
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Neurol. , 30 January 2023
Sec. Neurorehabilitation
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1115370
This article is part of the Research Topic Combining a non-invasive transcranial stimulation technique with another therapeutic approach: mechanisms of action, therapeutic interest and tolerance View all 10 articles
In the past decade researchers began to assess the potential beneficial effects of non-invasive brain stimulation (NIBS) combined with a behavioral task as a treatment approach for various medical conditions. Transcranial direct current stimulation (tDCS) applied to the motor cortex combined with another treatment approach has been assessed as analgesic treatment in neuropathic and non-neuropathic pain conditions, and was found to exert only modest pain relief. Our group results show that combined tDCS and mirror therapy dramatically reduced acute phantom limb pain intensity with long-lasting effects, potentially preventing pain chronification. A review of the scientific literature indicates that our approach differs from that of others: We applied the intervention at the acute stage of the disease, whereas other studies applied the intervention in patients whose disease had already been established. We suggest that the timing of administration of the combined intervention is critical. Unlike in patients with chronic painful condition, in which the maladaptive plasticity associated with pain chronification and chronicity is well-consolidated, early treatment at the acute pain stage may be more successful in counterbalancing the not-yet consolidated maladaptive plasticity. We encourage the research community to test our hypothesis, both in the treatment of pain, and beyond.
Although the use of electrical currents for medical treatment has been documented historically (1–3), technological developments in recent decades have enabled the use of electrical-based non-invasive brain stimulation techniques, such as transcranial magnetic stimulation and transcranial direct current stimulation (tDCS), to alleviate various symptoms, such as depression and pain. This perspective article focuses on the combination of tDCS plus an additional non-pharmacological neuromodulatory treatment aimed at relieving pain.
tDCS is believed to exert its effects by modulating the resting membrane potential of a neuron and thereby changing the threshold for generating action potentials (4). Anodal motor cortex stimulation is a common montage often tested for the treatment of pain. The analgesic effect of anodal tDCS of the motor cortex was proposed to originate from local and connectional effects in remote cortical and subcortical areas through enhanced neuronal excitability. Current evidence suggests that M1 stimulation modulates thalamic and somatosensory activity by descending corticothalamic pathways, brain areas of the fronto-striatal circuit, limbic brain areas, and the periaqueductal gray [i.e., (4–6)].
Although the past 20 years have seen much research on the effects of tDCS on both the brain and pain (7), the accumulated results of the early investigations highlighted only modest and short-term analgesic effects. More recently, researchers hypothesized that combining tDCS with another neuromodulatory treatment could enhance analgesic effects (7–11).
To address this hypothesis, researchers began to explore the analgesic effects of such combined treatments in various pain indications, including phantom limb pain (12–14), neuropathic pain (15–23), complex regional pain syndrome (24, 25), fibromyalgia (26–33), headache (34), chronic musculoskeletal pain (35), chronic low-back pain (36–40), knee osteoarthritis pain (41–45), temporomandibular disorders (46), burning mouth syndrome (47), chronic visceral pain (48), neurogenic pain (49), myofascial pain (50, 51), tendinopathy (52), and radiculopathy (53) (Table 1).
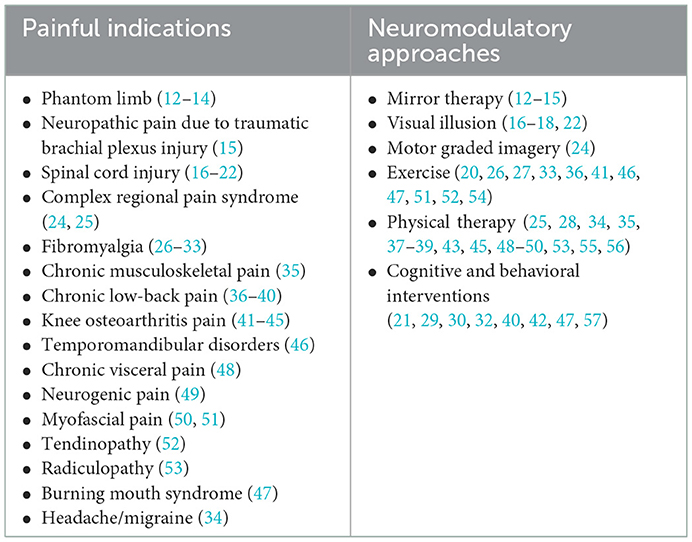
Table 1. Painful indications and the neuromodulatory approaches used in combination with transcranial direct current stimulation (tDCS).
