- 1Research Center of Health Policy and Innovation, Jiangxi Science and Technology Normal University, Nanchang, Jiangxi, China
- 2School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
- 3Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases of Ministry of Education, Gannan Medical University, Ganzhou, Jiangxi, China
- 4School of Public Health and Health Management, Gannan Medical University, Ganzhou, Jiangxi, China
- 5School of Economics and Management, East China Jiaotong University, Nanchang, Jiangxi, China
Introduction: Epidemiological studies have shown that tooth loss may be associated with an increased risk of cognitive decline and dementia. However, some results do not show a significant association. Therefore, we performed a meta-analysis to evaluate this association.
Methods: Relevant cohort studies were searched in PubMed, Embase, Web of Science (up to May 2022), and the reference lists of retrieved articles. The pooled relative risk (RR) and 95% confidence intervals were computed using a random-effects model (CI). Heterogeneity was evaluated using the I2 statistic. Publication bias was evaluated using the Begg's and Egger's tests.
Results: Eighteen cohort studies met the inclusion criteria. Original studies with 356,297 participants with an average follow-up of 8.6 years (ranging from 2 to 20 years) were included in this study. The pooled RRs of tooth loss on dementia and cognitive decline were 1.15 (95% CI: 1.10–1.20; P < 0.01, I2 = 67.4%) and 1.20 (95% CI: 1.14–1.26; P = 0.04, I2 = 42.3%), respectively. The results of the subgroup analysis showed an increased association between tooth loss and Alzheimer's disease (AD) (RR = 1.12, 95% CI: 1.02–1.23) and vascular dementia (VaD) (RR = 1.25, 95% CI: 1.06–1.47). The results of the subgroup analysis also showed that pooled RRs varied by geographic location, sex, use of dentures, number of teeth or edentulous status, dental assessment, and follow-up duration. None of the Begg's and Egger's tests or funnel plots showed evidence of publication bias.
Discussion: Tooth loss is associated with a significantly increased risk of cognitive decline and dementia, suggesting that adequate natural teeth are important for cognitive function in older adults. The likely mechanisms mostly suggested include nutrition, inflammation, and neural feedback, especially deficiency of several nutrients like vitamin D.
1. Introduction
Dementia, also known as severe neurocognitive disorder, describes a group of disorders characterized by very poor cognitive function that often affects older people (1). The most common forms are AD and vascular dementia (VaD), which are caused by stroke and other cerebrovascular diseases. Cognitive decline is not the only key symptom of dementia; it has been shown that in many patients it may exist long before the diagnosis of dementia (2, 3). Cognitive decline and dementia lead to impaired ability to perform basic activities of daily living, resulting in a tremendous disease burden and economic burden. According to the World Health Organization, the number of dementia patients currently stands at about 55 million and is increasing by nearly 10 million annually, resulting in care costs of more than $1 trillion worldwide (4, 5). Current treatments for dementia have no curative effect, whereas early prevention, diagnosis, and intervention may delay or even prevent disease progression. Although dementia is not an inevitable part of aging, age is clearly the greatest known risk factor. However, knowledge of risk factors for dementia and cognitive decline is currently limited. More attention should be paid to identifying modifiable risk factors.
Tooth loss is another public health problem in older adults worldwide (6). Recently, interest in the relationship between tooth loss and cognitive decline and dementia has increased. Kondo et al. (7) conducted a case-control study in Japan in 1994 to assess lifestyle factors and found that tooth loss was a significant risk factor for AD. Several types of observational studies have also reported that tooth loss is associated with increased prevalence or incidence of dementia (8). However, negative results have been reported (9). Additionally, tooth loss has also been shown to be related to systemic health, such as stroke (10), obesity (11), cardiovascular diseases (12), cancers (13), and mental illnesses (14, 15). This implies complicated mechanisms between tooth loss and health outcomes, including cognitive damage.
Several meta-analyses have also been conducted. In 2016, Shen et al. (16) published a meta-analysis of six cohort studies, three cross-sectional studies, and two case-control studies to examine the association between tooth loss and the incidence of dementia (odds ratio [OR] = 1.43; 95% CI: 1. 26–1.63). In the same year, a meta-analysis by Cerutti-Kopplin et al. (17) found a greater than 20% increased risk of developing cognitive decline with a hazard ratio (HR) of 1.26 (95% CI: 1.14–1.40) and dementia (HR = 1.22, 95% CI: 1.04–1.43) for people with suboptimal dentition (<20 teeth) based on eight cohort studies. Three meta-studies conducted in 2018 showed similar positive results from all types of observational studies (8) or cohort studies, only (18, 19). The five reviews had limitations, including insufficient (8, 16–19) or poor-qualified studies (19), or unrestricted cohort studies (8, 16). Since then, many new cohort studies have emerged looking at the risks of tooth loss to cognitive decline and dementia (20–23). Therefore, we conducted an updated analysis to further investigate the association between tooth loss and risk of cognitive decline and dementia using well-qualified large sample cohort studies that would provide evidence-based recommendations and guidance for a public health response to dementia.
2. Materials and methods
2.1. Search strategy
This meta-analysis was not registered and carried out in accordance with the checklist of the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines and not registered (24) Two researchers separately searched the PubMed, Embase, Web of Science, and China National Knowledge Infrastructure (CNKI) databases from their inception to May 2022 for pertinent studies published in any language. We used the following keywords “oral health,” “dental loss,” “tooth loss,” “periodontitis,” “edentulous,” “missing tooth,” “dental care,” “denture,” “oral disease,” or “poor tooth health” and “dementia,” “Alzheimer's disease,” “AD,” “cognitive impairment,” “mild cognitive impairment,” “MCI,” “cognitive decline,” “neurocognitive disorder,” “cognitive disorder,” “memory disorder,” “vascular dementia,” or “VaD” along with “cohort studies,” “follow-up studies,” “prospective studies,” or “longitudinal studies” (see Supplementary material for details). Additionally, all identified pertinent publications' reference lists were examined.
2.2. Inclusion and exclusion criteria
Two investigators independently assessed each cohort study's eligibility, and any disagreements were settled by consultation with a third investigator. Studies that met the following criteria were included in this meta-analysis: (1) tooth loss served as the exposure, and dementia and its subtypes or cognitive decline served as the outcome; (2) the study design was a cohort study, including nested case-control studies with a prospective design or a retrospective cohort study; and (3) the study provided HR or relative risk (RR) with corresponding 95% CI for the association between tooth loss and dementia and/or cognitive decline.
Studies were excluded if any of the following conditions were met: (1) the study was not published as full reports, such as comments, communication, conference abstracts, and letters to editors; (2) participants were patients with dementia at baseline; (3) the study design was cross-sectional or case-control; and (4) the study was not HR/RR or lacked sufficient data to calculate them.
2.3. Data extraction
Two researchers independently collected data from the included studies and discussed the discrepancies; then an entire research group reviewed and confirmed the data. The following information was extracted from the included studies: first author(s) name, year of publication, sex, sample, study name, country, type, baseline age, tooth loss, denture wearing, assessment method, outcome and assessment, sample size, follow-up period, and covariates adjusted for in statistical analysis.
2.4. Quality assessment
Utilizing three criteria from the Newcastle-Ottawa scale for cohort studies independently, two researchers evaluated the quality assessment of the meta-analysis (25). Scores of 0–3, 4–6, and 7–9 were assigned for low, moderate, and high quality of studies, respectively. By discussing with a third investigator, disagreements on quality assessment were resolved.
2.5. Statistical analysis
RR is regarded as a typical measure of the relationship between tooth loss and the risk of dementia and cognitive decline. We preferentially pooled multivariable adjusted risk estimates when such estimates were reported. We combined the unadjusted estimates in the absence of an adjusted analysis. For studies that reported outcomes separately for different age groups, education levels, dental conditions, or dementia types, we combined the estimates using a fixed-effects model to obtain an overall estimate that was combined in the primary meta-analysis (26). To examine the source of heterogeneity in the primary outcomes, subgroup analyses were conducted, stratified by geographic location, sex, use of dentures, number of teeth, edentulous status, methods of dental assessment, and duration of follow-up. In a sensitive analysis to assess robustness, we performed a leave-one-out analysis to observe the magnitude of the effect on the pooled RR of each study (24).
