- Shanghai East Hospital, Tongji University, Shanghai, China
Objective: This study aimed to investigate the effects of recombinant tissue plasminogen activator intravenous thrombolysis (IVT) on the core growth rate of acute ischemic stroke.
Methods: Stroke patients with large vessel occlusion and non-recanalization from IVT treatment were retrospectively included in this study and divided into two groups: IVT and non-IVT. The core growth rate was estimated by the acute core volume on perfusion CT divided by the last known well time from stroke to CT perfusion. The primary endpoint was the core growth rate, the tissue outcome was 24 h-ASPECTS, and the clinical outcome was a 3-month modified Rankin score.
Results: A total of 94 patients were included with 53 in the IVT group and 41 in the non-IVT group. There was no significant difference in age, gender, hypertension, diabetes, atrial fibrillation, acute NIHSS, and last known well time from stroke to CT perfusion acquisition between the two groups. The core growth rate in the IVT group was lower than that in the non-IVT group, which was statistically significant after multivariate adjustment (coefficient: −5.20, 95% CI= [−9.85, −0.56], p = 0.028). There was a significant interaction between the IVT and the collateral index in predicting the core growth rate. The analysis was then stratified according to the collateral index, and the results suggested that IVT reduced the core growth rate more significantly after the worsening of collateral circulation (coefficient: 15.38, 95% CI= [−26.25, −4.40], p = 0.007). The 3-month modified Rankin score and 24 h-ASPECTS were not statistically significant between the two groups.
Conclusion: Intravenous thrombolysis reduces the core growth rate in patients with AIS, especially those with poor collateral status.
1. Introduction
Intravenous thrombolysis (IVT) is an established treatment for acute ischemic stroke (AIS), and it can be rapidly initiated after clinical assessment and cranial CT scan (1–3). However, IVT also has significant limitations, such as the patients' need to receive IV tPA within 4.5 h of the onset, and the recanalization rates are low in patients with large vascular occlusion (LVO), that is, a meta-analysis reported approximately 35% for M1 MCA occlusions, 13% for ICA occlusions, and 13% for BA occlusions (4).
Since 2015, several clinical trials acknowledged the superiority of endovascular thrombectomy (EVT), which has a higher rate of recanalization of LVO and a longer treatment window (5–9). Therefore, the “bridge” therapy was proposed to use EVT to rescue patients with a lack of recanalization after IVT. Some clinical trials showed that early administration of alteplase can promote microvessel patency and that the rate of successful recanalization in bridge therapy was significantly higher than that in patients who only accept EVT (10, 11). However, IVT may increase the risk of bleeding (12) and promote thromboplastic migration (13). Hence, whether patients with LVO-AIS who arrive at the hospital within 4.5 h of the onset can benefit from the “bridge” therapy is a research hotspot (14, 15).
In patients with AIS, successful recanalization and core volume are the strongest predictors of outcome (16). Before vessel recanalization, the infarct core increases linearly within 6 h of stroke onset (17, 18). Assuming that the core at the time of stroke onset was 0, the infarction volume divided by the time from stroke onset to CTP can be used to estimate the speed of cerebral infarction progression. The core growth rate has been reported to be an independent predictor of clinical outcomes and is highly associated with the collateral status. Reducing the core growth rate may reduce the volume of core infarction and may have important therapeutic implications on AIS.
This study aimed to assess the efficacy of IVT on changing infarct core growth rate in patients with LVO-AIS who had not achieved recanalization.
2. Materials and methods
2.1. General information
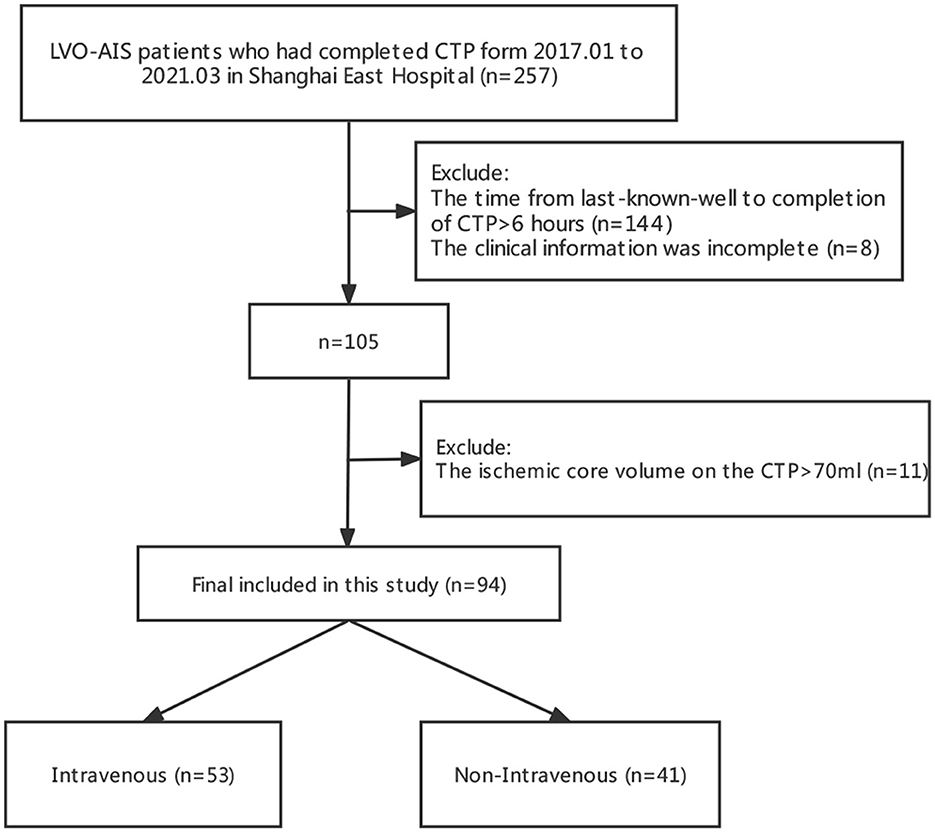
We conducted a retrospective cohort study that involved 94 AIS patients with LVO between January 2017 and March 2021 at the Shanghai East Hospital—Department of Neurology. All the patients were divided into two groups according to whether or not they received IVT.
