- 1Department of Anesthesiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2Anesthesiology and Physiology and Pharmacology Departments, State University of New York Downstate Health Sciences University, New York, NY, United States
Introduction: Patients with malignant brain tumors frequently exhibit hypercoagulation and are at a high risk of postoperative thrombosis-related complications. However, the risk factors for postoperative thrombosis-related complications remain unclear.
Methods: In this retrospective, observational study, we consecutively enrolled elective patients undergoing resection of malignant brain tumors from 26 November 2018 to 30 September 2021. The primary objective of the study was to identify risk factors for a composite of three major adverse events including postoperative lower limb deep venous thrombosis, pulmonary embolism, and cerebral ischemia.
Results: A total of 456 patients were enrolled in this study, where 112 (24.6%) patients had postoperative thrombosis-related complications, 84 (18.4%) with lower limb deep venous thrombosis, 0 (0.0%) with pulmonary embolism, and 42 (9.2%) with cerebral ischemia. In a multivariate model, age more than 60 years (OR: 3.98, 95% CI: 2.30–6.88, P < 0.001), preoperative abnormal APTT (OR: 2.81, 95% CI: 1.06–7.42, P = 0.037), operation duration longer than 5 h (OR: 2.36, 95% CI: 1.34–4.16, P = 0.003), and admission to ICU (OR: 2.49, 95% CI: 1.21–5.12, P = 0.013) were independent risk factors of the postoperative deep vein thrombosis. Intraoperative plasma transfusion (OR: 6.85, 95% CI: 2.73–17.18, P < 0.001) was associated with significantly increased odds of deep vein thrombosis.
Conclusion: Patients with craniocerebral malignant tumors have a high incidence of postoperative thrombosis-related complications. There is an increase in the odds of postoperative lower limb deep venous thrombosis in patients; over 60 years old, with preoperative abnormal APTT, undergoing surgeries longer than 5-h, admission to ICU, or receiving intraoperative plasma infusion. Fresh frozen plasma infusion should be used more cautiously, especially in patients with a high risk of thrombosis.
Introduction
Thrombosis, a severe postoperative complication, is a major concern following surgery. Patients with brain tumors, particularly those with gliomas or malignant tumors, are often considered to be at a high risk of thromboembolic complications (1, 2). The incidence of postoperative deep vein thrombosis was reported to be 10.3–21.3% in patients undergoing brain tumor resections (3, 4). In patients with malignant gliomas, the incidence increased from 16.1 to 33.3% (5, 6). Retrospective studies have reported a 0.5–1.5% incidence of pulmonary embolism after brain tumor resections (7, 8). Perioperative cerebral ischemia in patients with gliomas was reported as high as 27.7–50% (9, 10). The risk factors for postoperative venous thromboembolism and cerebral ischemia following brain tumor surgery included increased age (5, 7, 11, 12), prolonged operation time (5, 7, 11), abnormal preoperative coagulation function (5, 7), tumor size and location (10, 13), and intraoperative blood loss (10, 14).
Fresh frozen plasma is required to improve hemostasis when facing coagulation factors deficiency induced by excessive bleeding. However, fresh frozen plasma may be aggressively administered in some circumstances to reduce the massive bleeding in the operation field, although the volume of blood loss maybe even <20% of total blood volume, which predominantly occurred during malignant brain tumor resection. The balance between the risks and benefits of aggressive fresh frozen plasma administration during neurosurgery is not clear. One study reported that intraoperative fresh frozen plasma infusion increased the risk of postoperative deep vein thrombosis in patients undergoing extramedullary spinal masses resections (15), and the median estimated blood loss was higher in patients who received fresh frozen plasma transfusion. However, the impact of the aggressive use of fresh frozen plasma on postoperative thrombosis-related complications in malignant brain tumors remains unclear.
Therefore, we conducted this retrospective, observational study to explore the risk factors for thrombosis-related complications in patients who underwent malignant brain tumor resections.
Materials and methods
Study design and participants
This retrospective, observational cohort study involved patients who underwent elective intracranial malignant tumor resection from 26 November 2018 to 1 September 2021, at Beijing Tiantan Hospital, Capital Medical University. The study protocol was approved by the Medical Ethics Committee of Beijing Tiantan Hospital, Capital Medical University (KY2021-173-02, date of approval: 16 December 2021). Given the retrospective nature, the ethics committee waived the need for written informed consent. The article adhered to the applicable Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) standards for observational studies (16). Inclusion criteria were adult patients (≥18 years) and elective resection of intracranial malignant tumor, including World Health Organization (WHO) grade III-IV glioma, metastatic carcinoma, lymphoma, and atypical meningioma. Exclusion criteria included recurrent tumors, preoperative radiotherapy or chemotherapy, and postoperative coagulation measurement unavailable.
