- 1Department of Neurology, Xiangya Hospital, Central South University, Changsha, China
- 2National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Changsha, China
- 3Key Laboratory of Hunan Province in Neurodegenerative Disorders, Central South University, Changsha, China
Primary familial brain calcification (PFBC) is a disorder in which pathologic calcification of the basal ganglia, cerebellum, or other brain regions with bilateral symmetry occurs. Common clinical symptoms include dysarthria, cerebellar symptoms, motor deficits, and cognitive impairment. Genetic factors are an important cause of the disease; however autosomal recessive (AR) inheritance is rare. In 2018, the myogenesis-regulated glycosidase (MYORG) gene was the first to be associated with AR-PFBC. The present case is a 24-year-old woman with AR-PFBC that presented with migraine at the age of 16 years. Symmetrical patchy calcifications were seen in the bilateral cerebellopontine nuclei, thalamus, basal ganglia, and radiocoronal area on computed tomography and magnetic resonance imaging. AR-PFBC with migraine as the main clinical symptom is rare. Whole-exome sequencing revealed a compound heterozygous mutation in the MYORG gene, one of which has not been previously reported. Our case highlights the pathogenic profile of the MYORG gene, and demonstrates the need for exclusion of calcium deposits in the brain for migraine patients with AR inheritance.
Introduction
Primary familial brain calcification (PFBC, OMIM #213600) is a disorder in which the main pathological finding is calcification of the basal ganglia, cerebellum, or other brain regions with bilateral symmetry (1–3). Genetic components is an important cause of PFBC, which can exhibit both autosomal dominant (AD) and autosomal recessive (AR) inheritance. Autosomal recessive-primary familial brain calcification (AR-PFBC) generally has a higher clinical penetrance and a more severe pattern of calcifications compared to autosomal dominant-primary familial brain calcification (AD-PFBC) (1, 3, 4). The genes involved in AD-PFBC include SLC20A2 (NM_001257180.2), PDGFB (NM_002608.4), PDGFRB (NM_001355016.2), and XPR1 (NM_001135669.2), which are associated with calcium and phosphorus metabolism and the blood-brain barrier (5–7). Instead, genes involved in AR-PFBC include MYORG (NM_020702.5) and JAM2 (NM_001270407.2). The myogenesis-regulated glycosidase (MYORG) gene was first identified in 2018 (8). The typical indications of AR-PFBC are chronic progressive motor impairment, cognitive impairment, dysarthria, and cerebellar symptoms, whereas migraine presentation is rare (8–10). Here we report the case of a 24-year-old woman with AR-PFBC and migraine onset at the age of 16 years harboring MYORG compound heterozygous variants, one of which has not been previously reported.
Case presentation
A 24-year-old Chinese woman was admitted to our outpatient clinic with migraine headache, reportedly experienced for 8 years. She first developed recurrent episodes of headache at the age of 16, associated with no apparent trigger. These symptoms occurred one to two times a year, lasted for 1–2 days at a time, and resolved spontaneously. The profession of the patient was a middle school art teacher, and her academic performance during her school period had remained good, with no history of psychological illness or psychiatric disorders. The patient was born to non-consanguineous parents, and her physical examination showed no abnormality except for minor knee and bicep brisk reflexes.
The patient's endocrine examination showed normal serum allosteric parathyroid hormone, calcium, and phosphorus levels. Moreover, the results of the Foaming experimental and right heart contrast echocardiography were normal. Computed tomography (CT) and magnetic resonance imaging revealed symmetrical patchy calcifications in the bilateral cerebellopontine nuclei, thalamus, basal ganglia, and radiocoronal areas (Figure 1). The patient had a Montreal Cognitive Assessment score of 26 (delayed memory minus 4 points did not meet the diagnostic criteria for cognitive dysfunction), and a Mini-Mental State Examination score of 28. The patient also had a migraine-specific Quality of Life Questionnaire score of 21, and a Headache Impact Test-6 score of 51. We administered Oxiracetam and nicergoline for neurotrophic treatment and improvement of cerebrovascular circulation, and the patient reported symptom improvement. The patient's brother had also undergone CT examination at another hospital due to occasional headache, which revealed multiple symmetrical calcifications in the bilateral basal ganglia, dorsal thalamus, cerebellar hemispheres, and vermis. The calcification sites and morphology of both siblings exhibited a high degree of similarity (Figure 1).