2.2. Inclusion criteria
Patients were selected based on the following criteria: (1) All the patients met the diagnostic criteria of acute cerebral infarction due to LVO; according to the guidelines, the IVT group patients met the indications of IVT, and the contraindications of IVT were excluded; (2) the last known well time from stroke to completion of CTP was <6 h; (3) the ischemic core volume on the CTP was <70 ml, penumbra ≥10 ml, and radio > 1.2; and (4) the clinical information was complete.
2.3. Methods
The clinical data of patients included age, gender, and histories of hypertension, diabetes, heart issues, prior stroke, current smoking, and NIHSS recorded at the hospital arrival, as well as radiographic data. The DT collateral index was used to evaluate the collateral status (19).
The IVT group patients were given 0.9 mg/kg of rt-PA for thrombolysis: 10% of the total dose was given intravenously for 1 min, while the remaining dose was injected intravenously 1 h later.
2.4. Imaging acquisition and post-processing
Baseline CT imaging included brain non-contrast CT, CTP, and CTA, obtained with different CT scanners (64, 128, 256, or 320 detectors, with Toshiba [Tokyo, Japan], Siemens [Munich, Germany], or GE [Cleveland, OH, USA] scanners). The axial coverage ranged from 80 to 160 mm.
The CTP data were processed by commercial software MIStar (Apollo Medical Imaging Technology, Melbourne, Vic, Australia). CTP parameters were generated by applying the mathematical algorithm of singular value decomposition with delay and dispersion correction (20, 21). The following four CTP parameters were generated: cerebral blood flow (CBF), cerebral blood volume (CBV), mean transit time (MTT), and delay time (DT). The penumbra and core volume were measured on acute CTP with dual threshold setting (22): DT at the threshold of 3 s for whole ischemic lesion volume and CBF at the threshold setting of 30% for acute core volume. The collateral index was defined by the ratio of DT >6 s/DT >2 s volume.
2.5. Calculation of the ischemic core growth rate
The core growth rate was determined using the baseline core volume divided by the time between the symptom onset and the CTP. It was assumed that the core volume was zero just prior to symptom onset and would grow in a near-linear pattern within 6 h of stroke onset (23). This study calculated the core growth rate using the following approach:
Core growth rate = Acute core volume on CTP / Last known well time from stroke to CTP (19, 24).
2.6. Statistical analysis
Data were statistically analyzed using the statistical software SPSS 25.0. The normality of the continuous variables was examined using the Kolmogorov–Smirnov test. When the normality assumption was not met, a non-parametric test was used. The categorical variables were compared by the chi-square test. Multi-factor linear regression was used to analyze predictors of ischemic core growth rate. Variables with a p < 0.25 were included in the regression model. In addition, collateral circulation was included in the model as a significant predictor. A scatter diagram was used to describe the interaction between IVT and DT collateral index in predicting the core growth rate. Then, the core growth rate of IVT vs. non-IVT patients was plotted across the collateral index (with a 0.100 increment). Within each collateral index category, the predictive power of IVT (vs. non-IVT) on the patient core growth rate was assessed by regression models.
2.7. Tissue outcomes
Alberta stroke program early CT score (ASPECTS) was used to evaluate the extent of anterior circulation infarction (25). The posterior circulation stroke was assessed by pc-ASPECTS (26). A normal CT scan has an ASPECTS value of 10 points. ΔASPECTS is defined as 24 h-ASPECTS minus baseline ASPECTS, which would be used to describe the change in brain tissue. Multi-factor logistic and linear regression was used to describe the relationship between intravenous therapy and clinical outcomes and tissue outcomes.
