- 1Department of Neurology, Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital, Affiliated Hospital of University of Electronic Science and Technology of China, Chengdu, China
- 2Department of Neurology, Yunyang County People's Hospital, Chongqing, China
- 3Department of Neurology, Zigong Third People's Hospital, Zigong, China
- 4Department of Neurology, Chengdu Eighth People's Hospital, Chengdu, China
- 5Department of Neurology, Ya'an People's Hospital, Yaan, China
Background: Low serum levels of major lipid markers have been proved to be significantly associated with increased risks of hemorrhagic stroke (HS) and cerebral microbleeds (CMBs). However, there is no lipid modification guideline telling us how to maintain a balance between the prevention of ischemic stroke recurrence and the prevention of hemorrhagic events, especially in patients with acute ischemic stroke (AIS) and CMBs.
Aim: The Intracranial Hemorrhage Risk of Intensive Statin Therapy in Patients with Acute Ischemic Stroke combined with Cerebral Microbleeds (CHRISTMAS) trial evaluates the risk of intracranial hemorrhage (i.e., HS and CMBs) of high-dose statin therapy in patients with AIS combined with CMBs.
Methods and design: This is an investigator-initiated, multicenter, prospective, randomized controlled clinical trial design. Up to 344 eligible patients will be consecutively randomized to receive high-dose or low-dose atorvastatin in 1:1 ratio in 5 stroke centers in China.
Outcomes: CHRISTMAS trial has co-primary outcomes, namely, hemorrhage risk: the incidence of HS and the changes in degree of CMBs until the end of 36-month follow-up.
Discussion: The primary hypothesis of this study is that an excessive reduction in serum lipid levels by an intensive statin therapy in AIS patients with CMBs can increase the risk of intracranial hemorrhage. This study will shed light on new clinical decisions regarding the long-term serum lipid management in these patients with dilemma in clinical practice.
Clinical trial registration: Clinicaltrials.gov, identifier: NCT05589454.
Introduction
Cerebral microbleeds (CMBs) are a crucial radiological marker of cerebral small vessel disease (CSVD) (1) to illustrate the micropathology of perivascular hemosiderin deposition corresponding to past small foci of bleeding (2). The prevalence of CMBs increases with age and exceeds 20% in community population over 60 years old (3, 4). More importantly, CMBs are also a common comorbidity of stroke, brain trauma, Alzheimer's disease, and cerebral amyloid angiopathy (5, 6), and are associated with cognitive impairment, psychiatric symptoms, and decline of daily living ability (7).
CMBs have been identified to be indicative of bleeding-prone microangiopathy for their dynamical accumulation over time, with the burden (presence and number) of baseline CMBs predicting the development of new CMBs in both deep and lobar regions, and may predict spontaneous intracranial hemorrhage (ICH) (8–12). These pieces of evidence have suggested an important link between the presence of CMBs and future macrobleeding. However, CMBs have also been demonstrated in approximately one-third of patients with ischemic stroke (IS), that are associated with an increased risk of recurrent IS, symptomatic ICH and death (13). As the burden of CMBs in IS patients increased, the relative and absolute risk ratios for ICH increase steeply, although the absolute incidence of recurrent IS is consistently higher than that of ICH (13–16). Therefore, it raises clinical dilemmas, particularly regarding the safety of secondary prevention treatment of IS with concomitant presence of CMBs.
Statins have been widely used for secondary prevention of IS. The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) study had proposed that the reduction in the risk of IS was primarily related to the extent to which low-density lipoprotein cholesterol (LDL-C) levels are lowered instead of statin use (17). It was the first to demonstrate that 80 mg of atorvastatin per day reduced the overall incidence of strokes and cardiovascular events in patients with recent stroke or transient ischemic stroke (TIA) and without known coronary heart disease (17). However, the correlation between statin use of an intensive dose and ICH risk has not been elucidated so far (18–20). A recent meta-analysis on intensive LDL-C-lowering statin-based therapy also revealed an increased risk of ICH related to high-dose statins (17, 21), while other studies did not show the similar conclusions that statin use (22) or intensive reduction of LDL-C (23) increased the ICH risk. More importantly, none of these previous studies have explored the relationship between intensive statin therapy and hemorrhage risk from the perspective of CMBs.