The other neuromodulatory approaches that were combined with the tDCS could be grouped into 4 categories: The first category includes mirror therapy (12–15), visual illusion (16–18, 22) and motor graded imagery (24). These three interventions are sharing similar characteristic—in all these behavioral tasks the participants receive (or imagine) visual input (with, or without additional sensory-motor input) that is assumed to counterbalance the maladaptive plasticity associated with the painful condition. The second category of neuromodulatory approaches includes different exercises (20, 26, 27, 33, 36, 41, 46, 47, 51, 52, 54), in which participants were requested to use a treadmill to perform aerobic exercise or to produce a series of movements specifically intended to increase mobilization, strength and endurance of a painful limb. The therapeutic effects of these exercises are assumed to be produced via modulation of several systems, such as enhancement of corticothalamic excitability, and motor and attentional areas, increase in activity of the descending pain modulatory system and release of dopaminergic and endogenous opioids (58–60). The third category of neuromodulatory approaches comprised of other physical therapy interventions, included the use of transcutaneous electrical nerve stimulation, intramuscular electrical stimulation, mobilization through physical therapy, among other similar techniques, (25, 28, 34, 35, 37–39, 43, 45, 48–50, 53, 55, 56). These approaches assumed to activate descending pain inhibition systems and promote the release of endogenous opioid mechanisms (45, 61–63). The fourth category includes cognitive/behavioral interventions, in which participants perform cognitive tasks such as attentional, memory, executive functioning tasks, mindfulness-meditation, or breathing interventions which are also related to attention processes, processes that are commonly impaired in chronic pain patients (21, 29, 30, 32, 40, 42, 47, 57). These tasks target brain regions such as dorsolateral prefrontal cortex and limbic brain areas, that process cognitive and emotional demands of painful stimuli and exerts a role in modulating pain perception and related emotions (64–70). Summary of all neuromodulatory interventions that were assessed in conjunction with tDCS for the treatment of pain are summarized in Table 1.
In a paper published by our group (12), we compared the effects of mirror therapy stand alone or with either real or sham tDCS on phantom limb pain. The study included 30 lower limb amputees who had been amputated up to 8 weeks previously and who were in the acute phase of phantom pain. Participants were randomized into 1 of the 3 groups (mirror therapy, mirror therapy + sham tDCS, mirror therapy + real tDCS) receiving 10 sessions (5 per week). They were assessed at baseline, at the end of the intervention, and 1 and 3 months thereafter, with the change in pain intensity between baseline and 1 month following the end of treatment predefined as the primary end-point.
The analgesic effects seen in our study were overwhelming (Figure 1). 3 months after the end of the treatment, the combined-treatment group experienced a robust analgesic effect, with mean pain reduction of 5.4 ± 2.6 points (on a 0–10 scale), and in percentage of change, about an 80% reduction), significantly more than the other 2 study arms. The analgesic effects were so large that it virtually eliminated the development of chronic phantom pain, with 90 and 80% of participants reporting pain of ≤2/10 at 1 and 3 months after the end of treatment, respectively. The analgesic effects in the two control arms were, in line with the literature, only modest, leaving the participants with significant phantom pain (>5/10) 3 months after the end of treatment.
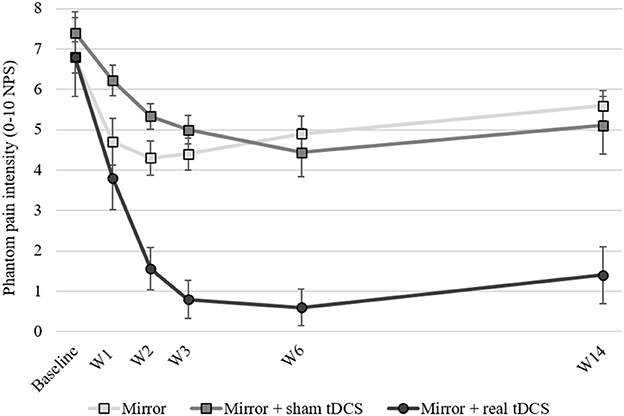
Figure 1. Phantom pain intensity at baseline, across treatment weeks and up to 3 months following the end of treatment. W1, during 1st week of treatment; W2, during 2nd week of treatment; W3, 1st week following end of treatment; W6, 1-month following the end of treatment; W14, 3-months following the end of treatment. tDCS, transcranial direct current stimulation; NPS, numerical pain scale. Error bars represent the Standard Error of the Mean (SEM).
While most methodological aspects of our study were identical or similar to all the other studies that tested the effects of tDCS combined with other neuromodulatory therapy, there was one clear distinction: our study was the only one in which the patients were at the acute stage of pain. Hence, the unprecedented huge analgesic effects seen in our study might be attributed to this characteristic—the short time between the onset of the phantom limb pain and the administration of the therapy. All the other studies included chronic pain patients—that is, those who had been experiencing pain for a long time, sometimes even years or decades.
To gain more insight on our hypothesis, we searched the literature for all relevant studies that used similar treatment approaches, including mirror therapy, visual illusion, and motor graded imagery combined with tDCS. We summarized the relevant studies results in Table 2. To support a fair comparison, only studies in which 10 treatment sessions (or more) were administrated were included in the table. The indications included in the table consist of phantom pain, spinal cord injury, neuropathic pain due to traumatic brachial plexus injury, and complex regional pain syndrome. While our study included only participants who were amputated < 8 weeks previously, all the other studies included only patients with chronic pain. Treatment characteristics were similar: All the studies except ours used anodal motor cortex stimulation at 2 mA. Our study used 1.5 mA in an attempt to support blinding. To compare the clinical effects of adding tDCS to the other therapy, we gathered the means (and standard deviations) of pain scores before (at baseline) and after each study arm. Whenever possible (not all studies included the two relevant study arms), we calculated the analgesic effects in terms of standardized effect sizes (Cohen's d), as follows: the change in pain in the combined treatment (real tDCS plus real other intervention) minus the change in pain in the sham tDCS plus real other intervention, divided by their pooled standard deviation.