2.8. Patient outcomes
The primary endpoint was the core growth rate. The clinical outcome was the modified Rankin score (mRS) at three months. The good clinical outcome was defined by an mRS score of 0–2 vs. an mRS score of 3–6, and the poor clinical outcome was an mRS score of 5–6 vs. an mRS score of 0–4.
3. Results
The flow diagram of the study is given in Figure 1. A total of 94 patients met the inclusion criteria, of whom 53 patients received IVT and 41 patients were treated without IVT.
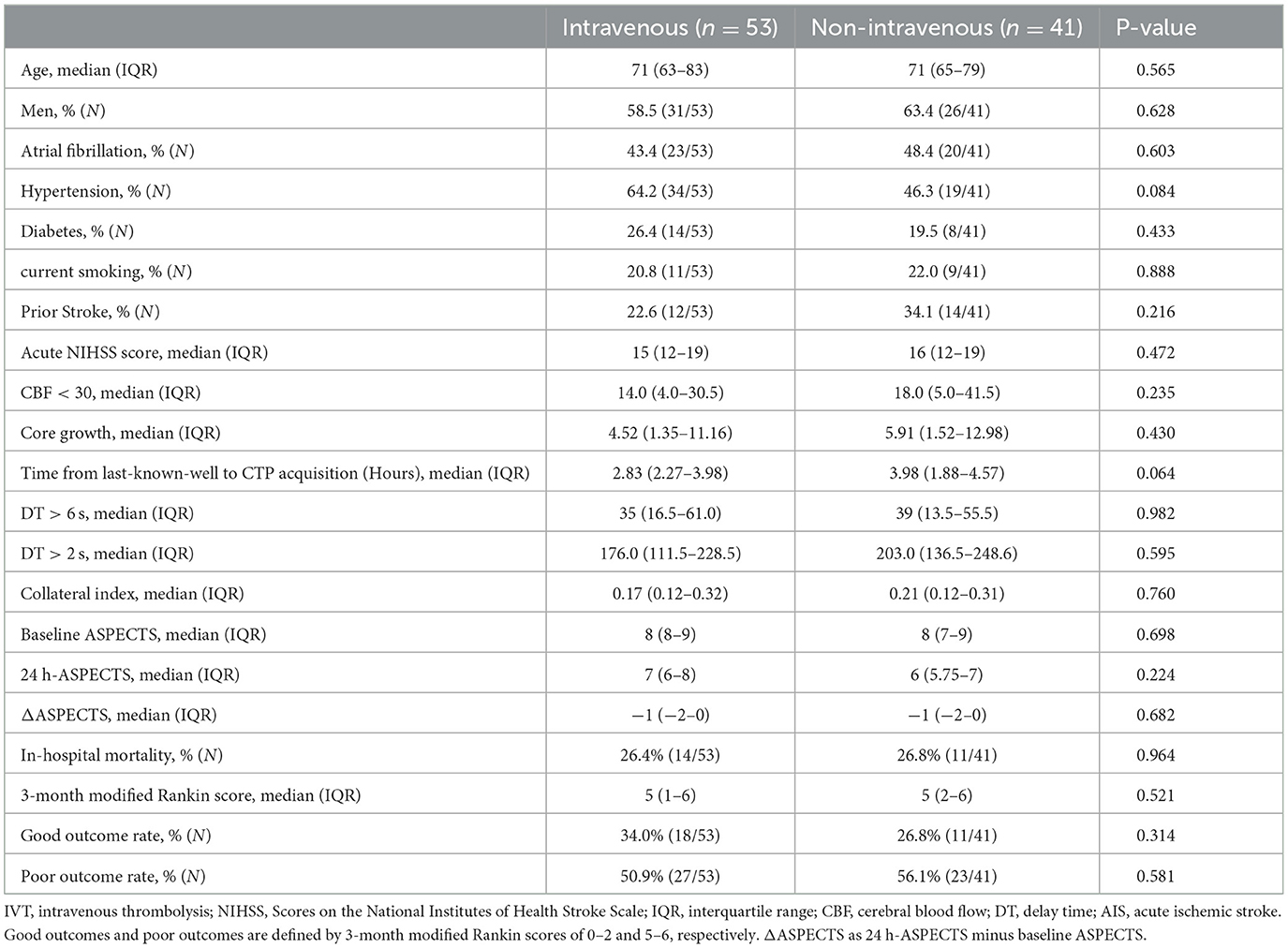
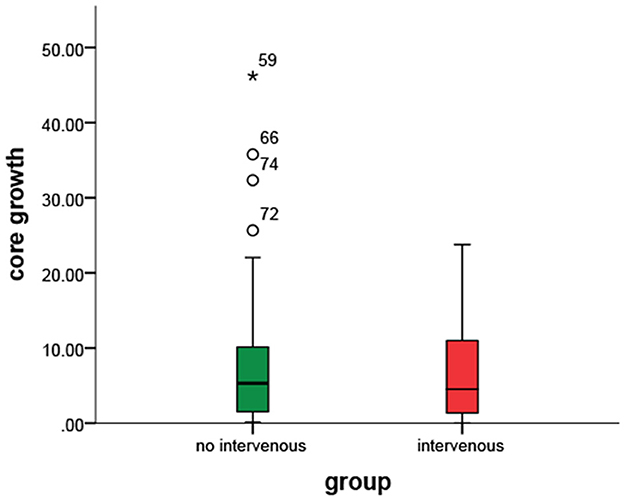
The baseline characteristics of the patients were similar in the two groups (Table 1). The average age of the patient was 39–98 years, and 57 patients (61%) were men. The median baseline NIHSS was 16 (IQR = 7). The median last known well time from stroke to CTP was 3.18 h (IQR, 2.27–3.98) in the IVT group and 3.4 h (IQR, 1.88–4.57) in the non-IVT group. There was no statistical difference between the two groups in age, gender, or AIS risk factors such as hypertension, diabetes mellitus, atrial fibrillation, previous history of stroke, or current smoking (P>0.05). Neither infarct volume nor collateral index showed a significant difference between the two groups (p = 0.982 and p = 0.760). No between-group differences were found at baseline ASPECTS or ΔASPECTS (p = 0.698 and p = 0.682). Although the difference was not statistically significant, 24 h-ASPECTS in the IVT group was slightly higher (7 vs. 6).
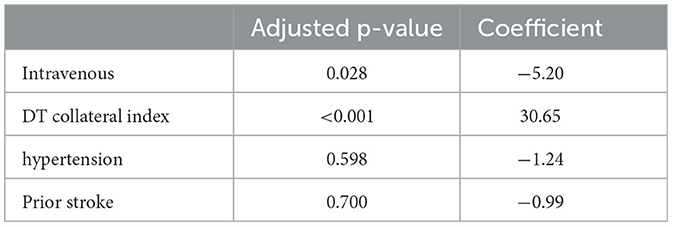