The I2 statistic was used to assess the statistical heterogeneity among studies, with cut-off values of 25%, 50%, and 75%, respectively, designating low, moderate, and high levels of heterogeneity (26). Potential publication bias was evaluated with a funnel plot, the Begg's test (27), and the Egger's test. Stata statistical software (version 12.0; College Station, TX, USA) was used to perform all statistical analyses. All tests were two-sided, and a statistically significant result was defined as P < 0.05.
2.6. Patient and public involvement statement
Patients and public were not involved in this study.
3. Results
3.1. Literature search
Figure 1 illustrates the study selection process. First, 38,521 articles were reviewed. Of these, 27,552 duplicate articles and 10,911 articles that were ineligible for this review after screening based on title and abstract were removed. Of the remaining 58 articles for full-text review, 31 were excluded based on a cross-sectional design (n = 19) or a case-control design (n = 12). Four studies did not provide sufficient data to calculate, and two studies that had dementia as a risk factor and tooth loss as outcomes were removed. Thus, 21 eligible cohort studies were included in this meta-analysis.
3.2. Characteristics of the study
The 21 included studies (9, 22, 23, 25, 28–44) were published between 2001 (28) and 2022 (43, 44), and characteristics of them are shown in Supplementary Table 1. The sample sizes of the cohorts ranged from 133 (30) to 209,806 (22) with a total of 356,297 and the average of 16,967. The length of follow-up duration ranged from 2 (29) to 20 (38) years in our study, with an average of 8.6 years. Among the 14 studies that reported dementia as outcome (9, 22, 23, 29–31, 33–35, 38, 39, 41–43), six studies also reported risk of tooth loss for AD (23, 29, 30, 39, 41, 42), and three studies reported VaD (23, 39, 41), and one reported comorbidity of AD and VaD (23). A various of dementia assessments were applied, including self- or professional-administrated questionnaires or interviews, medical records, and insurance records. A series of tests were also used, and the Mini-mental State Examination (MMSE) was applied in 11 included studies (25, 29, 30, 32, 33, 35–37, 39, 41, 44) as the most frequently used test, while other tests were only used in several studies. Eight included studies reported results with cognitive decline as outcome (25, 28, 32, 35–37, 40, 44), among which one study reported risks of tooth loss for dementia as well (35). Among these studies, ten studies were from Asia (22, 23, 25, 28, 29, 34, 41–44), six studies from North America (30–33, 36, 37), four studies from Europe (33, 38–40), and one study was conducted in 20 countries including Australasia, Asia, Europe and North America (35). Only four studies reported results for male (32, 33, 43), five studies independently reported results for female (30, 33, 39, 43), and the 13 remaining studies (9, 22, 23, 25, 28, 29, 31, 34–38, 40, 41, 44) did not report results by sex.
Supplementary Table 2 shows the results of the quality assessment. The median quality assessment score for the included cohort studies was 8 [range, 5 (31)−9 (22, 25, 28, 29, 41)]. The interobserver agreement (κ) between the two investigators was 0.95.
3.3. Quantitative synthesis
3.3.1. Association between tooth loss and dementia
An association between tooth loss and dementia risk was found in 12 independent studies, with a pooled RR of 1.15 (95% CI: 1.10–1.20) and moderate heterogeneity (I2 = 67.4%, P < 0.001). Forest plots are shown in Figure 2A.
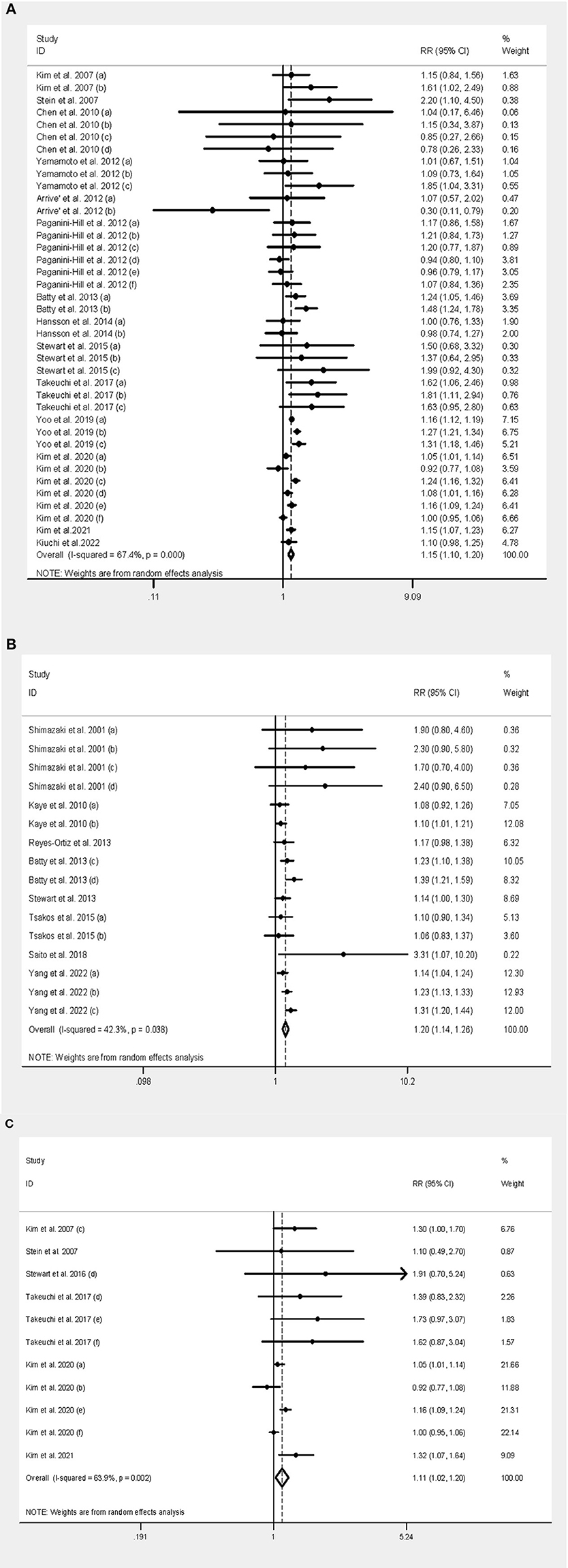
Figure 2. Forest plot of tooth loss and both dementia and cognitive decline. (A) Forest plot of tooth loss and dementia. (B) Forest plot of tooth loss and cognitive decline. (C) Forest plot of tooth loss and AD.
The pooled RR among the Asian population was 1.15 (95% CI: 1.10–1.21), but there was no statistical significance from Europe (RR = 1.06, 95% CI: 0.81–1.38) and North America (RR = 1.03, 95% CI: 0.94–1.13). The pooled RR among mixed-sex population was 1.16 (95% CI: 1.09–1.22), while statistical significance was also found among men (RR = 1.19, 95% CI: 1.09–1.30) and female (RR = 1.14, 95% CI: 1.01–1.29). The pooled RR without the denture use group was 1.70 (95% CI: 1.19–2.41), but there was no significant association in the population using dentures (RR = 1.04, 95% CI: 0.89–1.21). The pooled RR among edentulous people and people with natural teeth was 1.24 (95% CI: 1.06–1.46) and 1.12 (95% CI: 1.06–1.18), respectively. The risk was greater in people with <10 remaining natural teeth (RR = 1.22, 95% CI: 1.11–1.33) than in those with 20 or more teeth (RR = 1.16, 95% CI: 1.13–1.20), while it was not significant in those with 10–19 teeth (RR = 1.04, 95% CI: 0.95–1.13). The pooled RR was 1.16 (95% CI: 1.10–1.22) in studies in which dental assessment was conducted by a dentist, and 1.12 (95% CI: 1.02–1.22) in studies that reported dental status. When dividing studies into ≥5 and <5 years of follow-up, the risk was 1.18 (95% CI: 1.12–1.24) and 1.24 (95% CI: 1.01–1.51), respectively. For the type of dementia, the pooled RR was 1.12 (95% CI: 1.02–1.23) for the association between tooth loss and AD (Figure 2C), and 1.25 (95% CI: 1.06–1.47) for the association between tooth loss and VaD.