However, it is worth noting that low levels of serum triglycerides (TG), total cholesterol (TC), and LDL-C have been proved to be significantly associated with increased risks of hemorrhagic stroke (HS) and CMBs (17, 19, 24). Thus, the risk of hemorrhagic events appears to depend on the reduction extent and degree of blood lipid levels rather than on statin use per se (17–22, 24). Unfortunately, the current international guidelines on lipid modification are all committed to emphasize the intensive reduction for LDL-C to prevent ischemic events, with no instructions on a more propriate target range to balance the prevention of ischemic stroke recurrence and the prevention of hemorrhagic events, especially for patients requiring high-dose statin treatment. Therefore, it is of great scientific and practical significance to conduct prospective studies to reveal the clinical and neuroimaging characteristics of CMBs in acute ischemic stroke (AIS) patients, and to explore the correlation between intensive lipid-lowering therapy and hemorrhage risk.
Methods
Study design
The CHRISTMAS trial is a multicenter, prospective, randomized controlled trial design. Participants will be enrolled in five hospitals from two provinces in China (Sichuan and Chongqing). This investigator-initiated clinical trial will be conducted in adherence to the Good Clinical Practice guidelines and in compliance with the Declaration of Helsinki, including all revisions. The study has been approved by the ethics committee of Sichuan Academy of Medical Sciences & Sichuan Provincial People's Hospital (SAMS & SPPH) and all participating centers, and has been registered at Clinicaltrials.gov with the identifier: NCT05589454 (Supplementary material 1). Written informed consent from eligible patients or their legally authorized representatives will be obtained before patient enrollment.
Participants
By December 2023, 344 patients will have been enrolled. The inclusion and exclusion criteria are provided in Table 1. Briefly, patients are eligible if they (1) have a non-cardioembolic ischemic stroke within 14 days from the onset of the symptoms prior to the study participation; (2) are between the ages of 18 and 90; and (3) have the presence of CMBs (without differentiating the number or localization) on baseline susceptibility-weighted imaging (SWI) (Supplementary material 2). Key exclusion criteria include active hemorrhagic diseases, hemorrhagic tendency, severe stroke, or other severe conditions that are not eligible for clinical trials. In addition, patients who have contraindications to statins or need for intravenous thrombolysis, interventional therapy, anticoagulation, or dual antiplatelet therapy shall be excluded.
Randomization and blinding
All patients who are undergoing an AIS will be screened timely according to inclusion and exclusion criteria by a qualified investigator of each center. Then the eligible patients will be randomized into high-dose and low-dose atorvastatin arms in a 1:1 ratio (Figure 1). The concealment of allocation will be performed using sealed envelopes with numbers by the participants' physicians. Considering the non-subjective nature of the primary outcomes, as well as the reason that the investigator-initiated clinical trial without technical and financial support from any pharmaceutical manufacturer to develop placebos that meet the study design requirements in clinical practice, the study finally gave up the design and application of placebo, and only designed a single-blind manner for the study investigators. So, it's important to note that neither the participants nor their physicians conducting the drug intervention can be blind to the grouping, because the number of tablets administered in different groups will be different, so these physicians cannot be involved in the research process. All investigators and data collectors will not participate in the whole intervention.