Delay time collateral index (coefficient: 30.65, 95% CI= [14.37, 46.93], p<0.001) and intravenous therapy (coefficient: −5.20, 95% CI = [−9.85, −0.56], p = 0.028) showed significant differences. Intravenous may decrease the core growth rate of 5 ml/h for patients with stroke (Table 2).
Figure 2 depicts the scatter plots for the association between the DT collateral index and the core growth rate in two groups.
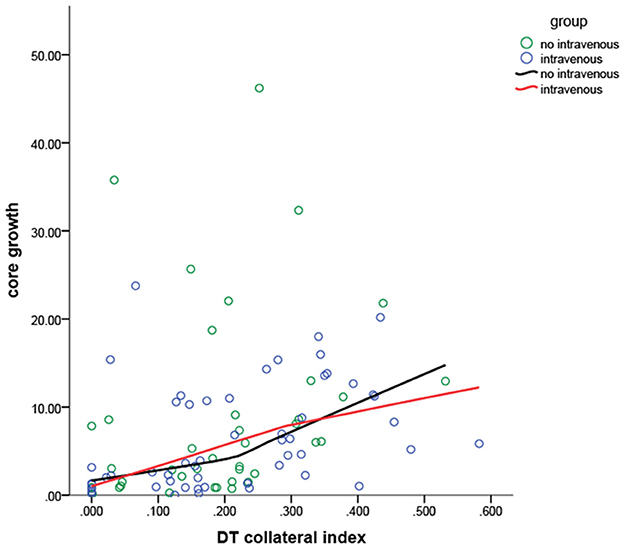
Figure 2. The core growth rate in different collateral indexes for the patients in the two groups. The IVT and non-IVT groups intersected two times, which suggested a significant interaction between the IVT and the collateral index, indicating that both of them interfere significantly with the core growth rate. The line of the non-IVT group showed a major increase when the DT collateral index was 0.250.
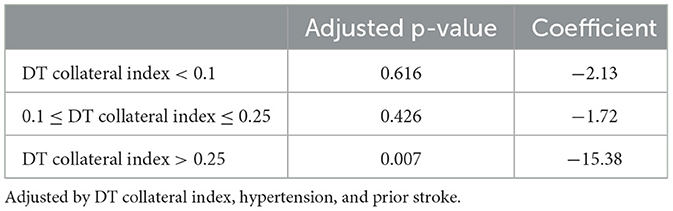
Delay time collateral index was classified into three categories based on the core growth rate of the IVT vs. the non-IVT group (Figure 2). For DT collateral index <0.100 and 0.100–0.250, there was no statistical significance in the effect of IVT on the core growth rate (p = 0.616 and p = 0.426). For DT collateral index >0.25, after adjusting for DT collateral index, hypertension, and prior stroke, the IVT showed a statistically significant result on the core growth rate (coefficient: 15.38, 95% CI= [−26.25, −4.40], p = 0.007) (Table 3). In other words, for patients with poor collateral index, IVT may significantly decrease the core growth rate (Figures 3, 4).