Data collection
Data were obtained from the medical records. The variables included age, gender, body mass index, blood type (ABO and Rh), preoperative comorbidities (diabetes, hypertension, hyperlipidemia, myocardial infarction, and cerebral infarction), leg paresis, preoperative Karnofsky Performance Status (KPS), intraoperative fluid volume (total fluid volume, crystal fluid, colloid fluid, urine volume, estimated blood loss, red blood cells, fresh frozen plasma, and platelet), duration of surgery, surgical position, perioperative coagulation-related medication (human fibrinogen, hemocoagulase, etc.), postoperative complications (pneumonia, hepatic dysfunction, renal dysfunction, central nervous system infection, anemia, and electrolyte disorder), pathological types of tumor, tumor WHO classification, admission to intensive care unit (ICU), duration of ICU stay, duration of hospital stay, and medical cost.
We also collected information about coagulation factors preoperatively and on postoperative day 1; these included fibrinogen degradation product (FDP), D-dimer, prothrombin time (PT), international standardized ratio (INR), activated partial thromboplastin time (APTT), and fibrinogen. We defined abnormal coagulation function as a coagulation index beyond the clinically recommended normal reference range.
Anesthesia procedure
Routine monitoring included non-invasive blood pressure, electrocardiography, pulse oxygen saturation, end-tidal carbon dioxide partial pressure, and body temperature. Anesthesia was induced with sufentanil, rocuronium or cis-atracurium, and propofol or etomidate and maintained with propofol, sevoflurane, and remifentanil. Analgesia was supplemented with sufentanil to attenuate the potential pain stimuli, during skull pin fixation, scalp incision, and dura suture.
After tracheal intubation, the tidal volume was fixed at 6–8 ml/kg, and the respiratory rate was adjusted to maintain end-tidal carbon dioxide partial pressure at 30–35 cm H2O. The standard for blood transfusion protocol at our institution is based on the NICE guidelines (17). Aggressive fresh frozen plasma infusion is defined as fresh frozen plasma transfusion at an estimated blood loss of <20% of total blood volume. Blood volume was calculated using the Gilcher quintile method, which estimated that a male patient has a blood volume of 70 ml/kg, while a female patient has a blood volume of 65 ml/kg (17, 18).
Outcomes
The primary outcome included those as follows: (1) postoperative deep vein thrombosis, assessed by a lower extremity venous ultrasonography manifesting an intravascular hypoechoic mass or a moderate echoic mass; (2) postoperative cerebral ischemia, located far from the tumor-surgery site, assessed by magnetic resonance imaging examination manifesting a hypertensive signal on the diffusion-weighted image or a restricted diffusion area on the apparent diffusion coefficient maps; (19) and (3) pulmonary embolism defined as a filling defect in the pulmonary artery found by computed tomography pulmonary angiogram. The study did not include diffusion restrictions related to the operative field and the edema zone in postoperative cerebral ischemia. The outcomes were collected before discharge.
The secondary outcomes included postoperative non-thrombosis complications (myocardial infarction and arrhythmias, pneumonia, abnormal liver and renal functions, central nervous system infection, anemia, and electrolyte disturbances, as defined in Supplementary Table 1), duration of stay in hospital and ICU, and hospitalization cost.
Statistical analysis
The continuous normal and skewed distribution variables were reported as mean (standard deviation, SD) and median (interquartile range, IQR), respectively. The categorical variables were expressed as count (percent). Patients were divided into two groups according to whether they exhibited postoperative deep vein thrombosis or cerebral ischemia. The difference in normally distributed variables and outcomes between groups was compared using the independent sample t-test, while skewed variables were compared using the Mann–Whitney U-test. The chi-square or Fisher exact test was used for categorical variables.
Multivariable logistic regression models were used to assess the association between intraoperative infusion of fresh frozen plasma and thrombosis-related complications. Confounders were defined as the statistically (P < 0.10 in the univariable analysis) and clinically significant variables. The Hosmer–Lemeshow goodness-of-fit test and the area under the curve were used to assess the model fit. The variable inflation factor was used to assess the correlation between predictors. The results were expressed as odds ratio (OR) and 95% confidence interval (CI). For all outcomes, a p-value of ≤0.05 was considered to be statistically significant. Statistical software package SPSS Statistics (version 23.0) was used for analysis.