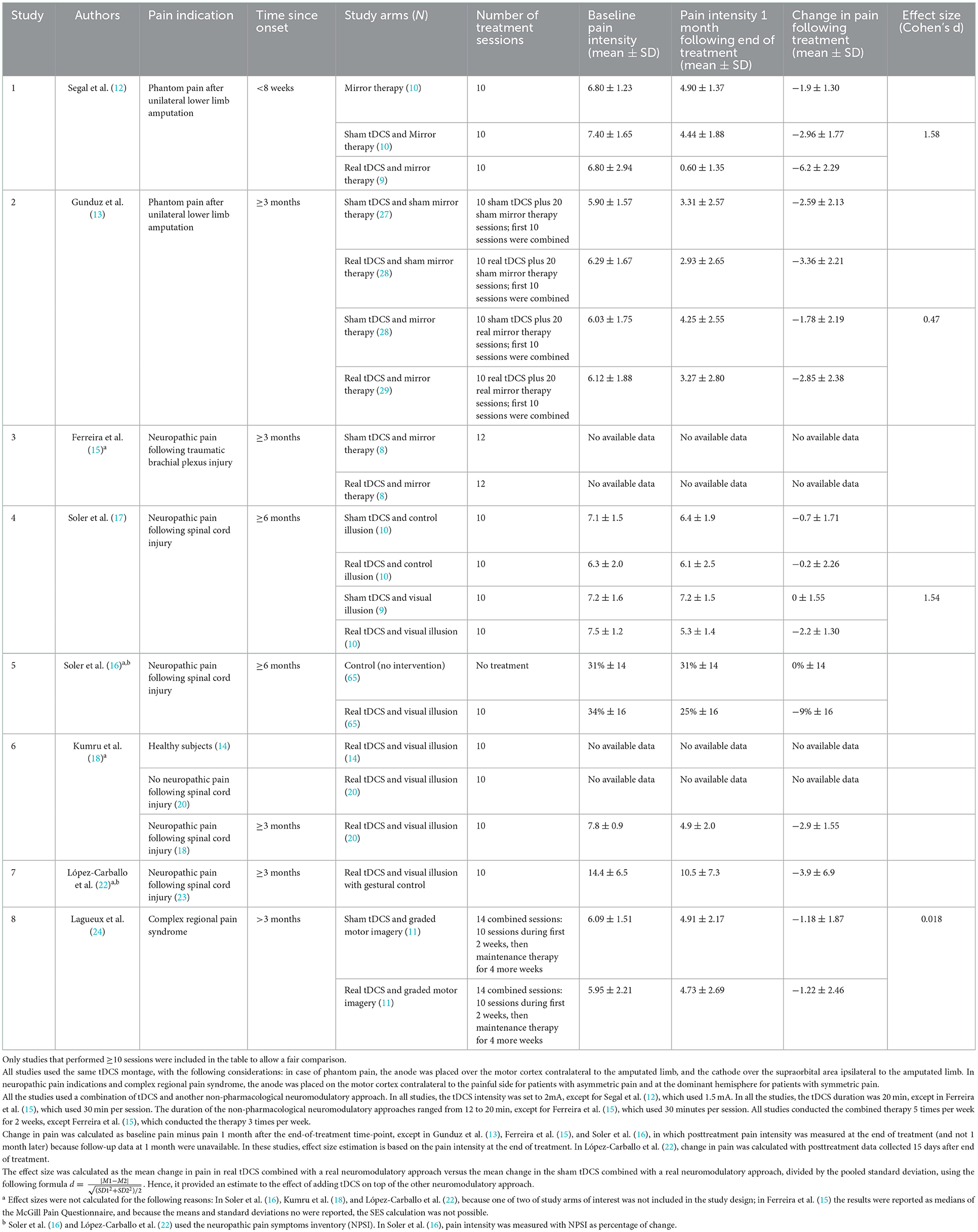
Table 2. Comparison of the analgesic effects among similar studies of tDCS combined with other therapies for pain.
In our study, at 1 month following the end of treatment, the analgesic effects were approximately twice as great as those found in the other studies. On the 0–10 scale, phantom pain intensity was reduced by an average of 6.2 points. Our study also showed much larger standardized effect size than did the other studies, except Soler et al. (17), which demonstrated similar effect size. Although Soler et al. (17) found modest average reductions in pain in the combined-treatment arm (−2.2 points on the 0–10 scale), they observed no change at all in the control arm. The lack of any pain reduction in the control produces a huge calculated effect size. In contrast, in our study, the reductions in pain in the 2 control arms were, as expected, in the magnitude of 2 and 3 points on the 0–10 scale in the mirror therapy alone and in the mirror therapy plus sham tDCS, respectively.