Figure 4. Differences in the core growth rate between within the IVT (or not) group and within a DT collateral index group. IVT significantly decreased the core growth rate in patients with poor collateral index.
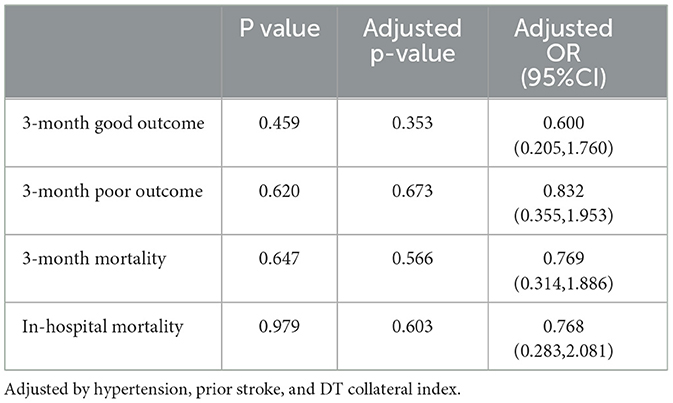
Univariate and multivariate regression analyses were used to explore the association between intravenous therapy and clinical outcomes. There was no statistical difference in the 3-month modified Rankin score (Table 1). After adjusting for hypertension, prior stroke, and DT collateral index, both the good outcome (OR = 0.60, 95% CI = [0.205, 1.760]) and poor outcome (OR = 0.83, 95% CI = [0.355,1.953]) showed no significant predictive power between the two groups (Table 4). No significant between-group differences were detected in 3-month mortality (26.4 vs. 26.8%; OR=0.77, 95% CI = [0.314, 1.886]).
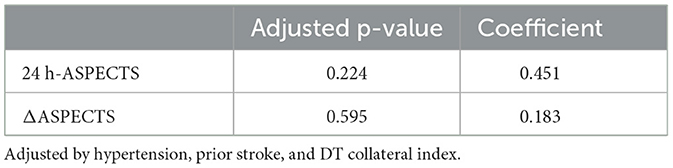
After multivariate adjustments, the differences in 24 h-ASPECTS (coefficient: 0.451, 95% CI= [-0.281, 1.183], p = 0.224) and ΔASPECTS (coefficient: 0.183, 95% CI = [−0.501, 0.868], p = 0.595) were not statistically significant (Table 5).
4. Discussion
In acute ischemic stroke (AIS) treatment, recanalization of the occluded vessel is crucial for a good clinical outcome (27). Intravenous thrombolysis (IVT) with rt-PA is a conventional treatment for AIS. However, the low vascular recanalization rate led to many conflicting views (4, 28). When clinical symptoms of patients with LVO-AIS did not significantly improve after receiving IVT, endovascular therapy was required to achieve recanalization. For such patients, the role of IVT remains controversial. This study showed that IVT may reduce the core growth rate in patients with AIS, even if the vessel did not achieve recanalization. Moreover, this effect was influenced by collateral circulation and was clearer in patients with poor collateral circulation.
Whether patients with AIS can benefit from the “bridge” therapy (BT) is a research hotspot. In a meta-analysis of 38 eligible observational studies, BT was associated with a higher likelihood of 3-month functional independence compared to direct mechanical thrombectomy (29). Whether IVT will extend the total time of recanalization therapy remains controversial. In recent years, numerous studies showed that the average recanalization time was not statistically different between the “bridge” therapy and direct thrombectomy (14, 15, 29), indicating that IVT did not delay the time to recanalization. In addition, with the application of tenecteplase, the time difference between the two treatment modalities was further reduced (30). This study demonstrated that IVT may reduce the core growth rate without vessel recanalization. Some studies also found that, in time-window patients who were transferred to an EVT capable center, the outcomes were better in patients who had previously received IVT (31). Therefore, IVT is considered the first-line treatment for patients with AIS even with LVO and IVT is required in those patients as early as possible.