Results
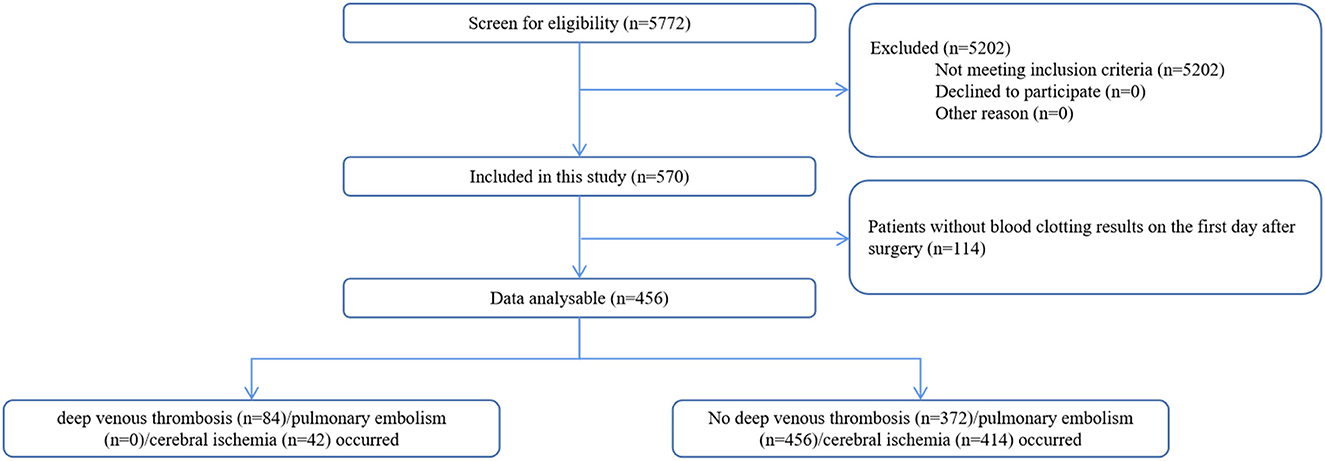
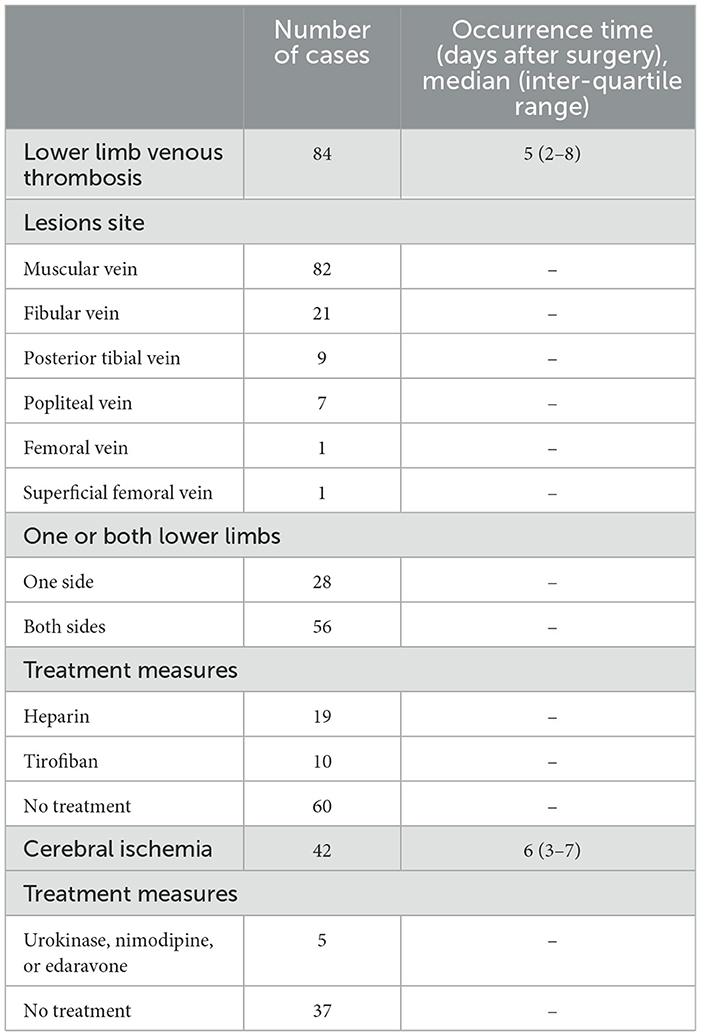
Between 26 November 2018 and 1 January 2021, a total of 456 patients with elective intracranial malignant tumor resections were included in this study (Figure 1). Overall, thrombosis-related complications occurred in 112 out of 456 (24.6%) patients. Deep vein thrombosis was present in 84 out of 456 (18.4%) patients, and 42 out of 456 patients (9.2%) had cerebral ischemia. No patients had definite pulmonary embolism on postoperative computed tomography pulmonary angiogram. The characteristics of the postoperative thrombosis-related complications are presented in Table 1.
The use of fresh frozen plasma was significantly higher in patients with postoperative cerebral ischemia (16.7 vs. 4.8%, P = 0.02) while urine volume was lower (4.14 vs. 5.04 mL/kg/h, P = 0.018) (see Supplementary Table 2).
For patients with postoperative deep vein thrombosis, the incidence of postoperative pneumonia, anemia, and electrolyte disturbances significantly increased (P < 0.05), as well as the proportion of ICU admission, the duration of ICU stays and hospital stay, and overall cost were all significantly higher in patients who had deep vein thrombosis (P < 0.05, see Table 2). Furthermore, patients with postoperative coagulation function disorder, including increased FDP and D-dimer and decreased APTT, also had a significantly increased incidence of DVT (P < 0.05).