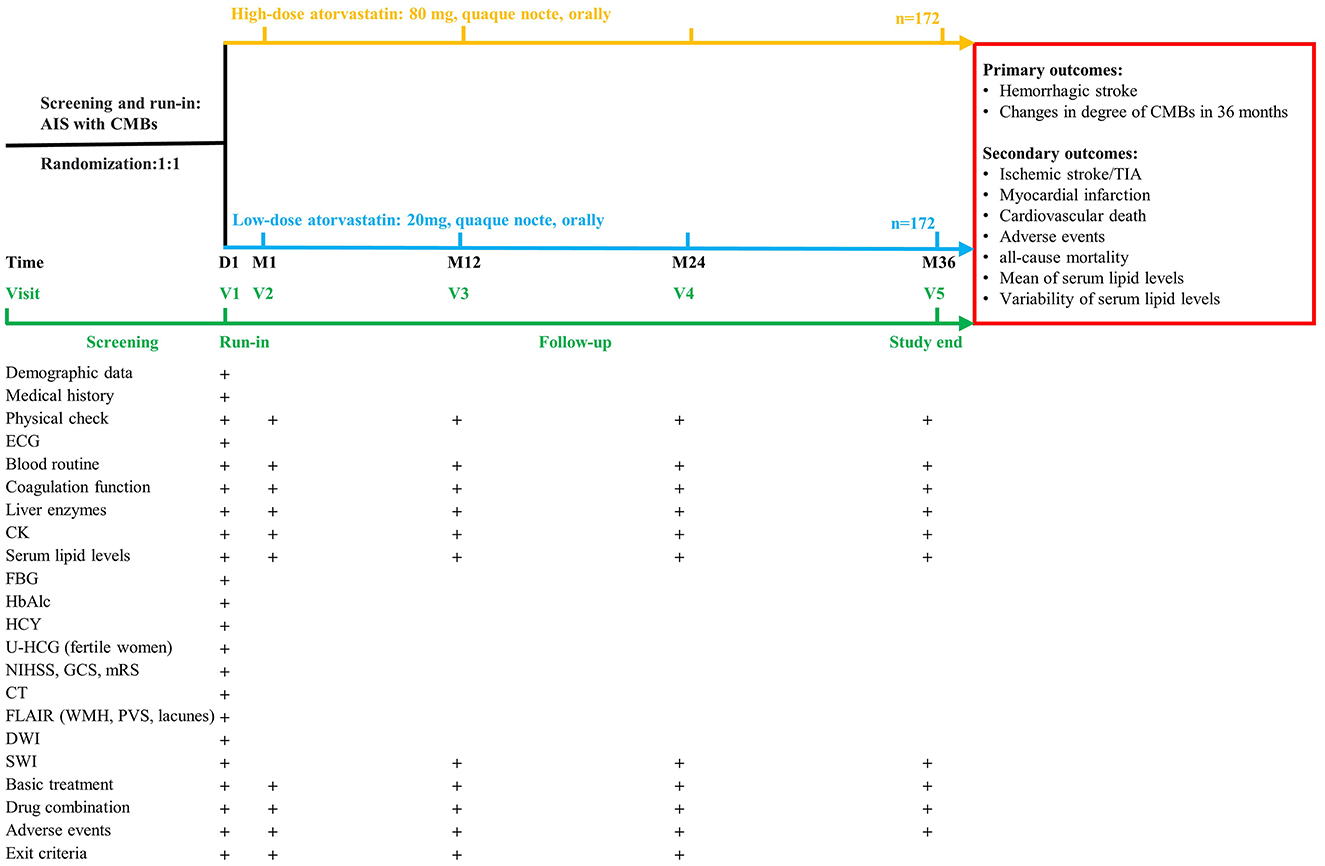
Figure 1. Design of the CHRISTMAS trial. AIS, acute ischemic stroke; CK, creatine kinase; CT, computed tomography; CMBs, cerebral microbleeds; DWI, diffusion weighted imaging; ECG, electrocardiograph; FBG, fasting blood-glucose; FLAIR, fluid-attenuated inversion-recovery sequency; GCS, Glasgow Coma Scale; mRS, modified Rankin Scale; HbAlc, glycosylated hemoglobin; HCY, homocysteine; NIHSS, National Institutes of Health Stroke Scale; PVS, perivascular spaces; SWI, susceptibility weighted imaging; TIA, transient ischemic attack; U-HCG, urine human chorionic gonadotropin; ULN, upper limit of normal; WMH, white matter hyperintensities.
Treatment
• High-dose atorvastatin arm: Atorvastatin calcium tablets four pills (80 mg), quaque nocte (24 ± 1 h between two doses), orally, until the end of follow-up.
• Low-dose atorvastatin arm: Atorvastatin calcium tablets one pill (20 mg), quaque nocte (24 ± 1 h between two doses), orally, until the end of follow-up.
It is strongly recommended in both arms for the optimal treatment including acute treatment and other secondary prevention agents in accordance with clinical practice guidelines and the best medical conditions in the health care unit.