Infarct core volume is an independent predictor for the outcome of patients with AIS. The smaller the infarct core, the better the likelihood of clinical outcomes (32). In recent years, several studies demonstrated that the infarct core grows linearly during the first 6 h of AIS (17, 18). Wheeler suggested that the early stroke core growth curves exhibited a nearly linear growth during the first 8 h after symptom onset for patients with <10% reperfusion (23). Therefore, the core growth rate in the first 6 h from the onset can predict the infarct core to some extent. In this study, all patients successfully completed the CTP examination in 6 h from last known well time. IVT was validated to reduce the infarct core growth rate in this study, and one possible mechanism is probably due to the reduction in thrombus volume by IVT (33, 34). In addition, the rt-PA can act on the distal microvasculature and reduce microvenous thrombosis (35). Thus, the blood supply in infarct areas can be improved. A retrospective study showed that the rate of successful recanalization was significantly higher in patients who received IVT before mechanical thrombectomy (11), and it might be implicated in those mechanisms. In this study, we found that IVT may reduce the infarct core growth rate, and patients with AIS who had a lower collateral circulation and underwent IVT exhibited slower infarct growth rates. The impact of collateral circulation on infarct core growth is well established (19). Better collateral circulation indicates slower infarct growth, while the effect of IVT is not perfect. However, in patients who had poor collateral circulation, along with a decrease in the thrombosis volume and thrombus load in the local microcirculation, the blood flow might have improved more in the ischemic penumbra.
There was no statistical difference in a 3-month modified Rankin score between the two groups. The potential explanation offered might be that, although the rate of core growth was decreased in the IVT group, the clinical outcome was largely decided by the developed infarct core volume and the degree of recanalization. Several studies confirmed that collateral circulation is the factor that has the greatest impact on the growth of infarct core (36, 37). Moreover, the factors associated with collateral circulation were age, smoking, hypertension (38), and the use of statins (39, 40). All these factors had no statistical significance in this study, which may have resulted in no difference in collateral circulation between the two groups. All patients in this trial had LVO, and they accepted mechanical thrombectomy after the CTP examination, and the intraoperative recanalization levels have been shown to impact the prognosis of stroke (41). Thus, the follow-up treatments might heavily influence the clinical outcome. It is difficult to assess the precise relationship between IVT with patient prognosis. Further studies may be needed to elucidate this relationship in a future study.
Although the 24 h-ASPECT in the IVT group was slightly higher than that of the non-IVT group, there was no significant difference in the tissue outcomes. Two considerations may have contributed to this result. On the one hand, despite this study finding that IVT may reduce the core growth rate in patients with LVO, the final infarct core was largely decided by the collateral circulation and the efficacy of endovascular therapy. On the other hand, the sample size in our study is relatively small, which needs to be expanded for a more in-depth research in future, and the effects of intravenous thrombolysis on histological changes would have likely been observed.
This retrospective single-center study has several limitations. First, the lack of randomized treatment allocation in this study was the main limitation. Second, there were no clear differences in baseline core growth rates between the two groups, which might be affected by the collateral circulation. We further adjusted it by the multivariate regression models and removed the effects of the collateral index, which showed positive results. Third, this study was conducted with a small sample size, which might have led to a certain degree of bias. Fourth, some of the patients had unwitnessed onset and the time of stroke onset was uncertain. Hence, the estimation of time from onset to CTP may be extended.
Although the difference in the long-term prognosis of the patients was not observed, the present study provided important new information about the benefit of IVT. “Time is brain” in this study, and IVT successfully reduced the core growth rate, suggesting the underlying application in the treatment of acute cerebral infarction. For patients with AIS who arrive at the hospital within the time window, we suggest IVT in the absence of contraindications and expect more benefits from the “bridge” therapy. Further studies are required to confirm these conclusions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of the Shanghai East Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YZ and ZH contributed to the conception and design of the present study. XW and HZ contributed to drafting a significant portion of the manuscript or figures. QW, HS, YX, and LX contributed to the acquisition and analysis of data. YL, CC, and GL helped perform the analysis with constructive discussions. All authors contributed to the article and approved the submitted version.