3.3.2. Association between tooth loss and cognitive decline
Of the seven independent studies, the pooled RR of cognitive decline was 1.20 (95% CI: 1.14–1.26) with moderate heterogeneity (I2 = 42.3%, P = 0.04) (Figure 2B).
The pooled RR among populations from Asia, North America and mixed countries was 1.26 (95% CI: 1.15–1.38), 1.12 (95% CI: 1.05–1.19) and 1.30 (95% CI: 1.15–1.46), respectively, but not significant in people from Europe (RR = 1.08, 95% CI: 0.93–1.27). The pooled RR among men and both sexes was 1.10 (95% CI: 1.01–1.18) and 1.22 (95% CI: 1.16–1.29), respectively. The pooled RR among those without dentures was 1.26 (95% CI: 1.18–1.34), but no statistical significance was observed among the population using dentures (RR = 1.02, 95% CI: 0.85–1.22). The pooled RR among people with 10–19 teeth and those with fewer than ten teeth was 1.23 (95% CI: 1.13–1.33) and 1.20 (95% CI: 1.00–1.44), respectively. From the results of the subgroup analysis for edentulous status, the populations without and with natural teeth had a pooled RR of 1.27 (95% CI: 1.14–1.41) and 1.20 (95% CI: 1.13–1.28), respectively. The pooled risk from studies that used professional dental assessments was 1.16 (95% CI: 1.04–1.31), and the risk from those using self-reported questionnaires was 1.17 (95% CI: 1.07–1.29). Most (6/8) of the included studies had follow-up for 5 years or more, and the pooled RR was 1.18 (95% CI: 1.09–1.27), while the risk was not significant in people who were followed up for <5 years (RR = 1.67, 95% CI: 0.61–4.53).
3.4. Sensitivity analysis
We excluded a single study and pooled the results of the remaining studies. In the sensitivity analysis between tooth loss and dementia risk, none of the individual studies had a significant effect on the pooled RR, which ranged from 1.15 (95% CI: 1.07–1.23) to 1.20 (95% CI: 1.11–1.29) when omitting individual studies individually. In the sensitivity analysis of tooth loss with cognitive decline, the pooled RR ranged from 1.17 (95% CI: 1.09–1.25) to 1.22 (95% CI: 1.11–1.34) when omitting individual studies individually. In summary, the results of the quantitative synthesis based on the sensitivity analysis were stable.
3.5. Publication bias assessment
The possibility of publication bias was assessed using a funnel plot. For the association between tooth loss and risk of dementia or cognitive decline, visual inspection of the funnel plots revealed no significant asymmetry (Figure 3). The Begg's and Egger's tests also showed no significant publication bias in the assessment of tooth loss and risk of dementia (P from Begg's test = 0.83, P from Egger's test = 0.53) or cognitive decline (P from Begg's test = 0.39, P from Egger's test = 0.11).
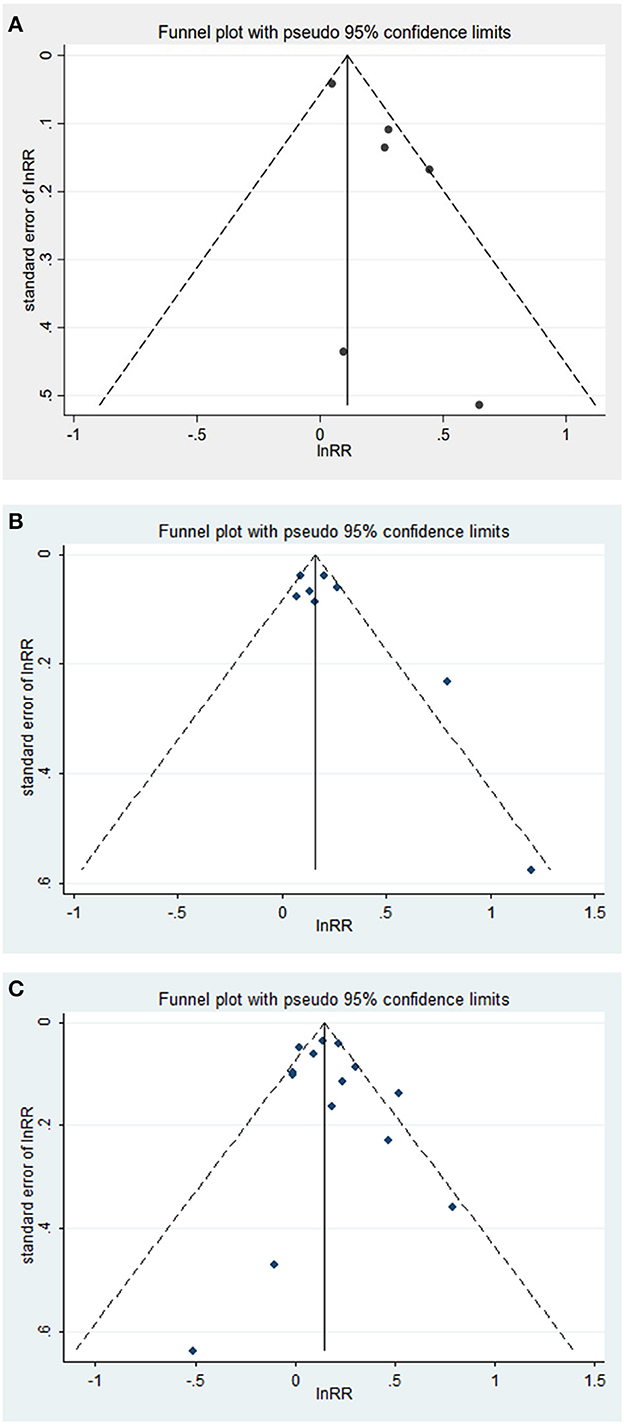
Figure 3. (A–C) Funnel plot with pseudo 95% confidence limits between tooth loss and dementia, cognitive decline.
4. Discussion
The results of this meta-analysis of 21 cohort studies showed that tooth loss was associated with the risk of cognitive decline (RR = 1.20; 95% CI: 1.14–1.26) and dementia (RR = 1.15; 95% CI: 1.10–1.20), respectively. These findings suggest that tooth loss is associated with an increased incidence of cognitive decline and dementia. Increased associations were also found between tooth loss and the two most common subtypes of dementia, that is, AD and VaD, with an RR of 1.12 (95% CI: 1.02–1.23) and 1.25 (95% CI: 1.06–1.47), respectively.
4.1. Comparison with previous studies
Our findings showed a positive correlation between tooth loss and dementia incidence (RR = 1.15; 95% CI: 1.10–1.20), which was not as strong as that from the previous meta-analysis (HR or RR of the included cohort studies between 1.22 and 2.07) (8, 16–19). Compared with these earlier studies, this meta-analysis included more cohort studies and a larger sample size, which significantly increased the statistical power to detect potential associations between tooth loss and dementia risk. The previous meta-analysis by Shen et al. (16) was based on 11 published articles with six cohort studies, three cross-control studies, and two cross-control studies. A previous review performed by Fang et al. (8) included seven cohort studies, eight cross-sectional studies, and two cross-control studies. The reviews by Cerutti-Kopplin et al. (17), Chen et al. (18), and Oh et al. (19) only included 8, 8, and 11 cohort studies, respectively. However, our review included 21 cohort studies with a large number of sample size (a total of 356,297) with a long follow-up period (8.6 years on average), which could decrease the sampling error to a great extent. Moreover, apart from dementia, our study also explored the relationship between tooth loss and the risk of cognitive decline and two common subtypes of dementia, AD and VaD. Additionally, the current meta-analysis investigated a more detailed subgroup analysis, including area, sex, dementia type, use of dentures, number of teeth and edentulous status, dental assessment methods, and follow-up duration of the associations between tooth loss and various cognitive disorders, including all types of dementia, cognitive decline, and dementia due to AD (see Table 1 and Supplementary Table 3).
