes. Noteworthy, heminodular lesions were found to be sufficient to trigger positional nystagmus. When presenting as triggered EVS (as in 1 out of 6 patients in our cohort), distinction from benign paroxysmal positional vertigo may be challenging.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board of the Chonnam National University Hospital (Gwangju, South Korea). The patients/participants provided their written informed consent to participate in this study.
S-HL: conception of the work, data collection, data analysis, interpretation of data for the work, drafting the manuscript, and revising the work critically for important intellectual content. J-MK: interpretation of data for the work, drafting the work, and revising it critically for important intellectual content. J-TK: data collection, interpretation of data for the work, and revising the work critically for important intellectual content. AT: conception of the work, data analysis, interpretation of data for the work, drafting the work, and revising it critically for important intellectual content. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated, resolved, and confirm that all persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
S-HL was supported by grants (BCRE18184 and 2022-00317) from the Chonnam National University Hospital Research Institute of Clinical Medicine, Korea.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Laurens J. The otolith vermis: a systems neuroscience theory of the Nodulus and Uvula. Front Syst Neurosci. (2022) 16:886284. doi: 10.3389/fnsys.2022.886284
3. Angelaki DE, Hess BJ. Inertial representation of angular motion in the vestibular system of rhesus monkeys. II Otolith-controlled transformation that depends on an intact cerebellar nodulus. J Neurophysiol. (1995) 73:1729–51. doi: 10.1152/jn.1995.73.5.1729
4. Waespe W, Cohen B, Raphan T. Dynamic modification of the vestibulo-ocular reflex by the nodulus and uvula. Science. (1985) 228:199–202. doi: 10.1126/science.3871968
5. Wiest G, Deecke L, Trattnig S, Mueller C. Abolished tilt suppression of the vestibulo-ocular reflex caused by a selective uvulo-nodular lesion. Neurology. (1999) 52:417–9. doi: 10.1212/WNL.52.2.417
6. Jeong HS, Oh JY, Kim JS, Kim J, Lee AY, Oh SY. Periodic alternating nystagmus in isolated nodular infarction. Neurology. (2007) 68:956–7. doi: 10.1212/01.wnl.0000257111.24769.d2
7. Moon IS, Kim JS, Choi KD, Kim MJ, Oh SY, Lee H, et al. Isolated nodular infarction. Stroke. (2009) 40:487–91. doi: 10.1161/STROKEAHA.108.527762
8. Sheliga BM, Yakushin SB, Silvers A, Raphan T, Cohen B. Control of spatial orientation of the angular vestibulo-ocular reflex by the nodulus and uvula of the vestibulocerebellum. Ann N Y Acad Sci. (1999) 871:94–122. doi: 10.1111/j.1749-6632.1999.tb09178.x
9. Cohen B, John P, Yakushin SB, Buettner-Ennever J, Raphan T. The nodulus and uvula: source of cerebellar control of spatial orientation of the angular vestibulo-ocular reflex. Ann N Y Acad Sci. (2002) 978:28–45. doi: 10.1111/j.1749-6632.2002.tb07553.x
10. Tarnutzer AA, Wichmann W, Straumann D, Bockisch CJ. The cerebellar nodulus: perceptual and ocular processing of graviceptive input. Ann Neurol. (2015) 77:343–7. doi: 10.1002/ana.24329
11. Amarenco P, Roullet E, Hommel M, Chaine P, Marteau R. Infarction in the territory of the medial branch of the posterior inferior cerebellar artery. J Neurol Neurosurg Psychiatry. (1990) 53:731–5. doi: 10.1136/jnnp.53.9.731
12. Lee H, Cho YW. A case of isolated nodulus infarction presenting as a vestibular neuritis. J Neurol Sci. (2004) 221:117–9. doi: 10.1016/j.jns.2004.03.022
13. Lee H, Sohn SI, Cho YW, Lee SR, Ahn BH, Park BR, et al. Cerebellar infarction presenting isolated vertigo: frequency and vascular topographical patterns. Neurology. (2006) 67:1178–83. doi: 10.1212/01.wnl.0000238500.02302.b4
14. Kattah JC, Talkad AV, Wang DZ, Hsieh YH, Newman-Toker DE. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. (2009) 40:3504–10. doi: 10.1161/STROKEAHA.109.551234
15. Newman-Toker DE, Kerber KA, Hsieh YH, Pula JH, Omron R, Saber Tehrani AS, et al. HINTS outperforms ABCD2 to screen for stroke in acute continuous vertigo and dizziness. Acad Emerg Med. (2013) 20:986–96. doi: 10.1111/acem.12223
16. Fracica E, Hale D, Gold DR. Diagnosing and localizing the acute vestibular syndrome - Beyond the HINTS exam. J Neurol Sci. (2022) 442:120451. doi: 10.1016/j.jns.2022.120451