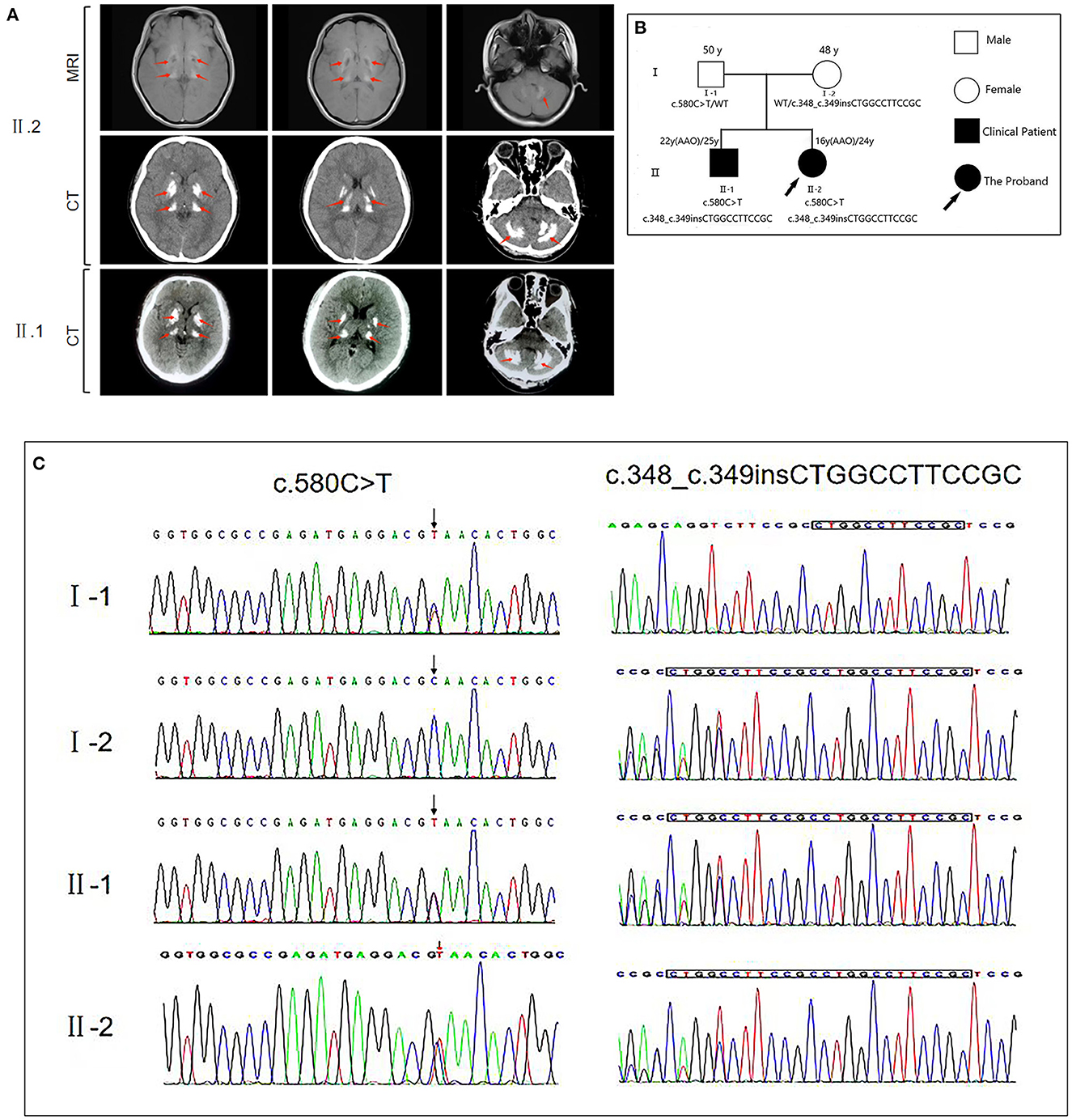
Figure 1. (A) Brain magnetic resonance imaging (MRI) and computed tomography (CT) for the proband (II.2) reveal bilateral calcification in the basal ganglia, thalamus, cerebellum, and pons. CT of the proband's brother (II.1) reveals bilateral calcification in the basal ganglia, thalamus, and cerebellum. The calcification sites showed a high degree of similarity between the patients. (B) The genetic pedigree for this case. (C) MYORG gene sequencing of the family members of this case. FM, family; AAO, age at onset; WT, wild type.