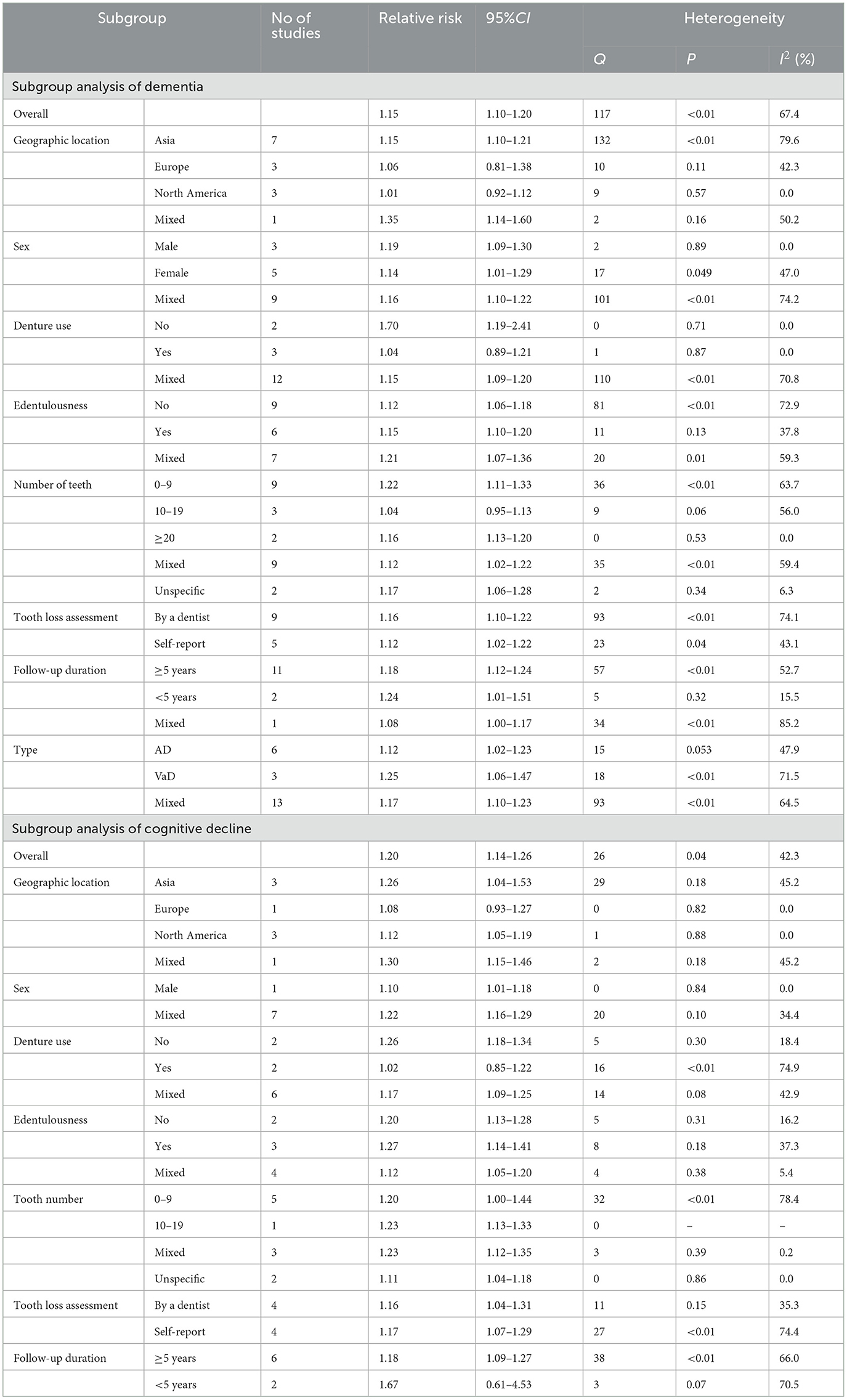
Table 1. Subgroup analysis of relative risks for the association between tooth loss and both dementia and cognitive decline.
More importantly, some valuable findings were observed. Our meta-analysis showed a significant association between an increased risk of dementia and cognitive decline in Asian populations with tooth loss, whereas the risks were lower or not statistically significant in people from Europe and North America with missing teeth. The World Health Organization's Global Burden of Disease Study 2019 (GBD 2019) anticipated an increasing trend in dementia prevalence due to population growth and aging, and found wide geographic heterogeneity in the projected increase in dementia cases across countries and regions (45). However, the present studies have been conducted in only a few countries and regions. Few studies were conducted in Africa and the Middle East, where the number of predicted dementia cases was greatest. For both dementia and cognitive decline, the risk of tooth loss was higher in people who did not wear dentures than in those with dentures. In subgroup analysis of the number of remaining natural teeth, the association between tooth loss and risk of dementia, cognitive decline, or AD was higher in the edentulous population than in the population with natural teeth, while higher AD and risk of all types of dementia were observed in people with only nine or fewer teeth than in people with 10–19 teeth. The results suggested a dose-response relationship between the number of natural teeth and the risk of dementia risk; however, further dose-response analyses are needed. In a quick summary, Daly et al. (46) noted that current epidemiologic research is generally based on self-report measures of oral health. Considering the low knowledge and awareness of oral health in the public, this would lead to a considerable risk of error and heterogeneity across studies.
Dementia is a chronic disease and has a longer prodromal period than other diseases. However, there is no firm evidence on the duration from exposure to tooth loss to the onset of dementia/cognitive decline, and there are no guidelines or instructions for a standard follow-up duration for research on this topic. The World Health Organization's Global Burden of Disease Study 2019 showed that the prevalence of dementia is increasing “doubling about every 5 years until 85 years of age in both 2019 and 2050” (45), so we divided the follow-up duration into <5 and ≥5 years. Most of the included studies (17/21) had a follow-up of more than 5 years, and their pooled RRs for all outcomes had narrow CI, allowing the results to provide precise estimates of population values. Some studies suggested a follow-up of 10 years (47, 48), so these risk factors in earlier life could be identified, patients in earlier stages of dementia be diagnosed, and reverse causation be minimized, especially in studies, except prospective cohorts. In our meta-analysis, more than half (11/21) of the included studies were followed for <10 years or more (at least 2 years). Future research should investigate the causality with a sufficiently long follow-up period.
Dementia is a disease that results in diminishing language abilities, judgement, memory loss, and behavioral abnormalities due to damage to cerebral neurons caused by progressive, degenerative, and cerebrovascular disease (49, 50). Although recent research suggests a link between poor dental health and accelerated dementia and cognitive decline, there are currently no clear treatments for dementia, and the mechanism by which cognitive impairment is associated with tooth loss remains unknown. Therefore, it is important to identify modifiable risk factors that support dementia prevention.
Several possible mechanisms have been proposed to explain the effects of tooth loss on cognitive function (51–58). Nutrition, inflammation, and neural feedback are the most commonly suggested factors (59). Theories of nutrition and neural feedback are related to masticatory dysfunction caused by tooth loss. Tooth loss means decreased chewing function, which leads to poorer digestive capacity and appetite, and then decreased nutrition, which ultimately damages brain functions such as cognition. Deficiency of several nutrients have been found to be related to the risk of cognitive decline and dementia, like vitamin D deficiency (60, 61) and iron deficiency (62). While higher intake of some nutrients and dietary interventions also seems to be associated with lower risk of cognitive dysfunction (63, 64). The causally link of vitamin D deficiency to dementia was observed, even after excluding the influence of genetic factors by using Mendelian randomization study design (65, 66). The metabolic, endocrine, immune functions and neuronal effects of 1 alpha, 25-dihydroxyvitamin D (1, 25(OH)2D) would be considered an essential contributor to the development of cognitive disorders (67, 68). Tooth loss (10–15) and vitamin D deficiency (61, 69–71) are both found to be related to many systemic health problems, and which further indicates an important role of low 1, 25(OH)2D concentration in the relationship between tooth loss and systemic diseases including cognitive damage. Low-oxygen supply (72) and brain iron dyshomeostasis (73) may explain the iron-related neuropathogenesis of cognitive impairment or dementia. This may also partly explain why evidence-based guidelines recommend dietary interventions to reduce the risk of cognitive decline and dementia (59). However, few interventional studies have proved causality between the deficiency of vitamin D and iron and the risk of dementia. Morever, attention should also be paid to the likely of prolonged supplementation or overload of vitamin D and iron (73, 74).