To conclude, the data summarized in Table 2 support further investigation of our hypothesis. The analgesic effects of non-invasive brain stimulation combined with other neuromodulator treatments seem to be much stronger when the interventions are administrated at an early phase of the condition. Given that the comparison derived from Table 2 is descriptive rather than statistical, the results of this preliminary investigation should be regarded as a hypothesis generator. At the early onset of the painful condition—the acute stage—the abnormal neuroplasticity that is associated with the development of a chronic pain condition might not yet have been consolidated. By enrolling patients as early as possible after their pain develops, we might be at a favorable window of opportunity to counterbalance the abnormal neuroplasticity.
The rationale for our hypothesis assumes that after a longer period of pain, the abnormal neuroplasticity that is seen in various painful indications is already consolidated (71, 72) and might be resistant to changes. In contrast, at the acute phase, the central neuroplastic changes have not yet consolidated and are more easily reversed or even prevented. The importance of conducting neuroplasticity-related treatments soon after an injury is well-accepted in the rehabilitation arena, such as in treating post-stroke movement disorders (73). Interestingly, already 20 years ago, McCabe et al. (74) found that the analgesic effects of mirror therapy in complex regional pain syndrome are better when administrated at an early stage (< 8 weeks after onset of pain) than when administered later (1 year or more) (74).
Given the currently inadequate treatments for phantom limb pain and other chronic painful conditions, the healthcare field urgently needs therapeutic interventions to prevent chronicity. A clearer understanding of how maladaptive plasticity is related to the development of chronic pain and how neuromodulation interference at the acute stage can prevent it will pave the way toward a new era of pain treatment: clinical adoption of neuromodulation targeting dysfunctional networks. We encourage the relevant research community to test our hypothesis and to assess the benefits of combined neuromodulatory approaches at earlier time-points of symptoms duration, whenever possible, both in the field of pain and beyond.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
MA performed the literature search and contributed to writing and reviewing the manuscript. IW and RT conceptualization, writing, reviewing, and editing. All authors contributed to the article and approved the submitted version.
We thank Patricia Boyd for assisting with manuscript reviewing and editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Cambiaghi M, Sconocchia S. Scribonius Largus (probably before 1CE–after 48CE). J Neurol. (2018) 265:2466–8. doi: 10.1007/s00415-018-8739-5
2. Sarmiento CI, San-Juan D, Prasath VBS. Letter to the editor: brief history of transcranial direct current stimulation (tDCS): from electric fishes to microcontrollers. Psychol Med. (2016) 46:3259–61. doi: 10.1017/S0033291716001926
4. Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. (2017) 128:56–92. doi: 10.1016/j.clinph.2016.10.08
5. Dasilva AF, Mendonca ME, Zaghi S, Lopes M, Dossantos MF, Spierings EL, et al. tDCS-induced analgesia and electrical fields in pain-related neural networks in chronic migraine. Headache. (2012) 52:1283–95. doi: 10.1111/j.1526-4610.2012.02141.x
6. Polanía R, Nitsche MA, Paulus W. Modulating functional connectivity patterns and topological functional organization of the human brain with transcranial direct current stimulation. Hum Brain Mapp. (2011) 32:1236–49. doi: 10.1002/hbm.21104
7. Pinto CB, Teixeira Costa B, Duarte D, Fregni F. Transcranial direct current stimulation as a therapeutic tool for chronic pain. J ECT. (2018) 34:e36–50. doi: 10.1097/YCT.0000000000000518
8. Massetti T, Crocetta TB, Silva TD da, Trevizan IL, Arab C, Caromano FA, et al. Application and outcomes of therapy combining transcranial direct current stimulation and virtual reality: a systematic review. Disabil Rehabil Assist Technol. (2016) 12:551–9. doi: 10.1080/17483107.2016.1230152
9. Cardenas-Rojas A, Pacheco-Barrios K, Giannoni-Luza S, Rivera-Torrejon O, Fregni F. Noninvasive brain stimulation combined with exercise in chronic pain: a systematic review and meta-analysis. Expert Rev Neurother. (2020) 20:401–12. doi: 10.1080/14737175.2020.1738927