We then performed whole-exome sequencing on the patient, identifying that the proband had potential compound heterozygous variants in the MYORG gene, including two heterozygous variants, namely c.348_c.349insCTGGCCTTCCGC (p.116_117insLAFR) and c.580G>T (p.Q194*) that were confirmed by Sanger sequencing (Figure 1). The insertion c.348_c.349insCTGGCCTTCCGC variant is a known pathogenic mutation (8). However, the loss-of-function c.580G > T MYORG gene variant has not been reported in ClinVar, and no publication has reported this mutation so far (1–3, 8, 11). Both variants were predicted to be damaging by multiple in silico prediction tools, including SIFT, Polyphen2, CADD, and MutationTaster. Additionally, the novel mutant (c.580G > T) is not listed in the 1,000 Genomes, NHLBI GO Exome Sequencing Project, and Exome Aggregation Consortium databases. According to the American College of Medical Genetics guidelines, the non-sense c.580G > T mutant is considered “potentially pathogenic: PVS1-Strong + PM2,” whereas the insertional c.348_c.349insCTGGCCTTCCGC mutant is considered “possibly pathogenic: PM3-Strong + PM4.” With consent, we also performed whole-exome sequencing on the patient's parents and brother. We found that the father carried only the non-sense c.580G > T variant, while the mother carried only the insertion c.348_c.349insCTGGCCTTCCGC variant. The brother carried both variants identified in the proband. Collectively, this information confirmed the two variants as the compound heterozygous variants in the MYORG gene, and as pathogenic MYORG variants associated with PFBC.
Discussion
Autosomal recessive-primary familial cerebral calcification (AR-PFBC) is caused by mutations in the MYORG gene, and has an age of onset between 38 and 53 years (1). The typical indications of AR-PFBC and of the MYORG variant are: verbal deficits, chronic progressive motor deficits, ataxia, cognitive deficits, and psychiatric symptoms (8, 10, 12). Among them, verbal impairment is often the first symptom of PFBC due to a MYORG variant (12, 13), while cognitive impairment is usually milder in AR-PFBC than in AD-PFBC and does not lead to major neurocognitive impairment (8, 14). Migraine as the main symptom is rare in AR-PFBC, with only one similar case having been reported in a 12-year-old Turkish girl with AR-PFBC (10). Cognitive deficits and depression have been documented as the main non-motor symptoms and signs of PFBC; instead headache is less common, and is observed in ~8% of cases (1).
Here, we report the case of a 24-year-old female whose symptoms began at the age of 16 years. The patient reported experiencing migraines over the last 8 years, together with some other cognitive symptoms but without motor symptoms. The patient had a Montreal Cognitive Assessment score of 26 with delayed memory affecting the score the most, although this did not meet pathological criteria. However, further tracking of cognitive capacity is warranted.