Besides, fewer interocclusal contacts related to decreased masticatory function indicate impaired proprioception and less somatosensory feedback, and such changes would lead to cognitive decline and dementia (75). Studies in animals and humans have shown the effects of dysfunction of the masticatory apparatus on cognitive damage and the reverse effect of masticatory activities on cognitive processes, indicating preventive and curative effects of chewing on cognitive performance (76–79). The number of teeth remaining rather than lost, e.g., 16, 20, or whatever, was used for the classification because they were thought to be sufficient for mastication. Number 20 without a third molar is usually recommended as the cut-off (23, 28, 34, 39, 41), since it is at least one posterior occlusal unit, the most important factor affecting a person's ability to chew (80). In fact, the number of lost teeth were assessed only in four out of the 18 involved studies (22, 29, 32, 33) in our meta-analysis. A 2018 Australian cross-sectional study found that the number of teeth (<20) was associated with a lower ability to chew hard foods and discomfort with eating, both of which were related to cognitive status, suggesting a chain of tooth loss, chewing ability, and cognitive damage (81). A cross-national study of three large cohorts in Europe and New Zealand confirmed in 2019 that lack of intact teeth and difficulty chewing are predictive of poor cognition and three other aspects of overall health in older people (82). However, there are some controversial results on whether tooth loss explains the association between cognition and mastication. Lexomboon et al. (83) conducted a study based on a nationally representative sample of an elderly Swedish population and reported that the likelihood of cognitive impairment, adjusted for sex, age, and education, was not significantly different between elderly people with and without multiple tooth loss, but remained significantly higher in those with difficulty chewing. The study suggests that difficulty chewing, not tooth loss, is an independent risk factor for cognitive impairment. Further evidence from studies based on a cohort design across different geographic areas and with adequate follow-up time is needed to confirm the relationship between tooth loss, chewing ability, and cognition.
Inflammation is one of the most commonly proposed theory to explain the effects of tooth loss on cognitive function. The theory is that without the protection of the teeth that results from tooth loss, the gingival tissue cannot protect the central nervous system (CNS) from constant exposure to pathogenic oral bacteria that can lead to or exacerbate inflammatory processes, causing endovascular inflammatory mediators and neurodegeneration. Thus, cognitive deficits and dementia may precede inflammation in the CNS (30, 84, 85). Periodontitis may also be related to cardiovascular and cerebrovascular disease, which are associated with the onset of dementia (10, 53, 86). This inflammation theory is also supported by epidemiological studies, which report a higher risk and severity (87, 88), clinical studies that observed bacterial evidence in patients with dementia and periodontitis (89), and animal experiments (90). Excessive nitric oxide levels (NO) are associated with excessive cell death and inflammation. Pang et al. (90) compared the effects of tooth loss with those of chronic cerebral ischaemia on cognitive function in Wistar rats and found that the effects were similar; NO, inducible nitric oxide (iNOS), and endothelial nitric oxide synthase (eNOS) in the hippocampus were involved in both cases. Some of the involved studies in our meta-analysis also paid attention to the problem of periodontitis and dental caries and found an increased risk for cognitive damage (22, 23). However, few studies have examined the relationship between the incidence of dementia and the number of teeth lost after accounting for other dental problems and/or behaviors, such as chewing difficulties and periodontitis (22, 41). It would be prudent for future studies to consider potential confounding or interactions with other dental problems to demonstrate an independent causative role of tooth loss on the risk cognitive impairment.
Further, poor cognitive status may also precede tooth loss. People having dementia are usually observed to have poor oral conditions and tooth loss, even in the early stage of the disease (91). Foley et al. (92) have conducted and demonstrated the effect of tooth loss on cognitive status. It may be caused by impaired cognitive function, memory, and physical ability. The risk of tooth loss resulting from poor cognitive function should also be paid attention to when discussing the relationship between tooth loss and cognitive impairment, i.e., the bi-direction association between them.
4.2. Strengths and limitations
The strength of our study is that it includes twice as many studies as previous reviews, with a large sample size of up to 35 thousand and an average follow-up time of 8 years. This makes our findings more reliable and generalisable. Additionally, this study demonstrates associations between tooth loss and various cognitive disorders, including subclinical cognitive decline, AD, VaD, and dementia of all types. Finally, we conducted a series of subgroup analyses focusing on the baseline characteristics of the subjects (sex and region), study design (follow-up period and dental assessment method), and type of cause and outcome, which could provide a wealth of insights for research on the relationship between dental health and cognitive status. The possible confounding effects of age on the relationship between tooth loss and cognitive decline had been also dealt with, as age was used as a covariate in most of the included studies (19/21, see Supplementary Table 1 “Characteristics of included studies in the meta-analysis”).
In interpreting this meta-analysis, the following limitations must be considered: first, the included studies used different methods and criteria to measure tooth loss and outcomes. For example, the number of teeth in some studies was self-reported by participants and not confirmed by a dentist or dental records, which could affect the accuracy of our results. Although we attempted to pool adjusted risk estimates, adjusted confounders were not available or varied widely among included studies. Although the mechanisms and risk factors of dementia and cognitive decline are far from clear, the incidence of dementia increases with age and varies between men and women. The evidence supporting 12 modifiable dementia risk factors was emphasized by the Lancet Commission on Dementia Prevention, Intervention, and Care (93). However, some of the above 12 potentially important confounders, such as sex, education, diet, smoking, hypertension, depression, dental health behaviors, and status, were neither fully adjusted for nor used as stratifying factors in some of the included studies, which may have influenced the assessment of the results. Third, the time of occurrence of tooth loss and the length of the edentulous period cannot be precisely determined, which may introduce bias in the incomparability of results to some extent (19).
5. Conclusions
This meta-analysis suggests that tooth loss is associated with increased risk of cognitive decline and dementia, as well as the two most common types of dementia, AD and VaD. Underlying factors that may be the cause of the observed associations include nutrition, inflammation, and neural feedback, especially deficiency of several nutrients like vitamin D. The results of our study suggest the importance of dental care in maintaining teeth adequately and protecting against cognitive impairment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
Article evaluation: LL, CW, QZ, YZ, and DY. Data extraction: CW, SY, and DY. Data analysis: CW, DY, SY, and YZ. Results interpretation: MJ, XW, LZ, and QL. Drafting the article: LL and CW. Critical revision of the manuscript: CW, LL, QZ, DY, SY, ZL, YG, and XZ. Final approval of the manuscript: all the authors. They have also agreed both to be personally accountable for their own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Funding
This study was supported by the Science and Technology Program of Jiangxi Education Committee (No: GJJ190814), the Open Project of Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases, Ministry of Education (Nos: XN201801 and XN201901), and the Project of Scientific and Technological Innovation Team of Gannan Medical College (TD201801). The contribution of topics and team projects to this article lies in research design and data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1103052/full#supplementary-material
Abbreviations
RR, relative risk; CI, confidence interval; AD, Alzheimer's disease; VaD, vascular dementia; HR, hazard ratio; MOOSE, meta-analysis of observational studies in epidemiology; CNKI, web of science and china national knowledge infrastructure; OR, odds ratio; MCI, mild cognitive impairment; CNS, central nervous system.