Follow-up
Detailed follow-up schedules and procedures are provided in Figure 1. Baseline characteristics, SWI and clinical laboratory tests will be obtained at enrollment. The following assessments will be performed at 1 month and annually follow-up: blood tests, SWI, study compliance, concomitant medication, adverse events, and withdrawal criteria. As it is not clear whether participants in this study would benefit more from following current guidelines or moderately relaxed lipid management targets. No further interventions which might violate the study protocol should be taken (such as suggesting withdrawal from the study or adjusting the treatment plan, etc.) if it is found that the serum lipid levels of the participants do not meet the current guidelines either for both groups. But the participants' doctor should be informed to strictly monitor their lipid levels and clinical conditions.
Outcomes
Primary outcomes
The trial has co-primary outcome, namely, hemorrhage risk.
The first part is the incidence of HS confirmed by brain computed tomography (CT) or magnetic resonance imaging (MRI).
The second part is the changes in degree of CMBs until the end of 36 month follow-up.
Secondary outcomes
The incidence of recurrent IS and TIA.
The incidence of myocardial infarction.
The incidence of cardiovascular death.
The incidence of adverse events.
The incidence of all-cause mortality.
The mean of serum lipid levels [TG, TC, LDL-C, and high-density lipoprotein cholesterol (HDL-C)] for 36 months with at least 3 measurements.
The variability of serum lipid levels (TG, TC, LDL-C, and HDL-C) for 36 months with at least 3 measurements.
Sample size estimates
Sample size calculations were based on the primary outcomes (the incidence of ICH). All participants are expected to be enrolled for 1 year and followed for 36 months. It will be tested whether the high-dose statin arm suffers a higher risk of hemorrhage than the low-dose statin arm in terms of the primary outcomes for 36 months. According to the SPARCL trial (the incidence of ICH: 25.2 vs. 12.0% in high-dose statin arm vs. low-dose statin arm with 4.9 years of mean follow-up duration) (17). The sample size was calculated by PASS software (version 15.0) with a two-sided type I error of 5% (α = 0.05) and 80% power, a sample size of 137 patients in each arm is required (total 274 patients). Assuming around 20% loss to follow-up, a total of 344 patients will be randomized.
Statistical analysis
SPSS 25.0 software will be used for statistical analysis by data analysts who are blind to the study. Two-sided P-values will be calculated with a significant level of 5%, and the parameters will be estimated by 95% confidence intervals. The primary analysis will be under intention-to-treat (ITT) principle and be performed on both the ITT population and per-protocol (PP) population.
Descriptive statistics of demographic data and other baseline eigenvalues will be performed on the ITT population for both groups. Continuous variables will be presented as mean (SD) or median (IQR). Categorical variables will be presented as numbers (%). For continuous variables and categorical variables, t-test/rank sum test and chi-square test/Fisher exact test will be used to detect possible disequilibrium between groups.
The outcomes will be performed on both ITT and PP population. Initially, the number and percentage of HS cases and patients with mild-to-severe CMBs at baseline and annul follow-up will be calculated and compared between the two arms by Chi-square test or Fisher's exact test. Then, for all time-to-event models, cumulative event rate during the follow-up will be estimated using the Kaplan-Meier method and will be compared with the log-rank test. If needed, Cox proportional hazard regression analysis will be used after testing proportional hazard assumption and important potential covariates will be adjusted for sensitivity analyses. The mean and variability of serum lipid levels in 36 month follow-up with at least three measurements will be calculated and compared by Student t-test or Wilcoxon rank sum test. Then generalized linear mixed model will be used to analyze the correlation between the proportion of CMBs with different degrees and serum lipid levels over time.
The interim analysis will be performed when 172 subjects have completed at least one follow-up SWI, and it will be focused on subject recruitment, comparability of baseline between groups, sample size assumptions to account for event incidence, follow-up shedding rate, adverse event data, and the effect of treatment on the primary and secondary outcomes.