We also reviewed the existing literature related to PFBC patients with MYORG variants (Table 1). By analyzing this information, we found that patients with dysphagia, verbal impairment, cognitive impairment, and dyskinesia as primary symptoms were mainly middle-aged and elderly (14–18). Conversely, patients with headache as the main clinical symptom are generally younger (10, 19). Therefore, we hypothesize that earlier age of onset is associated with increased risk of headache and migraine, and that other corresponding symptoms may then develop with age.
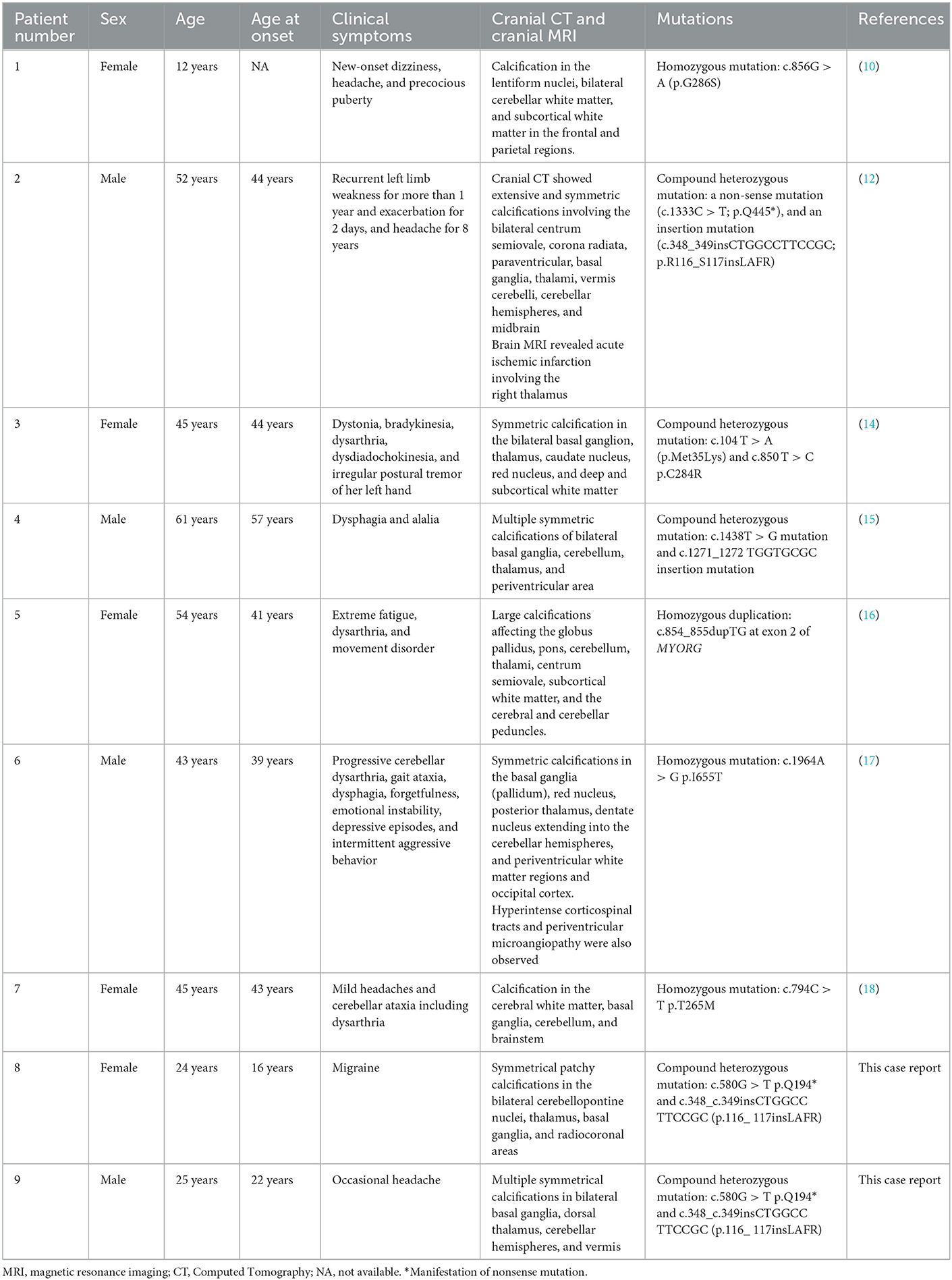
Table 1. Review of the previous literature related to AR-PFBC patients with MYORG mutations in comparison to the patients presented in the current report.