References
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 2nd ed. Arlington, VA: American Psychiatric Association (2013). doi: 10.1176/appi.books.9780890425596
2. Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. (2009) 302:385–93. doi: 10.1001/jama.2009.1064
3. National Institute on Aging at National Institutes of Health. What is Mild Cognitive Impairment? Ashburn: National Institute on Aging at National Institutes of Health (2010). Available online at: https://medlineplus.gov/mildcognitiveimpairment.html (accessed April 10, 2020).
4. World Health Organization. Dementia Key Facts. Geneva: World Health Organization (2009). Available online at: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed March 15, 2023).
5. World Health Organization. Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines. Geneva: World Health Organization (2019).
6. Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. (2012) 380:2197–223. doi: 10.1016/S0140-6736(12)61690-0
7. Kondo K, Niino M, Shido K. A case-control study of Alzheimer's disease in Japan: significance of life-styles. Dementia. (1994) 5:314–26. doi: 10.1159/000106741
8. Fang WL, Jiang MJ, Gu BB, Wei YM, Fan SN, Liao W, et al. Tooth loss as a risk factor for dementia: systematic review and meta-analysis of 21 observational studies. BMC Psychiatry. (2018) 18:345. doi: 10.1186/s12888-018-1927-0
9. Arrivé E, Letenneur L, Matharan F, Laporte C, Helmer C, Barberger-Gateau P, et al. Oral health condition of French elderly and risk of dementia: a longitudinal cohort study. Commun Dent Oral Epidemiol. (2012) 40:230–8. doi: 10.1111/j.1600-0528.2011.00650.x
10. Lafon A, Pereira B, Dufour T, Rigouby V, Giroud M, Béjot Y, et al. Periodontal disease and stroke: a meta-analysis of cohort studies. Eur J Neurol. (2014) 21:1155–61. doi: 10.1111/ene.12415
11. Nascimento GG, Peres KG, Mittinty MN, Mejia GC, Silva DA, Gonzalez-Chica D, et al. Obesity and periodontal outcomes: a population-based cohort study in Brazil. J Periodontol. (2017) 88:50–8. doi: 10.1902/jop.2016.160361
12. Cheng F, Zhang M, Wang Q, Xu H, Dong X, Gao Z, et al. Tooth loss and risk of cardiovascular disease and stroke: a dose-response meta-analysis of prospective cohort studies. PLoS ONE. (2018) 13:e0194563. doi: 10.1371/journal.pone.0194563
13. Shi J, Leng W, Zhao L, Deng C, Xu C, Wang J, et al. Tooth loss and cancer risk: a dose-response meta analysis of prospective cohort studies. Oncotarget. (2017) 9:15090–100. doi: 10.18632/oncotarget.23850
14. Marcenes W, Kassebaum NJ, Bernabé E, Flaxman A, Naghavi M, Lopez A, et al. Global burden of oral conditions in 1990–2010: a systematic analysis. J Dent Res. (2013) 92:592–7. doi: 10.1177/0022034513490168
15. Kisely S, Baghaie H, Lalloo R, Siskind D, Johnson NW. A systematic review and meta-analysis of the association between poor oral health and severe mental illness. Psychosom Med. (2015) 77:83–92. doi: 10.1097/PSY.0000000000000135
16. Shen T, Lv J, Wang L, Wang W, Zhang D. Association between tooth loss and dementia among older people: a meta-analysis. Int J Geriatr Psychiatry. (2016) 31:953–5. doi: 10.1002/gps.4396
17. Cerutti-Kopplin D, Feine J, Padilha DM, de Souza RF, Ahmadi M, Rompré P, et al. Tooth loss increases the risk of diminished cognitive function: a systematic review and meta-analysis. JDR Clin Trans Res. (2016) 1:10–9. doi: 10.1177/2380084416633102
18. Chen J, Ren C-J, Wu L, Xia L-Y, Shao J, Leng W-D, et al. Tooth loss is associated with increased risk of dementia and with a dose-response relationship. Front Aging Neurosci. (2018) 10:415. doi: 10.3389/fnagi.2018.00415
19. Oh B, Han D-H, Han K-T, Liu X, Ukken J, Chang C, et al. Association between residual teeth number in later life and incidence of dementia: a systematic review and meta-analysis. BMC Geriatr. (2018) 18:48. doi: 10.1186/s12877-018-0729-z
20. Kang J, Wu B, Bunce D, Ide M, Pavitt S, Wu J. Cognitive function and oral health among ageing adults. Commun Dent Oral Epidemiol. (2019) 47:259–66. doi: 10.1111/cdoe.12452
21. Kato H, Takahashi Y, Iseki C, Igari R, Sato H, Sato H, et al. Tooth loss-associated cognitive impairment in the elderly: a community-based study in Japan. Intern Med. (2019) 58:1411–6. doi: 10.2169/internalmedicine.1896-18
22. Yoo J-J, Yoon J-H, Kang M-J, Kim M, Oh N. The effect of missing teeth on dementia in older people: a nationwide population-based cohort study in South Korea. BMC Oral Health. (2019) 19:61. doi: 10.1186/s12903-019-0750-4
23. Kim DH, Jeong SN, Lee JH. Severe periodontitis with tooth loss as a modifiable risk factor for the development of Alzheimer, vascular, and mixed dementia: national health insurance service-national health screening retrospective cohort 2002–2015. J Periodontal Implant Sci. (2020) 50:303–12. doi: 10.5051/jpis.2000600030
24. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
25. Saito S, Ohi T, Murakami T, Komiyama T, Miyoshi Y, Endo K, et al. Association between tooth loss and cognitive impairment in community-dwelling older Japanese adults: a 4-year prospective cohort study from the Ohasama study. BMC Oral Health. (2018) 18:142. doi: 10.1186/s12903-018-0602-7
26. Irwig L, Macaskill P, Berry G, Glasziou P. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
27. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101.