10. Damercheli S, Ramne M, Ortiz-Catalan M. transcranial direct current stimulation (tDCS) for the treatment and investigation of Phantom Limb Pain (PLP). Psychoradiology. (2022) 2:23–31. doi: 10.1093/psyrad/kkac004
11. Nascimento RM do, Cavalcanti RL, Souza CG, Chaves G, Macedo LB. Transcranial direct current stimulation combined with peripheral stimulation in chronic pain: a systematic review and meta-analysis. Expert Rev Med Devices. (2022) 3:9623. doi: 10.1080/17434440.2022.2039623
12. Segal N, Pud D, Amir H, Ratmansky M, Kuperman P, Honigman L, et al. Additive analgesic effect of transcranial direct current stimulation together with mirror therapy for the treatment of phantom pain. Pain Med. (2021) 22:255–65. doi: 10.1093/pm/pnaa388
13. Gunduz ME, Pacheco-Barrios K, Bonin Pinto C, Duarte D, Vélez FGS, Gianlorenco ACL, et al. Effects of combined and alone transcranial motor cortex stimulation and mirror therapy in phantom limb pain: a randomized factorial trial. Neurorehabil Neural Repair. (2021) 35:704–16. doi: 10.1177/15459683211017509
14. Teixeira PEP, Pacheco-Barrios K, Gunduz ME, Gianlorenço AC, Castelo-Branco L, Fregni F. Understanding intracortical excitability in phantom limb pain: a multivariate analysis from a multicenter randomized clinical trial. Neurophysiol Clin. (2021) 51:161–73. doi: 10.1016/j.neucli.2020.12.006
15. Ferreira CM, de Carvalho CD, Gomes R, Bonifácio de. Assis ED, Andrade SM. Transcranial direct current stimulation and mirror therapy for neuropathic pain after brachial plexus avulsion: a randomized, double-blind, controlled pilot study. Front Neurol. (2020) 11:568261. doi: 10.3389/fneur.2020.568261
16. Soler D, Moriña D, Kumru H, Vidal J, Navarro X. Transcranial direct current stimulation and visual illusion effect according to sensory phenotypes in patients with spinal cord injury and neuropathic pain. J Pain. (2021) 22:86–96. doi: 10.1016/j.jpain.2020.06.004
17. Soler MD, Kumru H, Pelayo R, Vidal J, Tormos JM, Fregni F, et al. Effectiveness of transcranial direct current stimulation and visual illusion on neuropathic pain in spinal cord injury. Brain. (2010) 133:2565–77. doi: 10.1093/brain/awq184
18. Kumru H, Soler D, Vidal J, Navarro X, Tormos JM, Pascual-Leone A, et al. The effects of transcranial direct current stimulation with visual illusion in neuropathic pain due to spinal cord injury: an evoked potentials and quantitative thermal testing study. Eur J Pain. (2013) 17:55–66. doi: 10.1002/j.1532-2149.2012.00167.x
19. Roosink M, Robitaille N, Jackson PL, Bouyer LJ, Mercier C. Interactive virtual feedback improves gait motor imagery after spinal cord injury: An exploratory study. Restor Neurol Neurosci. (2016) 34:227–35. doi: 10.3233/RNN-150563
20. Yeh NC, Yang YR, Huang SF, Ku PH, Wang RY. Effects of transcranial direct current stimulation followed by exercise on neuropathic pain in chronic spinal cord injury: a double-blinded randomized controlled pilot trial. Spinal Cord. (2021) 59:684–92. doi: 10.1038/s41393-020-00560-x
21. Li S, Stampas A, Frontera J, Davis M. Combined transcranial direct current stimulation and breathing-controlled electrical stimulation for management of neuropathic pain after spinal cord injury. J Rehabil Med. (2018) 50:814–20. doi: 10.2340/16501977-2379
22. Lopez-Carballo J, Rodriguez N, Soler D, Opisso E, Sbert M. Gestural interaction and visual illusion for lower limbs' neuropathic pain treatment. IEEE Trans Neural Syst Rehabil Eng. (2018) 26:2217–25. doi: 10.1109/TNSRE.2018.2873593
23. McCallion E, Robinson CSH, Clark VP, Witkiewitz K. Efficacy of transcranial direct current stimulation-enhanced mindfulness-based program for chronic pain: a single-blind randomized sham controlled pilot study. Mindfulness. (2020) 11:895–904. doi: 10.1007/s12671-020-01323-8
24. Lagueux E, Bernier M, Bourgault P, Whittingstall K, Mercier C, Léonard G, et al. The effectiveness of transcranial direct current stimulation as an add-on modality to graded motor imagery for treatment of complex regional pain syndrome: a randomized proof of concept study. Clin J Pain. (2018) 34:145–54. doi: 10.1097/AJP.0000000000000522
25. Houde F, Harvey MP, Labrecque PFT, Lamarche F, Lefebvre A, Guillaume L. Combining transcranial direct current stimulation and transcutaneous electrical nerve stimulation to relieve persistent pain in a patient suffering from complex regional pain syndrome: a case report. J Pain Res. (2020) 13:467–73. doi: 10.2147/JPR.S226616