Discussion
CMBs have been identified as a potential neuroimaging biomarker of SCVD that are prone to spontaneous ICH (10). In IS cohorts, CMBs were found to be associated with the risks of both subsequent ICH and recurrent IS, while the relative risk of subsequent ICH seemed to rise more steeply than that of IS as the burden of CMBs increased (13, 14). This suggests that in the secondary prevention of IS, we need to pay attention to the risk of hemorrhagic events in addition to the benefit of ischemic events, especially for patients with CMBs.
At present, as a choice for secondary prevention of arteriosclerotic cardiovascular disease (ASCVD) patients and primary prevention of patients with elevated risk of ASCVD, statins have been widely used in clinical practice. The SPARCL study was the first to demonstrate that 80 mg of atorvastatin per day reduced the overall incidence of strokes and cardiovascular events in patients with recent stroke or TIA and without known coronary heart disease (17). But this study also revealed an increased risk of ICH related to high-dose statins (17). A recent meta-analysis showed that serum levels of major lipids (TC, TG, and LDL) were negatively correlated with CMBs, but there was no sufficient evidence for statin use as a risk factor for CMBs (19). Thus, it is reasonable to speculate that the risk of statin-related hemorrhage appears to depend on the extent to which certain serum lipid levels are reduced, rather than on statin use per se. Taken together, these findings present a paradoxical problem: high-dose statins have a protective effect on IS recurrence but are likely to be harmful for hemorrhage risk, including HS and CMBs.
In addition, in the secondary prevention of IS, national guidelines around the world delineate different lipid regulation criteria for different baseline conditions, emphasizing the use of high-intensity or maximum tolerated doses of statins to achieve serum LDL-C < 1.8 mmol/L (70 mg/dl) and/or a ≥50% reduction from baseline in those most likely to benefit (25, 26). However, current clinical studies and practice guidelines have failed to suggest lower limits for lipid management goals. Several studies have confirmed that lower serum TG, TC, and LDL-C levels increased the risk of CMBs (17, 19, 24, 27), and the presence of CMBs was associated with increased subsequent ICH risk and poor functional prognosis in patients with AIS (28). These studies suggest that unlimited reduction of serum major lipid levels, mainly LDL-C, is likely to be detrimental in the secondary prevention of IS, especially in IS with CMBs. Therefore, further studies are urgently needed to explore the safe lower limits of lipid management goals in IS with CMBs.
Conclusions
In conclusion, the CHRISTMAS trial is the first and largest secondary prevention trial about lipid-lowering therapy for patients at high risk of future HS. Our results will inform clinical decisions regarding the long-term lipid management in these patients, which is a growing dilemma in clinical practice.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
J-lZ and YX designed the study and revised the manuscript. J-lZ, WY, JT, LZ, YL, and SG drafted the protocol. YX, C-bA, LW, S-jY, JW, and T-qY coordinated the trial. All authors contributed to the article and approved the submitted manuscript.
Funding
CHRISTMAS is an investigator-initiated trial. This trial was financially sponsored by the Youth Science Foundation of Science and Technology Department of Sichuan Province (Grant No. 2022NSFSC1374), the Youth Talent Foundation of Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital (Grant No. 2022QN04), the National Natural Science Foundation for Youth Foundation of China (Grant No. 81601112), and the Huanhua Talent Project of Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital (Grant No. 30420220127).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1097078/full#supplementary-material