28. Shimazaki Y, Soh I, Saito T, Yamashita Y, Koga T, Miyazaki H, et al. Influence of dentition status on physical disability, mental impairment, and mortality in institutionalized elderly people. J Dent Res. (2001) 80:340–5. doi: 10.1177/00220345010800010801
29. Kim JM, Stewart R, Prince M, Kim SW, Yang SJ, Shin IS, et al. Dental health, nutritional status and recent-onset dementia in a Korean community population. Int J Geriatr Psychiatry. (2007) 22:850–5. doi: 10.1002/gps.1750
30. Stein PS, Desrosiers M, Donegan SJ, Yepes JF, Kryscio RJ. Tooth loss, dementia and neuropathology in the Nun study. J Am Dent Assoc. (2007) 138:1314–22. doi: 10.14219/jada.archive.2007.0046
31. Chen X, Shuman SK, Hodges JS, Gatewood LC, Xu J. Patterns of tooth loss in older adults with and without dementia: a retrospective study based on a Minnesota cohort. J Am Geriatr Soc. (2010) 58:2300–7. doi: 10.1111/j.1532-5415.2010.03192.x
32. Kaye EK, Valencia A, Baba N, Spiro A, Dietrich T, Garcia RI. Tooth loss and periodontal disease predict poor cognitive function in older men. J Am Geriatr Soc. (2010) 58:713–8. doi: 10.1111/j.1532-5415.2010.02788.x
33. Paganini-Hill A, White SC, Atchison KA. Dentition, dental health habits, and dementia: the Leisure World Cohort Study. J Am Geriatr Soc. (2012) 60:1556–63. doi: 10.1111/j.1532-5415.2012.04064.x
34. Yamamoto T, Kondo K, Hirai H, Nakade M, Aida J, Hirata Y. Association between self-reported dental health status and onset of dementia: a 4-year prospective cohort study of older Japanese adults from the Aichi Gerontological Evaluation Study (AGES) Project. Psychosom Med. (2012) 74:241–8. doi: 10.1097/PSY.0b013e318246dffb
35. Batty G-D, Li Q, Huxley R, Zoungas S, Taylor B-A, Neal B, et al. Oral disease in relation to future risk of dementia and cognitive decline: prospective cohort study based on the action in diabetes and vascular disease: preterax and diamicron modified-release controlled evaluation (ADVANCE) trial. Eur Psychiatry. (2013) 28:49–52. doi: 10.1016/j.eurpsy.2011.07.005
36. Reyes-Ortiz CA, Luque JS, Eriksson CK, Soto L. Self-reported tooth loss and cognitive function: data from the hispanic established populations for epidemiologic studies of the elderly (hispanic EPESE). Colomb Med. (2013) 44:139–45. doi: 10.25100/cm.v44i3.1248
37. Stewart R, Weyant RJ, Garcia ME, Harris T, Launer LJ, Satterfield S, et al. Adverse oral health and cognitive decline: the health, aging and body composition study. J Am Geriatr Soc. (2013) 61:177–84. doi: 10.1111/jgs.12094
38. Hansson P, Sörman DE, Bergdahl J, Bergdahl M, Nyberg L, Adolfsson R, et al. Dental status is unrelated to risk of dementia: a 20-year prospective study. J Am Geriatr Soc. (2014) 62:979–81. doi: 10.1111/jgs.12814
39. Stewart R, Stenman U, Hakeberg M, Hägglin C, Gustafson D, Skoog I. Associations between oral health and risk of dementia in a 37-year follow-up study: the prospective population study of women in Gothenburg. J Am Geriatr Soc. (2015) 63:100–5. doi: 10.1111/jgs.13194
40. Tsakos G, Watt RG, Rouxel PL, de Oliveira C, Demakakos P. Tooth loss associated with physical and cognitive decline in older adults. J Am Geriatr Soc. (2015) 63:91–9. doi: 10.1111/jgs.13190
41. Takeuchi K, Ohara T, Furuta M, Takeshita T, Shibata Y, Hata J, et al. Tooth loss and risk of dementia in the community: the Hisayama study. J Am Geriatr Soc. (2017) 65:e95–e100. doi: 10.1111/jgs.14791
42. Kim JH, Oh JK, Wee JH, Kim YH, Byun SH, Choi HG. Association between tooth loss and Alzheimer's disease in a nested case-control study based on a national health screening cohort. J Clin Med. (2021) 10:3763. doi: 10.3390/jcm10173763
43. Kiuchi S, Cooray U, Kusama T, Yamamoto T, Abbas H, Nakazawa N, et al. Oral status and dementia onset: mediation of nutritional and social factors. J Dent Res. (2022) 101:420–7. doi: 10.1177/00220345211049399
44. Yang HL Li FR, Chen PL, Cheng X, Mao C, Wu XB. Tooth loss, denture use, and cognitive impairment in Chinese older adults: a community cohort study. J Gerontol A Biol Sci Med Sci. (2022) 77:180–7. doi: 10.1093/gerona/glab056
45. GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. (2022) 7:e105–25. doi: 10.1016/S2468-2667(21)00249-8
46. Daly B, Thompsell A, Sharpling J, Rooney YM, Hillman L, Wanyonyi KL, et al. Evidence summary: the relationship between oral health and dementia. Br Dent J. (2018) 223:846–53. doi: 10.1038/sj.bdj.2017.992
47. Schilling S, Tzourio C, Soumaré A, Kaffashian S, Dartigues J-F, Ancelin M-L, et al. Differential associations of plasma lipids with incident dementia and dementia subtypes in the 3C study: a longitudinal, population-based prospective cohort study. PLoS Med. (2017) 14:e1002265. doi: 10.1371/journal.pmed.1002265
48. Iwagami M, Qizilbash N, Gregson J, Douglas I, Johnson M, Pearce N, et al. Blood cholesterol and risk of dementia in more than 18 million people over two decades: a retrospective cohort study. Lancet Healthy Long. (2021) 2:e498–506. doi: 10.1016/S2666-7568(21)00150-1
49. Sorbi S, Hort J, Erkinjuntti T. EFNS-ENS guidelines on the diagnosis and management of disorders associated with dementia. Eur J Neurol. (2012) 19:1159–79. doi: 10.1111/j.1468-1331.2012.03784.x
50. Sposato LA, Kapral MK, Fang J, Gill SS, Hackam DG, Cipriano LE, et al. Declining incidence of stroke and dementia: coincidence or prevention opportunity? JAMA Neurol. (2015) 72:1529–31. doi: 10.1001/jamaneurol.2015.2816
51. Kato T, Usami T, Noda Y, Hasegawa M, Ueda M, Nabeshima T. The effect of the loss of molar teeth on spatial memory and acetylcholine release from the parietal cortex in aged rats. Behav Brain Res. (1997) 83:239–42. doi: 10.1016/S0166-4328(97)86078-0
52. Ebersole JL, Cappelli D, Mathys EC, Steffen MJ, Singer RE, Montgomery M, et al. Periodontitis in humans and non-human primates: oral-systemic linkage inducing acute phase proteins. Ann Periodontol. (2002) 7:102–11. doi: 10.1902/annals.2002.7.1.102
53. Riviere GR, Riviere KH, Smith KS. Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer's disease. Oral Microbiol Immunol. (2002) 17:113–8. doi: 10.1046/j.0902-0055.2001.00100.x
54. Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, et al. Inflammatory proteins in plasma and the risk of dementia: the Rotterdam study. Arch Neurol. (2004) 61:668–72. doi: 10.1001/archneur.61.5.668
55. Meurman JH, Hämäläinen P. Oral health and morbidity: implications of oral infections on the elderly. Gerodontology. (2006) 23:3–16. doi: 10.1111/j.1741-2358.2006.00102.x
56. Kamer AR, Craig RG, Dasanayake AP, Brys M, Glodzik-Sobanska L, de Leon MJ. Inflammation and Alzheimer's disease: possible role of periodontal diseases. Alzheimers Dement. (2008) 4:242–50. doi: 10.1016/j.jalz.2007.08.004
57. Watts A, Crimmins EM, Gatz M. Inflammation as a potential mediator for the association between periodontal disease and Alzheimer's disease. Neuropsychiatr Dis Treat. (2008) 4:865–76. doi: 10.2147/NDT.S3610
58. Ramesh BN, Rao TS, Prakasam A, Sambamurti K, Rao KS. Neuronutrition and Alzheimer's disease. J Alzheimers Dis. (2010) 19:1123–39. doi: 10.3233/JAD-2010-1312
59. Thomson WM, Barak Y. Tooth loss and dementia: a critical examination. J Dent Res. (2021) 100:226–31. doi: 10.1177/0022034520957233
60. Jayedi A, Rashidy-Pour A, Shab-Bidar S. Vitamin D status and risk of dementia and Alzheimer's disease: a meta-analysis of dose-response. Nutr Neurosci. (2019) 22:750–9. doi: 10.1080/1028415X.2018.1436639