26. Mendonca ME, Simis M, Grecco LC, Battistella LR, Baptista AF, Fregni F. Transcranial direct current stimulation combined with aerobic exercise to optimize analgesic responses in fibromyalgia: a randomized placebo-controlled clinical trial. Front Hum Neurosci. (2016) 10:68. doi: 10.3389/fnhum.2016.00068
27. Desbiens S, Girardin-Rondeau M, Guyot-Messier L, Lamoureux D, Paris L, da Silva RA, et al. Effect of transcranial direct stimulation combined with a functional task on fibromyalgia pain: a case study. Neurophysiol Clin. (2020) 50:134–7. doi: 10.1016/j.neucli.2020.02.006
28. Yoo H. bin, Ost J, Joos W, van Havenbergh T, de Ridder D, Vanneste S. Adding prefrontal transcranial direct current stimulation before occipital nerve stimulation in fibromyalgia. Clin J Pain. (2018) 34:421–7. doi: 10.1097/AJP.0000000000000552
29. Gardoki-Souto I, Martín de la Torre O, Hogg B, Redolar-Ripoll D, Valiente-Gómez A, Martínez Sadurní L, et al. Augmentation of EMDR with multifocal transcranial current stimulation (MtCS) in the treatment of fibromyalgia: study protocol of a double-blind randomized controlled exploratory and pragmatic trial. Trials. (2021) 22:5042. doi: 10.1186/s13063-021-05042-w
30. Santos VS dos S dos, Zortea M, Alves RL, Naziazeno CC dos S, Saldanha JS, Carvalho S da CR de, et al. Cognitive effects of transcranial direct current stimulation combined with working memory training in fibromyalgia: a randomized clinical trial. Sci Rep. (2018) 8:1–11. doi: 10.1038/s41598-018-30127-z
31. Ramasawmy P, Khalid S, Petzke F, Antal A. Pain reduction in fibromyalgia syndrome through pairing transcranial direct current stimulation and mindfulness meditation: a randomized, double-blinded, sham-controlled pilot clinical trial. Front Med. (2022) 9:908133. doi: 10.3389/fmed.2022.908133
32. Riberto M, Alfieri F, Pacheco K, Leite V, Kaihami H, Fregni F, et al. Efficacy of transcranial direct current stimulation coupled with a multidisciplinary rehabilitation program for the treatment of fibromyalgia. Open Rheumatol J. (2011) 5:45–50. doi: 10.2174/1874312901105010045
33. Arroyo-Fernández R, Avendaño-Coy J, Velasco-Velasco R, Palomo-Carrión R, Bravo-Esteban E, Ferri-Morales A. Effectiveness of transcranial direct current stimulation combined with exercising in people with fibromyalgia: a randomized sham-controlled clinical trial. Arch Phys Med Rehabil. (2022) 103:1524–32. doi: 10.1016/j.apmr.2022.02.020
34. Alhassani G, Treleaven J, Schabrun SSM. Combined transcranial and trans-spinal direct current stimulation in chronic headache: a feasibility and safety trial for a novel intervention. Hong Kong Physiother J. (2017) 37:1–9. doi: 10.1016/j.hkpj.2016.11.001
35. Kim S, Salazar Fajardo JC, Seo E, Gao C, Kim R, Yoon BC. Effects of transcranial direct current stimulation on physical and mental health in older adults with chronic musculoskeletal pain: a randomized controlled trial. Eur Geriatr Med. (2022) 13:959–66. doi: 10.1007/s41999-022-00626-4
36. Straudi S, Buja S, Baroni A, Pavarelli C, Pranovi G, Fregni F, et al. The effects of transcranial direct current stimulation (tDCS) combined with group exercise treatment in subjects with chronic low back pain: A pilot randomized control trial. Clin Rehabil. (2018) 32:1348–56. doi: 10.1177/0269215518777881
37. Schabrun SM, Jones E, Elgueta Cancino EL, Hodges PW. Targeting chronic recurrent low back pain from the top-down and the bottom-up: a combined transcranial direct current stimulation and peripheral electrical stimulation intervention. Brain Stimul. (2014) 7:451–9. doi: 10.1016/j.brs.2014.01.058
38. Hazime FA, Baptista AF, de Freitas DG, Monteiro RL, Maretto RL, Hasue RH, et al. Treating low back pain with combined cerebral and peripheral electrical stimulation: a randomized, double-blind, factorial clinical trial. Eur J Pain. (2017) 21:1132–43. doi: 10.1002/ejp.1037
39. Schabrun SM, Burns E, Thapa T, Hodges P. The response of the primary motor cortex to neuromodulation is altered in chronic low back pain: a preliminary study. Pain Med. (2018) 19:1227–36. doi: 10.1093/pm/pnx168
40. Luedtke K, Rushton A, Wright C, Jürgens T, Polzer A, Mueller G, et al. Effectiveness of transcranial direct current stimulation preceding cognitive behavioral management for chronic low back pain: Sham controlled double blinded randomized controlled trial. BMJ. (2015) 350:1640. doi: 10.1136/bmj.h1640