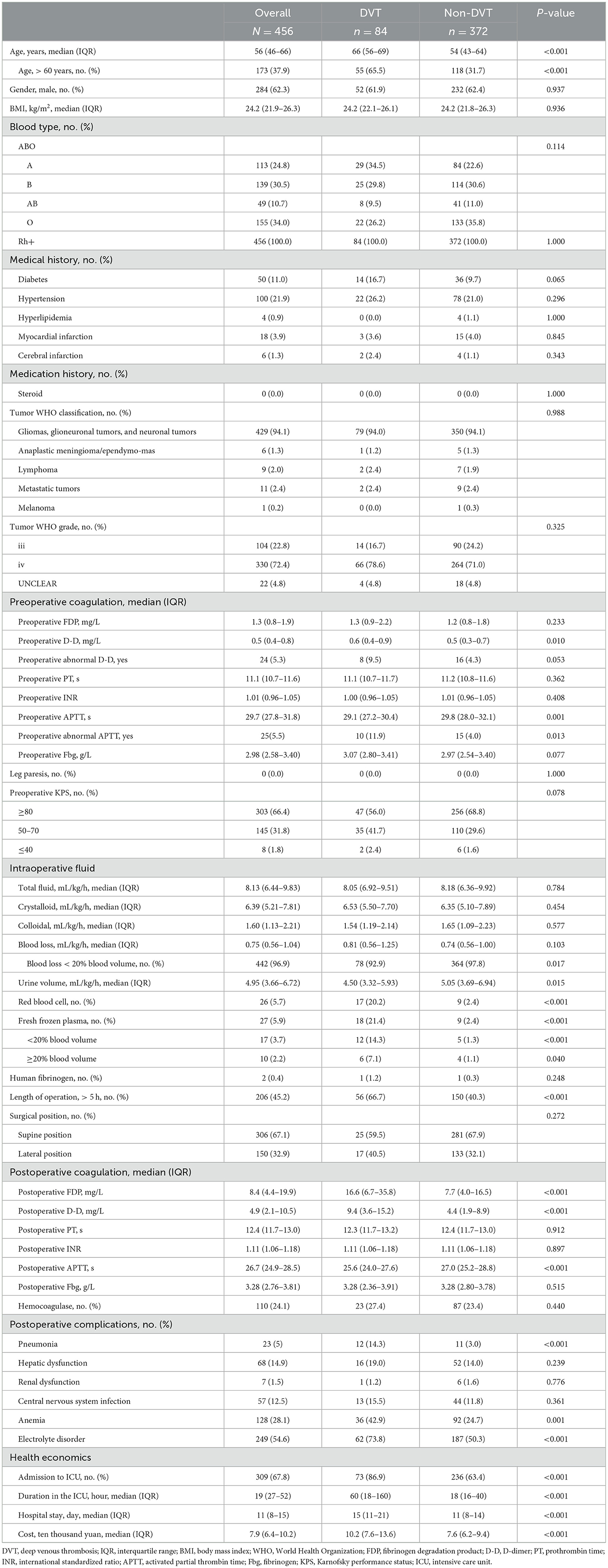
Table 2. Demographics, baseline values, and perioperative variables group by deep venous thrombosis.
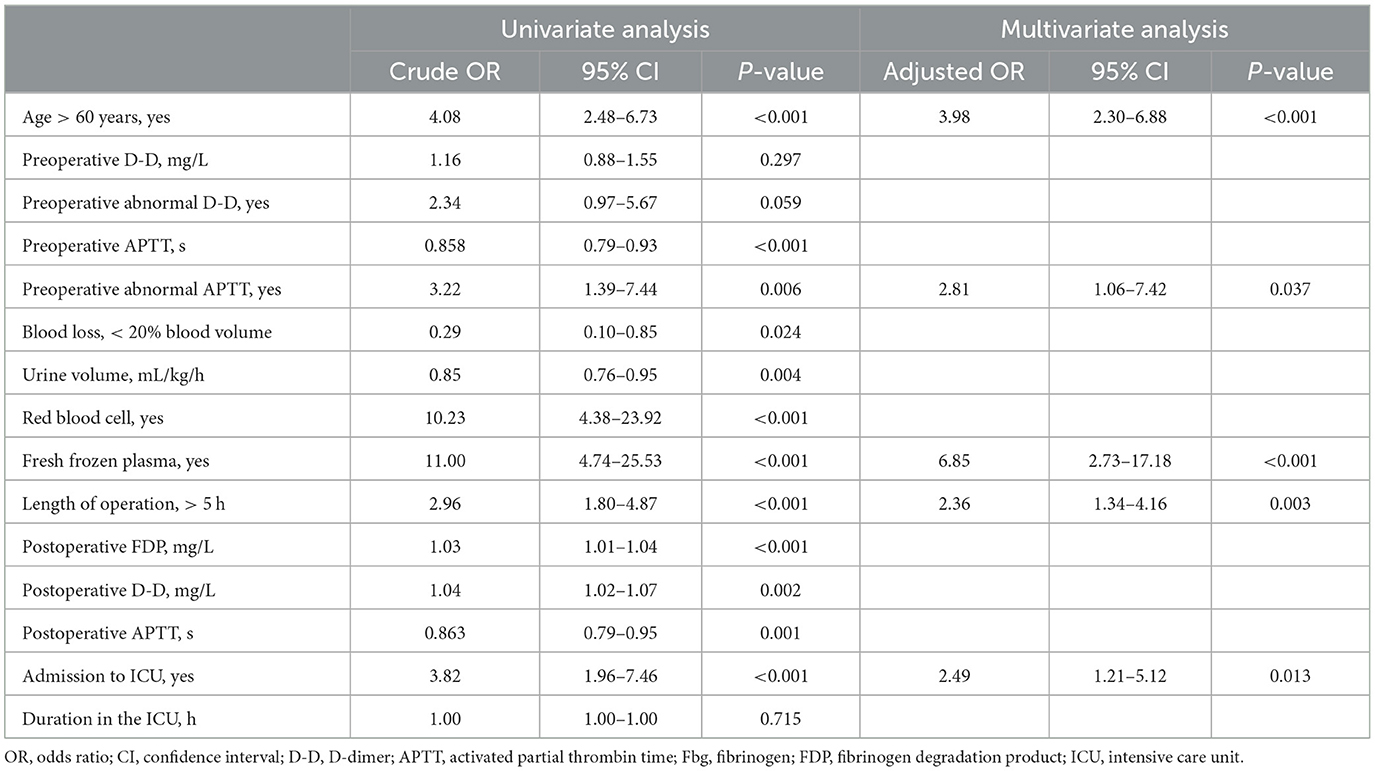
The demographics, baseline values, and perioperative variables are illustrated in Table 2. Patients older than 60 years or with preoperative abnormal APTT had a significantly higher incidence of DVT (P < 0.05). For patients with DVT, red blood cells, fresh frozen plasma transfusion or surgery duration longer than 5 h were significantly higher than those without (P < 0.05). The univariate and multivariate analyses for deep vein thrombosis are presented in Table 3. Finally, age more than 60 years (OR: 4.08, 95% CI: 2.48–6.73, P < 0.001), preoperative abnormal APTT (OR: 3.22, 95% CI: 1.39–7.44, P = 0.006), fresh frozen plasma used during the operation (OR: 11.0, 95% CI: 4.74–25.53, P < 0.001), duration of operation over 5 h (OR: 2.96, 95% CI: 1.80–4.87, P < 0.001), and admission to ICU (OR: 3.82, 95% CI: 1.96–7.46, P < 0.001) were independently associated with the incidence of deep vein thrombosis.
The multivariate model indicated that intraoperative fresh frozen plasma transfusion (OR: 6.85, 95% CI: 2.73–17.18, P < 0.001) significantly increased the odds of the incidence of outcomes adjusted by the previously mentioned factors (Table 3). While assessing the model fit, the models demonstrated an average area under the curve of 0.78 (95% CI: 0.73–0.84, P < 0.001, see Figure 2).

Figure 2. ROC curve of the risk factors associated with deep venous thrombosis. ROC, receiver operating characteristic curve; AUC, area under the curve. The blue curve represents the ROC curve of the multi-factor analysis model for postoperative risk factors associated with deep venous thrombosis. The AUC was 0.78 (95% CI: 0.73–0.84, P < 0.001).
Discussion
For patients who underwent brain malignant tumors resection, age more than 60 years, fresh frozen plasma infusion, preoperative abnormal APTT, surgery duration longer than 5 h, or admission to ICU were significantly associated with the DVT.
In previous studies, the incidence of postoperative deep venous thromboembolism in patients who underwent brain tumor surgery has been reported to vary from 10.3 to 21.3% (3–7, 12). The DVT incidence in our study is 18.4%, which was consistent with a previous study that investigated 263 patients with high-grade gliomas (6). Recently, Shi et al. (7) measured the frequency of postoperative