17. Choi JY, Kim HJ, Kim JS. Recent advances in head impulse test findings in central vestibular disorders. Neurology. (2018) 90:602–12. doi: 10.1212/WNL.0000000000005206
18. Chen L, Todd M, Halmagyi GM, Aw S. Head impulse gain and saccade analysis in pontine-cerebellar stroke and vestibular neuritis. Neurology. (2014) 83:1513–22. doi: 10.1212/WNL.0000000000000906
19. Park HK, Kim JS, Strupp M, Zee DS. Isolated floccular infarction: impaired vestibular responses to horizontal head impulse. J Neurol. (2013) 260:1576–82. doi: 10.1007/s00415-013-6837-y
20. Yacovino DA, Akly MP, Luis L, Zee DS. The floccular syndrome: dynamic changes in eye movements and vestibulo-ocular reflex in isolated infarction of the cerebellar flocculus. Cerebellum. (2018) 17:122–31. doi: 10.1007/s12311-017-0878-1
21. Lee SH, Kim SH, Kim JM, Tarnutzer AA. Vestibular dysfunction in Wernicke's encephalopathy: predominant impairment of the horizontal semicircular canals. Front Neurol. (2018) 9:141. doi: 10.3389/fneur.2018.00141
22. Macdougall HG, Mcgarvie LA, Halmagyi GM, Curthoys IS, Weber KP. The video Head Impulse Test (vHIT) detects vertical semicircular canal dysfunction. PLoS ONE. (2013) 8:e61488. doi: 10.1371/journal.pone.0061488
23. Lee SH, Newman-Toker DE, Zee DS, Schubert MC. Compensatory saccade differences between outward versus inward head impulses in chronic unilateral vestibular hypofunction. J Clin Neurosci. (2014) 21:1744–9. doi: 10.1016/j.jocn.2014.01.024
24. Mcgarvie LA, Macdougall HG, Halmagyi GM, Burgess AM, Weber KP, Curthoys IS. The Video Head Impulse Test (vHIT) of semicircular canal function - age-dependent normative values of VOR gain in healthy subjects. Front Neurol. (2015) 6:154. doi: 10.3389/fneur.2015.00154
25. Tarnutzer AA, Bockisch CJ, Buffone E, Weiler S, Bachmann LM, Weber KP. Disease-specific sparing of the anterior semicircular canals in bilateral vestibulopathy. Clin Neurophysiol. (2016) 127:2791–801. doi: 10.1016/j.clinph.2016.05.005
26. Kim JS, Newman-Toker DE, Kerber KA, Jahn K, Bertholon P, Waterston J, et al. Vascular vertigo and dizziness: diagnostic criteria. J Vestib Res. (2022) 32:205–22. doi: 10.3233/VES-210169
27. Carmona S, Martínez C, Zalazar G, Moro M, Batuecas-Caletrio A, Luis L, et al. The Diagnostic accuracy of truncal ataxia and HINTS as cardinal signs for acute vestibular syndrome. Front Neurol. (2016) 7:125. doi: 10.3389/fneur.2016.00125
28. Kattah JC, Martinez C, Zalazar G, Batuecas A, Lemos J, Carmona S. Role of incubitus truncal ataxia, and equivalent standing grade 3 ataxia in the diagnosis of central acute vestibular syndrome. J Neurol Sci. (2022) 441:120374. doi: 10.1016/j.jns.2022.120374
29. Choi JY, Glasauer S, Kim JH, Zee DS, Kim JS. Characteristics and mechanism of apogeotropic central positional nystagmus. Brain. (2018) 141:762–75. doi: 10.1093/brain/awx381
30. Kim JM, Lee SH, Kim HJ, Kim JS. Less talked variants of benign paroxysmal positional vertigo. J Neurol Sci. (2022) 442:120440. doi: 10.1016/j.jns.2022.120440
31. Lemos J, Strupp M. Central positional nystagmus: an update. J Neurol. (2022) 269:1851–60. doi: 10.1007/s00415-021-10852-8
32. Meng H, Blazquez PM, Dickman JD, Angelaki DE. Diversity of vestibular nuclei neurons targeted by cerebellar nodulus inhibition. J Physiol. (2014) 592:171–88. doi: 10.1113/jphysiol.2013.259614
33. Solomon D, Cohen B. Stimulation of the nodulus and uvula discharges velocity storage in the vestibulo-ocular reflex. Exp Brain Res. (1994) 102:57–68. doi: 10.1007/BF00232438
Keywords: cerebellum, acute stroke, vestibulo-ocular reflex, acute vestibular syndrome, episodic vestibular syndrome, vHIT
Citation: Lee S-H, Kim J-M, Kim J-T and Tarnutzer AA (2023) Video head impulse testing in patients with isolated (hemi)nodular infarction. Front. Neurol. 14:1124217. doi: 10.3389/fneur.2023.1124217
Received: 14 December 2022; Accepted: 18 January 2023;
Published: 06 February 2023.
Edited by:
David Samuel Zee, Johns Hopkins University, United StatesReviewed by:
Jorge Kattah, University of Illinois at Chicago, United StatesCopyright © 2023 Lee, Kim, Kim and Tarnutzer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander Andrea Tarnutzer,  YWxleGFuZGVyLnRhcm51dHplckBrc2IuY2g=
YWxleGFuZGVyLnRhcm51dHplckBrc2IuY2g=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.