References
1. Charidimou A, Shams S, Romero JR, Ding J, Veltkamp R, Horstmann S, et al. Clinical significance of cerebral microbleeds on MRI: a comprehensive meta-analysis of risk of intracerebral hemorrhage, ischemic stroke, mortality, and dementia in cohort studies (v1). Int J Stroke. (2018) 13:454–68. doi: 10.1177/1747493017751931
2. Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. (2009) 8:165–74. doi: 10.1016/S1474-4422(09)70013-4
3. Graff-Radford J, Aakre JA, Knopman DS, Schwarz CG, Flemming KD, Rabinstein AA, et al. Prevalence and heterogeneity of cerebrovascular disease imaging lesions. Mayo Clin Proc. (2020) 95:1195–205. doi: 10.1016/j.mayocp.2020.01.028
4. Ding J, Sigurdsson S, Garcia M, Phillips CL, Eiriksdottir G, Gudnason V, et al. Risk factors associated with incident cerebral microbleeds according to location in older people: the age, gene/environment susceptibility (AGES)-reykjavik study. JAMA Neurol. (2015) 72:682–8. doi: 10.1001/jamaneurol.2015.0174
5. Robles DJ, Dharani A, Rostowsky KA, Chaudhari NN, Ngo V, Zhang F, et al. Older age, male sex, and cerebral microbleeds predict white matter loss after traumatic brain injury. Geroscience. (2022) 44:83–102. doi: 10.1007/s11357-021-00459-2
6. Beaman C, Kozii K, Hilal S, Liu M, Spagnolo-Allende AJ, Polanco-Serra G, et al. Cerebral microbleeds, cerebral amyloid angiopathy, and their relationships to quantitative markers of neurodegeneration. Neurology. (2022) 98:e1605–16. doi: 10.1212/WNL.0000000000200142
7. Li L, Wu DH, Li HQ, Tan L, Xu W, Dong Q, et al. Association of cerebral microbleeds with cognitive decline: a longitudinal study. J Alzheimers Dis. (2020) 75:571–9. doi: 10.3233/JAD-191257
8. Charidimou A, Werring DJ. Cerebral microbleeds as a predictor of macrobleeds: what is the evidence? Int J Stroke. (2014) 9:457–9. doi: 10.1111/ijs.12280
9. Lee SH, Ryu WS, Roh JK. Cerebral microbleeds are a risk factor for warfarin-related intracerebral hemorrhage. Neurology. (2009) 72:171–6. doi: 10.1212/01.wnl.0000339060.11702.dd
10. Wilson D, Ambler G, Shakeshaft C, Brown MM, Charidimou A, Al-Shahi Salman R, et al. Cerebral microbleeds and intracranial haemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischaemic stroke or transient ischaemic attack (CROMIS-2): a multicentre observational cohort study. Lancet Neurol. (2018) 17:539–47. doi: 10.1016/S1474-4422(18)30145-5
11. Gregoire SM, Brown MM, Kallis C, Jager HR, Yousry TA, Werring DJ. MRI detection of new microbleeds in patients with ischemic stroke: five-year cohort follow-up study. Stroke. (2010) 41:184–6. doi: 10.1161/STROKEAHA.109.568469
12. Poels MM, Ikram MA, Van Der Lugt A, Hofman A, Krestin GP, Breteler MM, et al. Incidence of cerebral microbleeds in the general population: the rotterdam scan study. Stroke. (2011) 42:656–61. doi: 10.1161/STROKEAHA.110.607184
13. Shoamanesh A, Hart RG, Connolly SJ, Kasner SE, Smith EE, Marti-Fabregas J, et al. Microbleeds and the effect of anticoagulation in patients with embolic stroke of undetermined source: an exploratory analysis of the NAVIGATE ESUS randomized clinical trial. JAMA Neurol. (2021) 78:11–20. doi: 10.1001/jamaneurol.2020.3836
14. Wilson D, Ambler G, Lee KJ, Lim JS, Shiozawa M, Koga M, et al. Cerebral microbleeds and stroke risk after ischaemic stroke or transient ischaemic attack: a pooled analysis of individual patient data from cohort studies. Lancet Neurol. (2019) 18:653–65. doi: 10.1016/S1474-4422(19)30197-8
15. Shoamanesh A, Charidimou A, Sharma M, Hart RG. Should patients with ischemic stroke or transient ischemic attack with atrial fibrillation and microbleeds be anticoagulated? Stroke. (2017) 48:3408–12. doi: 10.1161/STROKEAHA.117.018467