61. Liu D, Meng X, Tian Q, Cao W, Fan X, Wu L, et al. Vitamin D and multiple health outcomes: an umbrella review of observational studies, randomized controlled trials, and Mendelian randomization studies. Adv Nutr. (2022) 13:1044–62. doi: 10.1093/advances/nmab142
62. Kung W-M, Yuan S-P, Lin M-S, Wu C-C, Islam MM, Atique S, et al. Anemia and the risk of cognitive impairment: an updated systematic review and meta-analysis. Brain Sci. (2021) 11:777. doi: 10.3390/brainsci11060777
63. Annweiler C, Rolland Y, Schott AM, Blain H, Vellas B, Herrmann FR, et al. Higher vitamin D dietary intake is associated with lower risk of Alzheimer's disease: a 7-year follow-up. J Gerontol A Biol Sci Med Sci. (2012) 67:1205–11. doi: 10.1093/gerona/gls107
64. Vlachos GS, Scarmeas N. Dietary interventions in mild cognitive impairment and dementia. Dialog Clin Neurosci. (2019) 21:69–82. doi: 10.31887/DCNS.2019.21.1/nscarmeas
65. Navale SS, Mulugeta A, Zhou A, Llewellyn DJ, Hyppönen E. Vitamin D and brain health: an observational and Mendelian randomization study. Am J Clin Nutr. (2022) 116:531–40. doi: 10.1093/ajcn/nqac107
66. Larsson SC, Traylor M, Markus HS, Michaëlsson K. Serum parathyroid hormone, 25-hydroxyvitamin D, and risk of Alzheimer's disease: a Mendelian randomization study. Nutrients. (2018) 10:1243. doi: 10.3390/nu10091243
67. Roy NM, Al-Harthi L, Sampat N, Al-Mujaini R, Mahadevan S, Adawi SA, et al. Impact of vitamin D on neurocognitive function in dementia, depression, schizophrenia and ADHD. Front Biosci. (2021) 26:566–611. doi: 10.2741/4908
68. Gáll Z, Székely O. Role of vitamin D in cognitive dysfunction: new molecular concepts and discrepancies between animal and human findings. Nutrients. (2021) 13:3672. doi: 10.3390/nu13113672
69. Zhou A, Selvanayagam JB, Hyppönen E. Non-linear Mendelian randomization analyses support a role for vitamin D deficiency in cardiovascular disease risk. Eur Heart J. (2022) 43:1731–9. doi: 10.1093/eurheartj/ehab809
70. Muñoz A, Grant WB. Vitamin D and cancer: an historical overview of the epidemiology and mechanisms. Nutrients. (2022) 14:1448. doi: 10.3390/nu14071448
71. Chan Y-H, Schooling CM, Zhao J, Yeung S-LA, Hai JJ, Thomas GN, et al. Mendelian randomization focused analysis of vitamin D on the secondary prevention of ischemic stroke. Stroke. (2021) 52:3926–37. doi: 10.1161/STROKEAHA.120.032634
72. Kececi H, Degirmenci Y. Quantitative EEG and cognitive evoked potentials in anemia. Neurophysiol Clin. (2008) 38:137–43. doi: 10.1016/j.neucli.2008.01.004
73. Belaidi AA, Bush AI. Iron neurochemistry in Alzheimer's disease and Parkinson's disease: targets for therapeutics. J Neurochem. (2016) 139:179–97. doi: 10.1111/jnc.13425
74. Lai RH, Hsu CC Yu BH, Lo YR, Hsu YY, Chen MH, et al. Vitamin D supplementation worsens Alzheimer's progression: animal model and human cohort studies. Aging Cell. (2022) 21:e13670. doi: 10.1111/acel.13670
75. Lin CS. Revisiting the link between cognitive decline and masticatory dysfunction. BMC Geriatr. (2018) 18:5. doi: 10.1186/s12877-017-0693-z
76. Watanabe K, Ozono S, Nishiyama K, Saito S, Tonosaki K, Fujita M, et al. The molarless condition in aged SAMP8 mice attenuates hippocampal Fos induction linked to water maze performance. Behav Brain Res. (2002) 128:19–25. doi: 10.1016/S0166-4328(01)00268-6
77. Hirano Y, Obata T, Kashikura K, Nonaka H, Tachibana A, Ikehira H, et al. Effects of chewing in work memory processing. Neurosci Lett. (2008) 436:189–92. doi: 10.1016/j.neulet.2008.03.033
78. Ono Y, Dowaki K, Ishiyama A, Onozuka M. Gum chewing maintains working memory acquisition. Int J Bioelectromagnetism. (2009) 11:130–4.
79. Narita N, Kamiya K, Yamamura K, Kawasaki S, Matsumoto T, Tanaka N. Chewing-related prefrontal cortex activation while wearing a partial denture prosthesis: pilot study. J Prosthodont Res. (2009) 53:126–35. doi: 10.1016/j.jpor.2009.02.005
80. Leake JL. An index of chewing ability. J Public Health Dent. (1990) 50:262–7. doi: 10.1111/j.1752-7325.1990.tb02133.x
81. Wright FAC, Law GG, Milledge KL, Chu SK-Y, Hsu B, Valdez E, et al. Chewing function, general health and the dentition of older Australian men: the concord health and ageing in men project. Commun Dent Oral Epidemiol. (2019) 47:134–41. doi: 10.1111/cdoe.12435
82. de Almeida Mello J, Tran TD, Krausch-Hofmann S, Meehan B, van Hout H, Turcotte L, et al. Cross-country validation of the association between oral health and general health in community-dwelling older adults. J Am Med Dir Assoc. (2019) 20:1137–42.e2. doi: 10.1016/j.jamda.2019.02.020
83. Lexomboon D, Trulsson M, Wårdh I, Parker MG. Chewing ability and tooth loss: association with cognitive impairment in an elderly population study. J Am Geriatr Soc. (2012) 60:1951–6. doi: 10.1111/j.1532-5415.2012.04154.x
84. Gatz M, Mortimer JA, Fratiglioni L, Johansson B, Berg S, Reynolds CA, et al. Potentially modifiable risk factors for dementia in identical twins. Alzheimers Dement. (2006) 2:110–7. doi: 10.1016/j.jalz.2006.01.002
85. Tonsekar PP, Jiang SS, Yue G. Periodontal disease, tooth loss and dementia: Is there a link? A systematic review. Gerodontology. (2017) 34:151–63. doi: 10.1111/ger.12261
86. Lee J-H, Lee J-S, Choi J-K, Kweon H-I, Kim Y-T, Choi S-H. National dental policies and socio-demographic factors affecting changes in the incidence of periodontal treatments in Korean: a nationwide population-based retrospective cohort study from 2002 to 2013. BMC Oral Health. (2016) 16:118. doi: 10.1186/s12903-016-0310-0
87. Ghezzi EM, Ship JA. Dementia and oral health. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2000) 89:2–5. doi: 10.1016/S1079-2104(00)80003-7
88. Philip P, Rogers C, Kruger E, Tennant M. Oral hygiene care status of elderly with dementia and in residential aged care facilities. Gerodontology. (2012) 29:e306–11. doi: 10.1111/j.1741-2358.2011.00472.x
89. Beydoun MA, Beydoun HA, Hossain S, El-Hajj ZW, Weiss J, Zonderman AB. Clinical and bacterial markers of periodontitis and their association with incident all-cause and Alzheimer's disease dementia in a large national survey. J Alzheimers Dis. (2020) 75:157–72. doi: 10.3233/JAD-200064
90. Pang Q, Wu Q, Hu X, Zhang J, Jiang Q. Tooth loss, cognitive impairment and chronic cerebral ischemia. J Dent Sci. (2020) 15:84–91. doi: 10.1016/j.jds.2019.09.001
91. Nakamura T, Zou K, Shibuya Y, Michikawa M. Oral dysfunctions and cognitive impairment/dementia. J Neurosci Res. (2021) 99:518–28. doi: 10.1002/jnr.24745
92. Foley N, Affoo R, Siqueira W, Martin R. A systematic review examining the oral health status of persons with dementia. JDR Clin Trans Res. (2017) 2:330–42. doi: 10.1177/2380084417714789
Keywords: dementia, cognitive decline, meta-analysis, Alzheimer's disease, tooth loss
Citation: Li L, Zhang Q, Yang D, Yang S, Zhao Y, Jiang M, Wang X, Zhao L, Liu Q, Lu Z, Zhou X, Gan Y and Wu C (2023) Tooth loss and the risk of cognitive decline and dementia: A meta-analysis of cohort studies. Front. Neurol. 14:1103052. doi: 10.3389/fneur.2023.1103052
Received: 03 December 2022; Accepted: 13 March 2023;
Published: 17 April 2023.
Edited by:
Vida Demarin, International Institute for Brain Health, CroatiaReviewed by:
William B. Grant, Sunlight Nutrition and Health Research Center, United StatesWei Yue, Tianjin Huanhu Hospital, China
Copyright © 2023 Li, Zhang, Yang, Yang, Zhao, Jiang, Wang, Zhao, Liu, Lu, Zhou, Gan and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunmei Wu, wuchunmei@gmu.edu.cn
 Liqing Li
Liqing Li