Funding
YZ received funding from the Science and Technology Development Fund of Shanghai Pudong New Area (Grant No. PKJ2021-Y07), China. GL received funding from the Shanghai Key Clinical Discipline (Grant No. shslczdzk06103), China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. (1995) 333:1581–7. doi: 10.1056/NEJM199512143332401
2. Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 45 hours after acute ischemic stroke. N Engl J Med. (2008) 359:1317–29. doi: 10.1056/NEJMoa0804656
3. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
4. Seners P, Turc G, Maïer B, Mas JL, Oppenheim C, Baron JC. Incidence and predictors of early recanalization after intravenous thrombolysis: a systematic review and meta-analysis. Stroke. (2016) 47:2409–12. doi: 10.1161/STROKEAHA.116.014181
5. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. (2015) 372:1009–18. doi: 10.1056/NEJMoa1414792
6. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. (2015) 372:11–20. doi: 10.1056/NEJMoa1411587
7. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. (2015) 372:1019–30. doi: 10.1056/NEJMoa1414905
8. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. (2015) 372:2296–306. doi: 10.1056/NEJMoa1503780
9. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. (2015) 372:2285–95. doi: 10.1056/NEJMoa1415061
10. Guedin P, Larcher A, Decroix JP, Labreuche J, Dreyfus JF, Evrard S, et al. Prior IV thrombolysis facilitates mechanical thrombectomy in acute ischemic stroke. J Stroke Cerebrovasc Dis. (2015) 24:952–7. doi: 10.1016/j.jstrokecerebrovasdis.2014.12.015
11. Behme D, Kabbasch C, Kowoll A, Dorn F, Liebig T, Weber W, et al. Intravenous thrombolysis facilitates successful recanalization with stent-retriever mechanical thrombectomy in middle cerebral artery occlusions. J Stroke Cerebrovasc Dis. (2016) 25:954–9. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.007
12. Werner M, Lopez-Rueda A, Zarco F, Román LS, Blasco J, Amaro S, et al. Mechanical thrombectomy in acute basilar artery occlusion: a safety and efficacy single centre study. Interv Neuroradiol. (2016) 22:310–7. doi: 10.1177/1591019916631145
13. Ohara T, Menon BK, Al-Ajlan FS, Horn M, Najm M, Al-Sultan A, et al. Thrombus migration and fragmentation after intravenous alteplase treatment: the INTERRSeCT study. Stroke. (2021) 52:203–12. doi: 10.1161/STROKEAHA.120.029292
14. Suzuki K, Matsumaru Y, Takeuchi M, Morimoto M, Kanazawa R, Takayama Y, et al. Effect of mechanical thrombectomy without vs with intravenous thrombolysis on functional outcome among patients with acute ischemic stroke: the SKIP randomized clinical trial. JAMA. (2021) 325:244–53. doi: 10.1001/jama.2020.23522
15. Zi W, Qiu Z, Li F, Sang H, Wu D, Luo W, et al. Effect of endovascular treatment alone vs intravenous alteplase plus endovascular treatment on functional independence in patients with acute ischemic stroke: the DEVT randomized clinical trial. JAMA. (2021) 325:234–43. doi: 10.1001/jama.2020.23523
16. Panni P, Gory B, Xie Y, Consoli A, Desilles JP, Mazighi M, et al. Acute stroke with large ischemic core treated by thrombectomy. Stroke. (2019) 50:1164–71. doi: 10.1161/STROKEAHA.118.024295
17. Markus R, Reutens DC, Kazui S, Read S, Wright P, Chambers BR, et al. Topography and temporal evolution of hypoxic viable tissue identified by 18F-fluoromisonidazole positron emission tomography in humans after ischemic stroke. Stroke. (2003) 34:2646–52. doi: 10.1161/01.STR.0000094422.74023.FF
18. Christoforidis GA, Vakil P, Ansari SA, Dehkordi FH, Carroll TJ. Impact of pial collaterals on infarct growth rate in experimental acute ischemic stroke. AJNR Am J Neuroradiol. (2017) 38:270–5. doi: 10.3174/ajnr.A5003
19. Lin L, Chen C, Tian H, Bivard A, Spratt N, Levi CR, et al. Perfusion computed tomography accurately quantifies collateral flow after acute ischemic stroke. Stroke. (2020) 51:1006–9. doi: 10.1161/STROKEAHA.119.028284
20. Lin L, Bivard A, Kleinig T, Spratt NJ, Levi CR, Yang Q, et al. Correction for delay and dispersion results in more accurate cerebral blood flow ischemic core measurement in acute stroke. Stroke. (2018) 49:924–30. doi: 10.1161/STROKEAHA.117.019562
21. Bivard A, Levi C, Krishnamurthy V, McElduff P, Miteff F, Spratt NJ, et al. Perfusion computed tomography to assist decision making for stroke thrombolysis. Brain. (2015) 138:1919–31. doi: 10.1093/brain/awv071
22. Lin L, Bivard A, Krishnamurthy V, Levi CR, Parsons MW. Whole-brain CT perfusion to quantify acute ischemic penumbra and core. Radiology. (2016) 279:876–87. doi: 10.1148/radiol.2015150319
23. Wheeler HM, Mlynash M, Inoue M, Tipirnini A, Liggins J, Bammer R, et al. The growth rate of early DWI lesions is highly variable and associated with penumbral salvage and clinical outcomes following endovascular reperfusion. Int J Stroke. (2015) 10:723–9. doi: 10.1111/ijs.12436