41. Chang WJ, Bennell KL, Hodges PW, Hinman RS, Young CL, Buscemi V, et al. Addition of transcranial direct current stimulation to quadriceps strengthening exercise in knee osteoarthritis: a pilot randomized controlled trial. PLoS ONE. (2017) 12:e0180328. doi: 10.1371/journal.pone.0180328
42. Ahn H, Zhong C, Miao H, Chaoul A, Park L, Yen IH, et al. Efficacy of combining home-based transcranial direct current stimulation with mindfulness-based meditation for pain in older adults with knee osteoarthritis: a randomized controlled pilot study. J Clin Neurosci. (2019) 70:140–5. doi: 10.1016/j.jocn.2019.08.047
43. Li X, Yu W, Li H, Wang B, Xu J. Prospective, single-center comparison of transcranial direct current stimulation plus electro acupuncture and standard analgesia in patients after total knee arthroplasty: effect on rehabilitation and functional recovery. Med Sci Monit. (2021) 27:e930363. doi: 10.12659/MSM.930363
44. Pollonini L, Montero-Hernandez S, Park L, Miao H, Mathis K, Ahn H. Functional near-infrared spectroscopy to assess central pain responses in a nonpharmacologic treatment trial of osteoarthritis. J Neuroimaging. (2020) 30:808–14. doi: 10.1111/jon.12782
45. da Graca-Tarragó M, Lech M, Angoleri LDM, Santos DS, Deitos A, Brietzke AP, et al. Intramuscular electrical stimulus potentiates motor cortex modulation effects on pain and descending inhibitory systems in knee osteoarthritis: a randomized, factorial, sham-controlled study. J Pain Res. (2019) 12:209–21. doi: 10.2147/JPR.S181019
46. Oliveira LB, Lopes TS, Soares C, Maluf R, Goes BT, Sá KN, et al. Transcranial direct current stimulation and exercises for treatment of chronic temporomandibular disorders: a blind randomised-controlled trial. J Oral Rehabil. (2015) 42:723–32. doi: 10.1111/joor.12300
47. Sánchez-Cuesta FJ, González-Zamorano Y, Arroyo-Ferrer A, Avellanal M, Fernández-Carnero J, Romero JP. Transcranial direct current stimulation (tDCS) combined with therapeutic exercise and cognitive rehabilitation to treat a case of burning mouth syndrome (BMS) related pain. Appl Sci. (2021) 11:11538. doi: 10.3390/app112311538
48. Thibaut A, Russo C, Hurtado-Puerto AM, Morales-Quezada JL, Deitos A, Petrozza JC, et al. Effects of transcranial direct current stimulation, transcranial pulsed current stimulation, and their combination on brain oscillations in patients with chronic visceral pain: a pilot crossover randomized controlled study. Front Neurol. (2017) 8:576. doi: 10.3389/fneur.2017.00576
49. Boggio PS, Amancio EJ, Correa CF, Cecilio S, Valasek C, Bajwa Z, et al. Transcranial DC stimulation coupled with TENS for the treatment of chronic pain: a preliminary study. Clin J Pain. (2009) 25:691–5. doi: 10.1097/AJP.0b013e3181af1414
50. Choi YH, Jung SJ, Lee CH, Lee SU. Additional effects of transcranial direct-current stimulation and trigger-point injection for treatment of myofascial pain syndrome: a pilot study with randomized, single-blinded trial. J Altern Complement Med. (2014) 20:698–704. doi: 10.1089/acm.2013.0243
51. Sakrajai P, Janyacharoen T, Jensen MP, Sawanyawisuth K, Auvichayapat N, Tunkamnerdthai O, et al. Pain reduction in myofascial pain syndrome by anodal transcranial direct current stimulation combined with standard treatment: a randomized controlled study. Clin J Pain. (2014) 30:1076–83. doi: 10.1097/AJP.0000000000000069
52. Belley AF, Mercier C, Bastien M, Léonard G, Gaudreault N, Roy JS. Anodal transcranial direct-current stimulation to enhance rehabilitation in individuals with rotator cuff tendinopathy: a triple-blind randomized controlled trial. J Orthop Sports Phys Ther. (2018) 48:541–51. doi: 10.2519/jospt.2018.7871
53. Chen Z, Zhang W, Yu Y, Tan T. A retrospective comparative cohort study of the effects of neural mobilization (NM) alone and NM combined with transcranial direct current stimulation in patients with cervical radiculopathy. Ann Palliat Med. (2022) 11:2961–7. doi: 10.21037/apm-22-746
54. Borovskis J, Cavaleri R, Blackstock F, Summers SJ. Transcranial direct current stimulation accelerates the onset of exercise-induced hypoalgesia: a randomized controlled study. J Pain. (2021) 22:263–74. doi: 10.1016/j.jpain.2020.08.004
55. Jafarzadeh A, Ehsani F, Yosephi MH, Zoghi M, Jaberzadeh S. Concurrent postural training and M1 anodal transcranial direct current stimulation improve postural impairment in patients with chronic low back pain. J Clin Neurosci. (2019) 68:224–34. doi: 10.1016/j.jocn.2019.07.017