16. Wilson D, Charidimou A, Ambler G, Fox ZV, Gregoire S, Rayson P, et al. Recurrent stroke risk and cerebral microbleed burden in ischemic stroke and TIA: a meta-analysis. Neurology. (2016) 87:1501–10. doi: 10.1212/WNL.0000000000003183
17. Amarenco P, Bogousslavsky J, Callahan A III, Goldstein LB, Hennerici M, Rudolph AE, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. (2006) 355:549–59. doi: 10.1056/NEJMoa061894
18. Katsanos AH, Lioutas VA, Charidimou A, Catanese L, Ng KKH, Perera K, et al. Statin treatment and cerebral microbleeds: a systematic review and meta-analysis. J Neurol Sci. (2021) 420:117224. doi: 10.1016/j.jns.2020.117224
19. Feng X, Tang Q, Cheng C, Xu S. Low serum lipid levels, use of statin and cerebral microbleeds: a systematic review and meta-analysis. J Clin Neurosci. (2021) 94:216–25. doi: 10.1016/j.jocn.2021.10.032
20. Szejko N, Kirsch E, Falcone GJ. Genetic determinants of LDL cholesterol and risk of intracerebral haemorrhage. Curr Opin Lipidol. (2021) 32:244–8. doi: 10.1097/MOL.0000000000000761
21. Lee M, Cheng CY, Wu YL, Lee JD, Hsu CY, Ovbiagele B. Association between intensity of low-density lipoprotein cholesterol reduction with statin-based therapies and secondary stroke prevention: a meta-analysis of randomized clinical trials. JAMA Neurol. (2022) 79:349–58. doi: 10.1001/jamaneurol.2021.5578
22. Hackam DG, Woodward M, Newby LK, Bhatt DL, Shao M, Smith EE, et al. Statins and intracerebral hemorrhage: collaborative systematic review and meta-analysis. Circulation. (2011) 124:2233–42. doi: 10.1161/CIRCULATIONAHA.111.055269
23. Amarenco P, Kim JS, Labreuche J, Charles H, Abtan J, Bejot Y, et al. A comparison of two LDL cholesterol targets after ischemic stroke. N Engl J Med. (2020) 382:9–19. doi: 10.1056/NEJMoa1910355
24. Wang X, Dong Y, Qi X, Huang C, Hou L. Cholesterol levels and risk of hemorrhagic stroke: a systematic review and meta-analysis. Stroke. (2013) 44:1833–9. doi: 10.1161/STROKEAHA.113.001326
25. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis. (2019) 290:140–205. doi: 10.1016/j.atherosclerosis.2019.08.014
26. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. (2019) 73:e285–350. doi: 10.1016/j.jacc.2018.11.003
27. Lee SH, Bae HJ, Yoon BW, Kim H, Kim DE, Roh JK. Low concentration of serum total cholesterol is associated with multifocal signal loss lesions on gradient-echo magnetic resonance imaging: analysis of risk factors for multifocal signal loss lesions. Stroke. (2002) 33:2845–9. doi: 10.1161/01.STR.0000036092.23649.2E
Keywords: acute ischemic stroke (AIS), cerebral microbleeds (CMBs), intracranial hemorrhage (ICH), statin, randomized controlled trial, protocol
Citation: Zhao J-l, Ai C-b, Wang L, Yang S-j, Wang J, Yang W, Tang J, Zhang L, Li Y, Yan T-q, Gou S, Xie G-g and Xiang Y (2023) A multicenter, prospective, randomized controlled trial of intracranial hemorrhage risk of intensive statin therapy in patients with acute ischemic stroke combined with cerebral microbleeds (CHRISTMAS): Study protocol. Front. Neurol. 14:1097078. doi: 10.3389/fneur.2023.1097078
Received: 19 November 2022; Accepted: 26 January 2023;
Published: 09 February 2023.
Edited by:
Marco Pasi, Centre Hospitalier Universitaire de Tours, FranceReviewed by:
Anna Bonkhoff, Massachusetts General Hospital and Harvard Medical School, United StatesDilek Necioglu Orken, Istanbul Arel University, Türkiye
Julia Ferrari, Krankenhaus der Barmherzigen Brüder Wien, Austria
Copyright © 2023 Zhao, Ai, Wang, Yang, Wang, Yang, Tang, Zhang, Li, Yan, Gou, Xie and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Xiang,  eGlhbmd5YW5ndHR0QDEyNi5jb20=
eGlhbmd5YW5ndHR0QDEyNi5jb20=
 Jia-ling Zhao1
Jia-ling Zhao1 Li Wang
Li Wang Jian Wang
Jian Wang Wei Yang
Wei Yang Yang Xiang
Yang Xiang