DVT in 373 high-grade glioma patients and reported an incidence of 16.1%. They only examined patients who exhibited symptoms or had increased D-dimer levels with ultrasound which might ignore asymptomatic patients with venous thrombosis. The incidence of postoperative DVT was found to be lower in patients with all types of brain tumors, and it ranged from 10.3 to 12.3% (3, 7, 12). One retrospective study reported that nine (0.54%) patients developed postoperative pulmonary embolism in 1,670 patients undergoing brain tumor surgery (7). Another retrospective study identified 107 (1.5%) postoperative pulmonary embolisms in 7,276 patients undergoing brain tumor surgery (8). However, no patients with postoperative pulmonary embolism were reported in our study. This difference may be due to the following reasons. We may fail to identify those patients with small pulmonary embolisms and mild symptoms which would be detected by computed tomography pulmonary angiogram. As computed tomography pulmonary angiogram was not routine in daily clinical practice, the incidence of pulmonary embolisms might be underestimated, which is also contributed by the relatively small sample size. Meanwhile, in previous reports, the incidence of perioperative cerebral ischemia in patients with glioma was reported to be 27.7–50% (9, 10), which included surgical cavity-related restricted diffusion. The incidence of cerebral ischemia in the current study was 9.2%, which was much lower than the previous study because we excluded the surgical cavity-related restricted diffusion.
For patients who underwent intracranial tumor resection, the mechanism of cerebral ischemia is more complicated. Vasospasms and blood vessel damage during surgery can cause local or extensive ischemia (13, 20). In patients with malignant brain tumors, in addition to the tumor compression of peripheral blood vessels or tumor blood steal (21, 22), cerebral ischemia may additionally be caused by tumor-induced inflammatory factors released into the periphery during tumor growth. However, the surgical procedure and inflammatory factors-related ischemia usually exist within or around the surgical field (23). To avoid interference with the surgical procedure, we defined postoperative cerebral ischemia as the one that is remote from the surgical cavity, excluding peritumoral ischemia. Therefore, surrounding vascular structures and the surgical approach were not selected for the model.