56. Rahimi F, Nejati V, Nassadj G, Ziaei B, Mohammadi HK. The effect of transcranial direct stimulation as an add-on treatment to conventional physical therapy on pain intensity and functional ability in individuals with knee osteoarthritis: a randomized controlled trial. Neurophysiol Clin. (2021) 51:507–16. doi: 10.1016/j.neucli.2021.06.002
57. Powers A, Madan A, Hilbert M, Reeves ST, George M, Nash MR, et al. Effects of Combining a brief cognitive intervention with transcranial direct current stimulation on pain tolerance: a randomized controlled pilot study. Pain Med. (2018) 19:677–85. doi: 10.1093/pm/pnx098
58. Neva JL, Brown KE, Mang CS, Francisco BA, Boyd LA. An acute bout of exercise modulates both intracortical and interhemispheric excitability. Eur J Neurosci. (2017) 45:1343–55. doi: 10.1111/ejn.13569
59. Tajerian M, David Clark J. Nonpharmacological interventions in targeting pain-related brain plasticity. Neural Plast. (2017) 2017:8573. doi: 10.1155/2017/2038573
60. Wakaizumi K, Kondo T, Hamada Y, Narita M, Kawabe R, Narita H, et al. Involvement of mesolimbic dopaminergic network in neuropathic pain relief by treadmill exercise: a study for specific neural control with Gi-DREADD in mice. Mol Pain. (2016) 12:1567. doi: 10.1177/1744806916681567
61. Leonard G, Goffaux P, Marchand S. Deciphering the role of endogenous opioids in high-frequency TENS using low and high doses of naloxone. Pain. (2010) 151:215–9. doi: 10.1016/j.pain.2010.07.012
62. Choi JC, Kim J, Kang E, Lee JM, Cha J, Kim YJ, et al. Brain mechanisms of pain relief by transcutaneous electrical nerve stimulation: a functional magnetic resonance imaging study. Eur J Pain. (2016) 20:92–105. doi: 10.1002/ejp.696
63. Schabrun SM, Chipchase LS. Priming the brain to learn: the future of therapy? Man Ther. (2012) 17:184–6. doi: 10.1016/j.math.2011.12.001
64. Seminowicz DA, Shpaner M, Keaser ML, Krauthamer GM, Mantegna J, Dumas JA, et al. Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J Pain. (2013) 14:1573–84. doi: 10.1016/j.jpain.2013.07.020
65. McCracken LM, Turk DC. Behavioral and cognitive-behavioral treatment for chronic pain: outcome, predictors of outcome, and treatment process. Spine. (2002) 27:2564–73. doi: 10.1097/00007632-200211150-00033
66. Pardos-Gascón EM, Narambuena L, Leal-Costa C, van-der Hofstadt-Román CJ. Differential efficacy between cognitive-behavioral therapy and mindfulness-based therapies for chronic pain: systematic review. Int J Clin Health Psychol. (2021) 21:100197. doi: 10.1016/j.ijchp.2020.08.001
67. Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. (2013) 14:502. doi: 10.1038/nrn3516
68. Zeidan F, Emerson NM, Farris SR, Ray JN, Jung Y, McHaffie JG, et al. Mindfulness meditation-based pain relief employs different neural mechanisms than placebo and sham mindfulness meditation-induced analgesia. J Neurosci. (2015) 35:15307–25. doi: 10.1523/JNEUROSCI.2542-15.2015
69. Ong WY, Stohler CS, Herr DR. Role of the prefrontal cortex in pain processing. Mol Neurobiol. (2019) 56:1137. doi: 10.1007/s12035-018-1130-9
70. Haase L, Thom NJ, Shukla A, Davenport PW, Simmons AN, Stanley EA, et al. Mindfulness-based training attenuates insula response to an aversive interoceptive challenge. Soc Cogn Affect Neurosci. (2016) 11:182. doi: 10.1093/scan/nsu042
71. Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumers N, et al. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. (1995) 375:482–4. doi: 10.1038/375482a0
72. Ramachandran VS, Rogers-Ramachandran D, Stewart M, Pons TP. Perceptual correlates of massive cortical reorganization. Science. (1992) 258:1159–60. doi: 10.1126/science.1439826
73. Krakauer J, Carmichael T. Broken Movement The Neurobiology of Motor Recovery after Stroke. Cambridge, MA: MIT Press (2017).
Keywords: neuromodulation, non-invasive brain stimulation, combined therapy, analgesic therapy, mirror therapy
Citation: Agostinho M, Weissman Fogel I and Treister R (2023) Time since onset might be of essence: A recommendation to assess the effects of combination of non-pharmacological neuromodulatory approaches at early stage since symptoms onset. Front. Neurol. 14:1115370. doi: 10.3389/fneur.2023.1115370
Received: 03 December 2022; Accepted: 11 January 2023;
Published: 30 January 2023.
Edited by:
Simone Rossi, University of Siena, ItalyReviewed by:
Jean-Pascal Lefaucheur, Univ Paris-Est Créteil, AP-HP, FranceCopyright © 2023 Agostinho, Weissman Fogel and Treister. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roi Treister,  cnRyZWlzdGVyQHVuaXYuaGFpZmEuYWMuaWw=
cnRyZWlzdGVyQHVuaXYuaGFpZmEuYWMuaWw=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.