The patient's cranial CT and MRI revealed symmetrical patches of calcified foci in the bilateral cerebellar dentate nuclei, thalamus, basal ganglia, and radiocoronal area, which are typical of PFBC. However, the degree of cerebral calcification in the patient was relatively mild, which may be related to age, disease duration, and mutational pattern. According to previous reports, following onset, the degree of brain calcification in PFBC with MYORG gene variants gradually becomes more severe with time (4, 16, 20). Endocrine analysis found normal levels of serum allotropic parathyroid hormone, calcium, and phosphorus. This is also an important point of differentiation between the MYORG mutant type of PFBC and AD-PFBC, which involves genes such as PIT2 (also known as SLC20A2) and XPR1 that are associated with parathyroid regulation of calcium and phosphorus metabolism. Consequently, AD-PFBC is often accompanied by hypoparathyroidism or pseudohypoparathyroidism and intracellular deposition of calcium and phosphorus (5, 7).
Whole-exome gene sequencing revealed compound heterozygous mutations on the MYORG gene: namely, a non-sense mutation c.580G > T (p.Q194*), and an insertional mutation c.348_c.349insCTGGCC TTCCGC (p.116_117ins LAFR). Both mutations are located in the C-terminal tubulin site of MYORG (8, 13). We summarized the variants in previous reports (Table 1), including compound heterozygous and homozygous mutations, and identified that the distribution of MYORG mutations is mainly segregated in the C-terminal luminal fragment of the MYORG protein, which is related to its glycosidase domain (Figure 2). In addition, we found that 9/42 of the mutations we summarized were loss-of-function (LOF) mutations, whereas 27/42 were missense mutations (Figure 2A). MYORG is specifically expressed in astrocytes, and may regulate protein glycosylation in the endoplasmic reticulum of brain astrocytes (8, 13, 15). Inactivation of the MYORG glycosidase function may lead to abnormal protein glycosylation and metabolism, which may lay the foundation for the formation of brain calcification (8). Additionally, astrocytes are a key component of the neurovascular unit. Mutations in MYORG may cause damage to the neurovascular unit, and accelerate the deposition of calcium and other minerals in small arteries, capillaries, small veins, and perivascular spaces; such neurovascular unit impairment could cause damage to the blood-brain barrier (8, 17). MYORG mutations have also been reported in brain hypoperfusion and cerebral infarction (12, 14, 21). However, it has been mentioned in the vascular theory that intracranial vasoconstriction causes migraine aura symptoms, followed by intracranial and extracranial vasodilatation leading to pulsatile headache production. It is also worth noting that, based on multiple recent imaging studies, vascular dilation is not considered to be necessarily present during migraine attacks (22, 23). Furthermore, because the membranes of astrocytes are rich in sodium and potassium pumps, astrocytes maintain a stable K+ concentration in the extracellular fluid. Thus, when astrocytes are damaged the electrical activity of neurons may be affected. However, the exact mechanisms by which MYORG mutations lead to migraine is unclear, and further studies will help elucidate these mechanisms.