Preoperative lower extremity venous thrombosis is a high-risk factor for postoperative DVT. Ultrasound examination should be performed in patients with preoperative elevated D-dimer or a high risk of DVT (24). Among the 456 enrolled patients, 24 patients had preoperative abnormal D-dimer, of these four had a preoperative lower extremity venous ultrasound examination. DVT was confirmed in two patients as thrombosis progressed during postoperative reexamination of lower extremity venous ultrasound. Because preoperative venous ultrasonography was not performed routinely in our center, we were unable to detect the association between preoperative ultrasonography and postoperative DVT.
Due to the large size of the tumor, rich blood supply, and long operation time, it would be inevitable that the amount of intraoperative blood loss or extensive bleeding in the operative field would increase. Surgeons generally advocate the supplementation of fresh frozen plasma to reduce bleeding. Fresh frozen plasma contains all coagulation factors and is often used to supplement the deficiency of coagulation factors due to massive blood loss. However, over-aggressive infusion of fresh frozen plasma, such as using fresh frozen plasma when the bleeding volume is below the transfusion standard, may increase the risk of thrombosis. Our study indicated that for patients undergoing surgery for malignant craniocerebral tumors, intraoperative infusion of fresh frozen plasma significantly increased the incidence of thrombosis-related complications, when blood loss was less than 20% of blood volume. In our study, patients who received plasma infusion had significantly lower postoperative APTT (25.3 vs. 26.8 s, P = 0.014), suggesting the risk of postoperative hypercoagulability in transfused plasma. Our findings are consistent with the study of Kaewborisutsakul et al. (15), who examined 103 patients with extramedullary tumors undergoing surgical treatment and found that intraoperative use of fresh frozen plasma significantly increased the risk of postoperative thrombotic events (hazard ratio: 16.38, 95% CI: 1.47–182.23).
Hemocoagulase is often used to reduce the risk of hematoma after neurosurgery. It shortens the bleeding time with hemostatic function and does not affect the amount of prothrombin. However, hemocoagulase has been reported to have a tendency to increase the risk of deep venous thrombosis after abdominal surgery, although the difference was not statistically significant (23.3 vs. 10.0%, P > 0.05) (25). In our study, we did not find any impact of hemocoagulase use on postoperative thrombosis-related complications. In addition, human fibrinogen also affects coagulation function (26). In our cohort, only two patients were administered with human fibrinogen, so we could not draw any conclusion.
Patients with brain tumors may be in a hypercoagulable state. Brain parenchyma contains high expression levels of the tissue factor, a recognized procoagulant (27, 28). Inflammation and damage to the brain parenchyma may lead to the release of clotting agents in the blood of brain tumors. These clotting factors cause hypercoagulation throughout the body and increase the risk of developing blood clots. In our study, 25 (5.5%) patients already had APTT shortened preoperatively, representing endogenous coagulation abnormalities and the prethrombotic state. Our study indicates that patients with preoperative shortened APTT are more likely to develop deep vein thrombosis. At the same time, patients with deep vein thrombosis also had significantly shortened APTT after surgery, accompanied by significant increases in FDP and D-dimer. However, thrombosis-related complications may have started during postoperative coagulation examination, so the measurement of postoperative coagulation function could not be regarded as a risk factor or enter the model for the prediction of postoperative deep vein thrombosis.
Several studies have confirmed that the risk of postoperative deep vein thrombosis increases significantly with age (5, 11, 12). In our study, elderly patients (≥60 years old) were at a high risk of postoperative deep vein thrombosis. The duration of the operation also affects the incidence of postoperative deep vein thrombosis. A retrospective study found that surgical duration > 350 min was significantly associated with an increased risk of postoperative deep vein thrombosis (OR: 1.82, 95% CI: 1.27–2.60, p = 0.001) (7). In our cohort, we set the cutoff value at 5 h based on the median surgical duration of patients (4.8 h), and the results showed that surgery longer than 5 h significantly increased the odds of deep vein thrombosis, which is consistent with previous studies. Patients with brain malignant tumors often exhibit severe brain edema which is frequently treated with corticosteroids (29). However, some studies have shown that corticosteroids significantly increase the risk of postoperative venous thrombosis (30). Alternatively, mannitol is routinely administered to reduce cerebral edema in our center, and none of the patients we enrolled had long-term use of corticosteroids.
Our study has certain limitations. First, the enrolled patients were relatively healthier, with few preoperative complications. Second, as an observational study, it is impossible to determine the causal association. Third, this study only focused on coagulation parameters on the first postoperative day, more extended tracking of coagulation function is desirable for future studies. Finally, we failed to detect the risk of cerebral ischemia caused by thrombus detachment or paradoxical embolism through routine ultrasonography screening of the lower extremity veins or echocardiography. Similar to cerebral angiography, the preexisting brain arterial stenosis or occlusion that contributed to cerebral ischemia was not evaluated.
In summary, patients with malignant brain tumors have a high incidence of postoperative deep vein thrombosis and cerebral ischemia. Advanced age, preoperative abnormal APTT, surgery duration of more than 5 h, and admission to ICU were the independent risk factors of deep vein thrombosis. At the same time, intraoperative plasma infusion was associated with postoperative deep vein thrombosis and cerebral ischemia and therefore it should be cautiously administered. Perioperative monitoring should be intensified if fresh frozen plasma is indeed required. A further prospective study is needed to explore the influence of blood products on thrombosis-related complications after malignant brain tumor resection.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of Beijing Tiantan Hospital, Capital Medical University (KY2021-173-02). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
YP designed the study. XZ, XL, MZ, and TM participated in data collection and manuscript preparation. XZ, ML, SL, LZ, and YP conducted the analysis. XZ, IK, and YP contributed to manuscript preparation. All authors reviewed and approved the manuscript.
Funding
This study was supported by the Ministry of Science and Technology of the People's Republic of China funding (2018YFC2001901) and the Beijing Municipal Science and Technology Commission (Z191100006619068).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1108596/full#supplementary-material
References
1. Yust-Katz S, Mandel JJ, Wu J, Yuan Y, Webre C, Pawar TA, et al. Venous thromboembolism (Vte) and glioblastoma. J Neurooncol. (2015) 124:87–94. doi: 10.1007/s11060-015-1805-2
2. Edwin NC, Elson P, Ahluwalia MS, Khorana AA. venous thromboembolism in patients with glioblastoma: risk factors and prognostic importance. J Clin Oncol. (2015) 33(15_suppl.):e13027. doi: 10.1200/jco.2015.33.15_suppl.e13027
3. Rethinasamy R, Alias A, Kandasamy R, Raffiq A, Looi MC, Hillda T. Deep vein thrombosis and the neurosurgical Patient. Malays J Med Sci. (2019) 26:139–47. doi: 10.21315/mjms2019.26.5.13
4. Nakano F, Matsubara T, Ishigaki T, Hatazaki S, Mouri G, Nakatsuka Y, et al. Incidence and risk factor of deep venous thrombosis in patients undergoing craniotomy for brain tumors: a Japanese single-center, retrospective study. Thromb Res. (2018) 165:95–100. doi: 10.1016/j.thromres.2018.03.016
5. Shi S, Cheng J, Zhao Y, Chen W. Incidence, and preoperative and intraoperative prognostic factors of deep venous thrombosis in patients with glioma following craniotomy. Clin Neurol Neurosurg. (2021) 210:106998. doi: 10.1016/j.clineuro.2021.106998
6. Smith TR, Nanney AD 3rd, Lall RR, Graham RB, McClendon J Jr, Lall RR, et al. Development of venous thromboembolism (vte) in patients undergoing surgery for brain tumors: results from a single center over a 10 year period. J Clin Neurosci. (2015) 22:519–25. doi: 10.1016/j.jocn.2014.10.003
7. Shi S, Cheng J, Chen H, Zhang Y, Zhao Y, Wang B. Preoperative and intraoperative predictors of deep venous thrombosis in adult patients undergoing craniotomy for brain tumors: a chinese single-center, retrospective study. Thromb Res. (2020) 196:245–50. doi: 10.1016/j.thromres.2020.09.005
8. Senders JT, Muskens IS, Cote DJ, Goldhaber NH, Dawood HY, Gormley WB, et al. Thirty-day outcomes after craniotomy for primary malignant brain tumors: a national surgical quality improvement program analysis. Neurosurgery. (2018) 83:1249–59. doi: 10.1093/neuros/nyy001
9. Jakola AS, Berntsen EM, Christensen P, Gulati S, Unsgård G, Kvistad KA, et al. Surgically acquired deficits and diffusion weighted mri changes after glioma resection–a matched case-control study with blinded neuroradiological assessment. PLoS ONE. (2014) 9:e101805. doi: 10.1371/journal.pone.0101805
10. Strand PS, Berntsen EM, Fyllingen EH, Sagberg LM, Reinertsen I, Gulati S, et al. Brain infarctions after glioma surgery: prevalence, radiological characteristics and risk factors. Acta Neurochir. (2021) 163:3097–108. doi: 10.1007/s00701-021-04914-z
11. Senders JT, Goldhaber NH, Cote DJ, Muskens IS, Dawood HY, De Vos F, et al. Venous thromboembolism and intracranial hemorrhage after craniotomy for primary malignant brain tumors: a national surgical quality improvement program analysis. J Neurooncol. (2018) 136:135–45. doi: 10.1007/s11060-017-2631-5
12. Guo X, Zhang F, Wu Y, Gao L, Wang Q, Wang Z, et al. Coagulation alteration and deep vein thrombosis in brain tumor patients during the perioperative period. World Neurosurg. (2018) 114:e982–e91. doi: 10.1016/j.wneu.2018.03.128
13. Gempt J, Förschler A, Buchmann N, Pape H, Ryang YM, Krieg SM, et al. Postoperative ischemic changes following resection of newly diagnosed and recurrent gliomas and their clinical relevance. J Neurosurg. (2013) 118:801–8. doi: 10.3171/2012.12.JNS12125
14. Lange N, Urich J, Barz M, Aftahy K, Wagner A, Albers L, et al. Metabolic parameters influence brain infarction and outcome after resection of brain metastases. Cancers. (2020) 12:1127. doi: 10.3390/cancers12051127
15. Kaewborisutsakul A, Tunthanathip T, Yuwakosol P, Inkate S, Pattharachayakul S. Postoperative venous thromboembolism in extramedullary spinal tumors. Asian J Neurosurg. (2020) 15:51–8. doi: 10.4103/ajns.AJNS_279_19
16. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (strobe) statement: guidelines for reporting observational studies. Ann Intern Med. (2007) 147:573–7. doi: 10.7326/0003-4819-147-8-200710160-00010
17. Padhi S, Kemmis-Betty S, Rajesh S, Hill J, Murphy MF. Blood transfusion: summary of nice guidance. Bmj. (2015) 351:h5832. doi: 10.1136/bmj.h5832
18. R.O. G. Apheresis: principles and practices. In: Rossi EC, Moss GS, Gould SA, editors. Principles of Transfusion Medicine. Baltimore, MD: Williams and Wilkins (1996). p. 537–46.