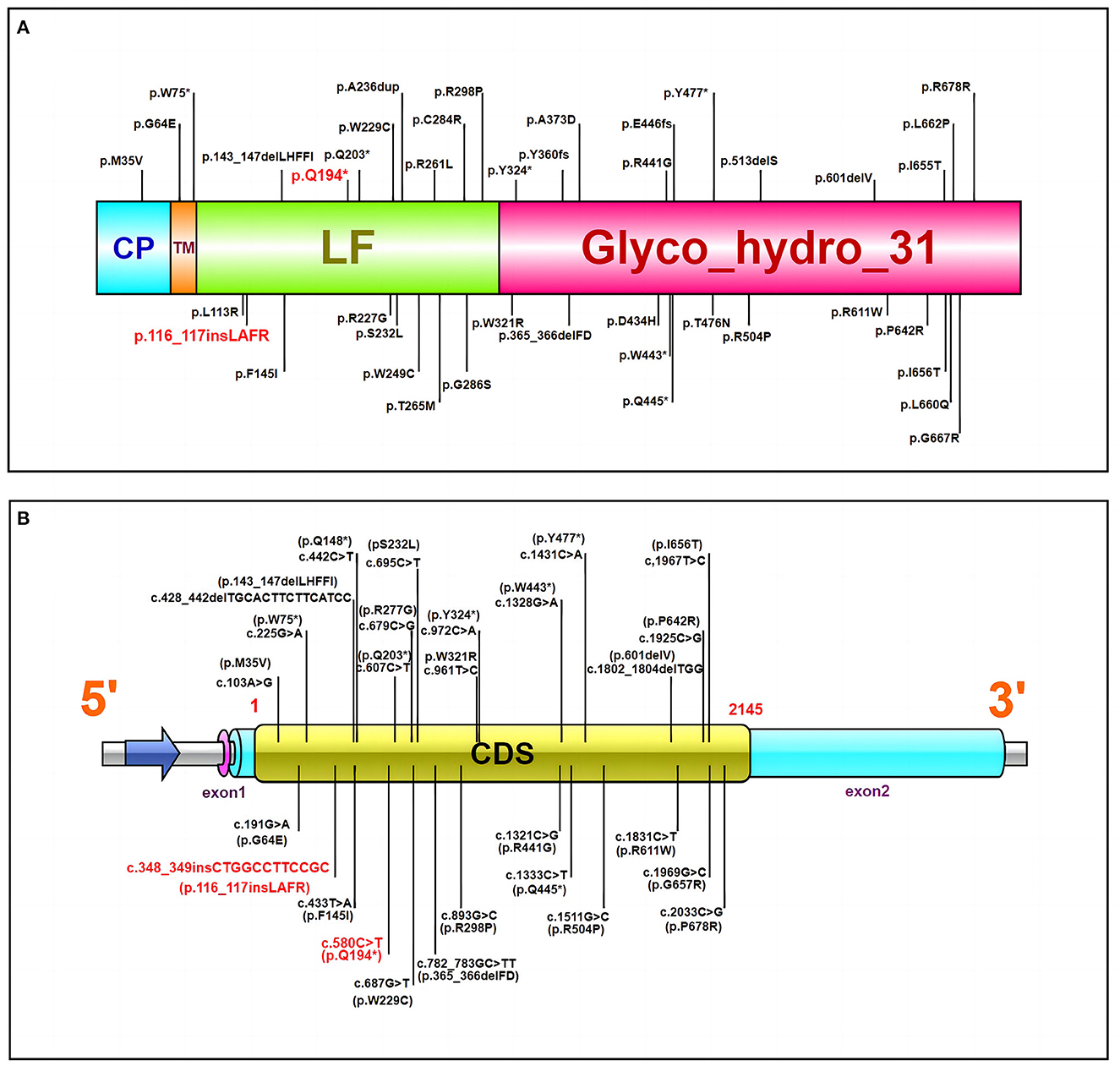
Figure 2. (A) The MYORG protein and reported variants. Domain architecture of the MYORG protein and previously reported variants; the variant in our case is marked in red. CP, cytoplasmic domain; TM, transmembrane domain; LF, C-terminal luminal fragment with a glycosidase domain. (B) The MYORG cDNA and reported variants. The variant in our case is marked in red. CDS Coding sequence (the length is ~2,145 bps). *Manifestation of nonsense mutation.
Conclusion
A patient with a novel mutation in MYORG was diagnosed with AR-PFBC at the age of 24 years, with migraine being the only main clinical symptom. Our case highlights the pathogenic profile of the MYORG gene and the clinical phenotype of MYORG mutations. It also demonstrates the need for exclusion of calcium deposits in the brain for migraine patients with AR inheritance.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Xiangya Hospital, Central South University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
QX conceived the study. TS drafted the manuscript. TS, YZ, and GW participated in the clinical management of patients and data collection. QX and JD revised the manuscript. QX accepts responsibility for final approval. All authors approved the final version of the article.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 82071437), the Natural Science Foundation of Hunan Province (Grant No. 2021JJ31115), the National Key Research and Development Program of China (Grant No. 2021YFC2501200), and the Project Program of National Clinical Research Center for Geriatric Disorders (Xiangya Hospital) (Grant No. 2021KFJJ10).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Balck A, Schaake S, Kuhnke NS, Domingo A, Madoev H, Margolesky J, et al. Genotype-phenotype relations in primary familial brain calcification: systematic MDSGene review. Mov Disord. (2021) 36:2468–80. doi: 10.1002/mds.28753
2. Tadic V, Westenberger A, Domingo A, Alvarez-Fischer D, Klein C, Kasten M. Primary familial brain calcification with known gene mutations: a systematic review and challenges of phenotypic characterization. JAMA Neurol. (2015) 72:460–7. doi: 10.1001/jamaneurol.2014.3889
3. Westenberger A, Balck A, Klein C. Primary familial brain calcifications: genetic and clinical update. Curr Opin Neurol. (2019) 32:571–8. doi: 10.1097/WCO.0000000000000712
4. Grangeon L, Wallon D, Charbonnier C, Quenez O, Richard AC, Rousseau S, et al. Biallelic MYORG mutation carriers exhibit primary brain calcification with a distinct phenotype. Brain. (2019) 142:1573–86. doi: 10.1093/brain/awz095
5. López-Sánchez U, Tury S, Nicolas G, Wilson MS, Jurici S, Ayrignac X, et al. Interplay between primary familial brain calcification-associated SLC20A2 and XPR1 phosphate transporters requires inositol polyphosphates for control of cellular phosphate homeostasis. J Biol Chem. (2020) 295:9366–78. doi: 10.1074/jbc.RA119.011376
6. Nicolas G, Pottier C, Maltête D, Coutant S, Rovelet-Lecrux A, Legallic S, et al. Mutation of the PDGFRB gene as a cause of idiopathic basal ganglia calcification. Neurology. (2013) 80:181–7. doi: 10.1212/WNL.0b013e31827ccf34
7. Zavatta G, Clarke BL. Basal ganglia calcification in hypoparathyroidism and pseudohypoparathyroidism: local and systemic metabolic mechanisms. J Endocrinol Invest. (2021) 44:245–53. doi: 10.1007/s40618-020-01355-w
8. Yao XP, Cheng X, Wang C, Zhao M, Guo XX, Su HZ, et al. Biallelic mutations in MYORG cause autosomal recessive primary familial brain calcification. Neuron. (2018) 98:1116–23. doi: 10.1016/j.neuron.2018.05.037
9. Ferreira LD, de Oliveira JRM. Overlapping diseases in a Brazilian subject with brain calcification linked to novel phenotypes. J Mol Neurosci. (2020) 70:1255–6. doi: 10.1007/s12031-020-01534-7
10. Tekin Orgun L, Besen S, Sangün Ö, Bisgin A, Alkan Ö, Erol I. First pediatric case with primary familial brain calcification due to a novel variant on the MYORG gene and review of the literature. Brain Dev. (2021) 43:789–97. doi: 10.1016/j.braindev.2021.04.002
11. Xu X, Sun H, Luo J, Cheng X, Lv W, Luo W, et al. The pathology of primary familial brain calcification: implications for treatment. Neurosci Bull. (2022). doi: 10.1007/s12264-022-00980-0. [Epub ahead of print].