19. Leiva-Salinas C, Wintermark M, Kidwell CS. Neuroimaging of cerebral ischemia and infarction. Neurotherapeutics. (2011) 8:19–27. doi: 10.1007/s13311-010-0004-2
20. Kumar G, Dumitrascu OM, Chiang CC, O'Carroll CB, Alexandrov AV. Prediction of delayed cerebral ischemia with cerebral angiography: a meta-analysis. Neurocrit Care. (2019) 30:62–71. doi: 10.1007/s12028-018-0572-2
21. Ghosh MK, Chakraborty D, Sarkar S, Bhowmik A, Basu M. the interrelationship between cerebral ischemic stroke and glioma: a comprehensive study of recent reports. Signal Transduct Target Ther. (2019) 4:42. doi: 10.1038/s41392-019-0075-4
22. Strand PS, Sagberg LM, Gulati S, Solheim O. Brain infarction following meningioma surgery-incidence, risk factors, and impact on function, seizure risk, and patient-reported quality of life. Neurosurg Rev. (2022) 45:3237–44. doi: 10.1007/s10143-022-01840-1
23. Berger A, Tzarfati G, Costa M, Serafimova M, Korn A, Vendrov I, et al. Incidence and impact of stroke following surgery for low-grade gliomas. J Neurosurg. (2021) 134:153–61. doi: 10.3171/2019.10.JNS192301
24. Key NS, Khorana AA, Kuderer NM, Bohlke K, Lee AYY, Arcelus JI, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: asco clinical practice guideline update. J Clin Oncol. (2020) 38:496–520. doi: 10.1200/JCO.19.01461
25. Zeng ZY, Chen XS. Impact of hemocoagulase on coagulatory function and deep venous thrombosis after abdominal surgery. Zhonghua Wei Chang Wai Ke Za Zhi. (2012) 15:353–6. doi: 10.3760/cma.j.issn.1671-0274.2012.04.012
26. Shams Hakimi C, Singh S, Hesse C, Jeppsson A. Effects of fibrinogen and platelet transfusion on coagulation and platelet function in bleeding cardiac surgery patients. Acta Anaesthesiol Scand. (2019) 63:475–82. doi: 10.1111/aas.13295
27. Chaichana KL, Pendleton C, Jackson C, Martinez-Gutierrez JC, Diaz-Stransky A, Aguayo J, et al. Deep venous thrombosis and pulmonary embolisms in adult patients undergoing craniotomy for brain tumors. Neurol Res. (2013) 35:206–11. doi: 10.1179/1743132812Y.0000000126
28. Ornstein DL, Meehan KR, Zacharski LR. The coagulation system as a target for the treatment of human gliomas. Semin Thromb Hemost. (2002) 28:19–28. doi: 10.1055/s-2002-20561
29. Schramm MWJ, Currie S, Lee MT, Livermore LJ, Solanki SP, Mathew RK, et al. Do animal models of brain tumors replicate human peritumoral edema? A systematic literature search. J Neurooncol. (2023) 161:451–67. doi: 10.1007/s11060-023-04246-1
Keywords: malignant brain tumors, thrombosis, cerebral ischemia, deep vein thrombosis (DVT), fresh frozen plasma (FFP)
Citation: Liu X, Zhang X, Ma T, Li M, Zhang L, Li S, Zeng M, Kass IS and Peng Y (2023) Risk factors for postoperative thrombosis-related complications in patients undergoing malignant brain tumor resection: a retrospective cohort study. Front. Neurol. 14:1108596. doi: 10.3389/fneur.2023.1108596
Received: 26 November 2022; Accepted: 27 March 2023;
Published: 18 April 2023.
Edited by:
Teresa Somma, Federico II University Hospital, ItalyReviewed by:
Domenico Solari, Federico II University Hospital, ItalyVincenzo Meglio, Federico II University Hospital, Italy
Copyright © 2023 Liu, Zhang, Ma, Li, Zhang, Li, Zeng, Kass and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuming Peng, ZmxvcmFweW03NjZAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Xiaoyuan Liu
Xiaoyuan Liu Xingyue Zhang
Xingyue Zhang Tingting Ma
Tingting Ma Muhan Li1
Muhan Li1 Ira S. Kass
Ira S. Kass Yuming Peng
Yuming Peng