12. Yang Q, Li J, Jiao B, Weng L. Primary familial brain calcification in a patient with a novel compound heterozygous mutation in presenting with an acute ischemic stroke: a case report. Ann Transl Med. (2022) 10:423. doi: 10.21037/atm-21-4883
13. Zeng YH, Lin BW, Su HZ, Guo XX, Li YL, Lai LL, et al. Mutation analysis of MYORG in a Chinese cohort with primary familial brain calcification. Front Genet. (2021) 12:732389. doi: 10.3389/fgene.2021.732389
14. Chen SY, Lin WC, Chang YY, Lin TK, Lan MY. Brain hypoperfusion and nigrostriatal dopaminergic dysfunction in primary familial brain calcification caused by novel MYORG variants: case report. BMC Neurol. (2020) 20:329. doi: 10.1186/s12883-020-01910-1
15. Fei BN, Su HZ, Yao XP, Ding J, Wang X. Idiopathic basal ganglia calcification associated with new mutation site: a case report. World J Clin Cases. (2021) 9:7169–74. doi: 10.12998/wjcc.v9.i24.7169
16. Ferreira LD, de Oliveira JRM. New homozygous indel in MYORG linked to brain calcification, thyroidopathy and neuropathy. Brain. (2019) 142:e51. doi: 10.1093/brain/awz225
17. Forouhideh Y, Müller K, Ruf W, Assi M, Seker T, Tunca C, et al. A biallelic mutation links MYORG to autosomal-recessive primary familial brain calcification. Brain. (2019) 142:e4. doi: 10.1093/brain/awy343
18. Kume K, Takata T, Morino H, Matsuda Y, Ohsawa R, Tada Y. The first Japanese case of primary familial brain calcification caused by an MYORG variant. J Hum Genet. (2020) 65:917–20. doi: 10.1038/s10038-020-0779-x
19. Sun H, Cao Z, Gao R, Li Y, Chen R, Du S, et al. Severe brain calcification and migraine headache caused by SLC20A2 and PDGFRB heterozygous mutations in a five-year-old Chinese girl. Mol Genet Genomic Med. (2021) 9:e1670. doi: 10.1002/mgg3.1670
20. Chen Y, Cen Z, Chen X, Wang H, Chen S, Yang D, et al. MYORG mutation heterozygosity is associated with brain calcification. Mov Disord. (2020) 35:679–86. doi: 10.1002/mds.27973
21. Gao L, Chen J, Dong H, Li X. A novel mutation in leads to primary familial brain calcification and cerebral infarction. Int J Neurosci. (2022) 132:1182–6. doi: 10.1080/00207454.2020.1869000
22. Goadsby P, Holland P, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. (2017) 97:553–622. doi: 10.1152/physrev.00034.2015
Keywords: primary familial brain calcification, novel MYORG mutation, migraine, case report, literature review
Citation: Song T, Zhao Y, Wen G, Du J and Xu Q (2023) A novel MYORG mutation causes primary familial brain calcification with migraine: Case report and literature review. Front. Neurol. 14:1110227. doi: 10.3389/fneur.2023.1110227
Received: 28 November 2022; Accepted: 13 January 2023;
Published: 02 February 2023.
Edited by:
Huifang Shang, Sichuan University, ChinaReviewed by:
Martin Paucar, Karolinska University Hospital, SwedenSaima Siddiqi, Institute of Biomedical and Genetic Engineering (IBGE), Pakistan
Copyright © 2023 Song, Zhao, Wen, Du and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Xu,  eHl4dXFpYW4yMDE1QDE2My5jb20=
eHl4dXFpYW4yMDE1QDE2My5jb20=
 Tingwei Song
Tingwei Song Yuwen Zhao
Yuwen Zhao Guo Wen2
Guo Wen2 Juan Du
Juan Du Qian Xu
Qian Xu