24. Sarraj A, Hassan AE, Grotta J, Blackburn S, Day A, Abraham M, et al. Early infarct growth rate correlation with endovascular thrombectomy clinical outcomes: analysis from the SELECT study. Stroke. (2021) 52:57–69. doi: 10.1161/STROKEAHA.120.030912
25. Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS study group Alberta stroke programme early CT score. Lancet. (2000) 355:1670–4. doi: 10.1016/S0140-6736(00)02237-6
26. Puetz V, Sylaja PN, Coutts SB, Hill MD, Dzialowski I, Mueller P, et al. Extent of hypoattenuation on CT angiography source images predicts functional outcome in patients with basilar artery occlusion. Stroke. (2008) 39:2485–90. doi: 10.1161/STROKEAHA.107.511162
27. Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. (2007) 38:967–73. doi: 10.1161/01.STR.0000258112.14918.24
28. Asadi H, Dowling R, Yan B, Wong S, Mitchell P. Advances in endovascular treatment of acute ischaemic stroke. Intern Med J. (2015) 45:798–805. doi: 10.1111/imj.12652
29. Katsanos AH, Malhotra K, Goyal N, Arthur A, Schellinger PD, Köhrmann M, et al. Intravenous thrombolysis prior to mechanical thrombectomy in large vessel occlusions. Ann Neurol. (2019) 86:395–406. doi: 10.1002/ana.25544
30. Huang X, Cheripelli BK, Lloyd SM, Kalladka D, Moreton FC, Siddiqui A, et al. Alteplase versus tenecteplase for thrombolysis after ischaemic stroke (ATTEST) : a phase 2, randomised, open-label, blinded endpoint study. Lancet Neurol. (2015) 14:368–76. doi: 10.1016/S1474-4422(15)70017-7
31. Sarraj A, Grotta J, Albers GW, Hassan AE, Blackburn S, Day A, et al. Clinical and neuroimaging outcomes of direct thrombectomy vs bridging therapy in large vessel occlusion: analysis of the SELECT cohort study. Neurology. (2021) 96:e2839–e53. doi: 10.1212/WNL.0000000000012063
32. Padroni M, Bernardoni A, Tamborino C, Roversi G, Borrelli M, Saletti A, et al. Cerebral blood volume ASPECTS is the best predictor of clinical outcome in acute ischemic stroke: a retrospective, combined semi-quantitative and quantitative assessment. PLoS ONE. (2016) 11:e0147910. doi: 10.1371/journal.pone.0147910
33. Rossi R, Fitzgerald S, Molina S, Mereuta OM, Douglas A, Pandit A, et al. The administration of rtPA before mechanical thrombectomy in acute ischemic stroke patients is associated with a significant reduction of the retrieved clot area but it does not influence revascularization outcome. J Thromb Thrombolysis. (2021) 51:545–51. doi: 10.1007/s11239-020-02279-1
34. Rossi R, Molina S, Mereuta OM, Douglas A, Fitzgerald S, Tierney C, et al. Does prior administration of rtPA influence acute ischemic stroke clot composition? Findings from the analysis of clots retrieved with mechanical thrombectomy from the RESTORE registry. J Neurol. (2022) 269:1913–20. doi: 10.1007/s00415-021-10758-5
35. Desilles JP, Loyau S, Syvannarath V, Gonzalez-Valcarcel J, Cantier M, Louedec L, et al. Alteplase reduces downstream microvascular thrombosis and improves the benefit of large artery recanalization in stroke. Stroke. (2015) 46:3241–8. doi: 10.1161/STROKEAHA.115.010721
36. Lin L, Yang J, Chen C, Tian H, Bivard A, Spratt NJ, et al. Association of collateral status and ischemic core growth in patients with acute ischemic stroke. Neurology. (2021) 96:e161–e70. doi: 10.1212/WNL.0000000000011258
37. Puhr-Westerheide D, Tiedt S, Rotkopf LT, Herzberg M, Reidler P, Fabritius MP, et al. Clinical and imaging parameters associated with hyperacute infarction growth in large vessel occlusion stroke. Stroke. (2019) 50:2799–804. doi: 10.1161/STROKEAHA.119.025809
38. Wufuer A, Mijiti P, Abudusalamu R, Dengfeng H, Jian C, Jianhua M, et al. Blood pressure and collateral circulation in acute ischemic stroke. Herz. (2019) 44:455–9. doi: 10.1007/s00059-018-4691-5
39. Ovbiagele B, Saver JL, Starkman S, Kim D, Ali LK, Jahan R, et al. Statin enhancement of collateralization in acute stroke. Neurology. (2007) 68:2129–31. doi: 10.1212/01.wnl.0000264931.34941.f0
40. Lee MJ, Bang OY, Kim SJ, Kim GM, Chung CS, Lee KH, et al. Role of statin in atrial fibrillation-related stroke: an angiographic study for collateral flow. Cerebrovasc Dis. (2014) 37:77–84. doi: 10.1159/000356114
41. Dargazanli C, Fahed R, Blanc R, Gory B, Labreuche J, Duhamel A, et al. Modified thrombolysis in cerebral infarction 2C/thrombolysis in cerebral infarction 3 reperfusion should be the aim of mechanical thrombectomy: insights from the ASTER trial (contact aspiration vs. stent retriever for successful revascularization). Stroke. (2018) 49:1189–96. doi: 10.1161/STROKEAHA.118.020700
Keywords: acute ischemic stroke, intravenous thrombolysis, core growth rate, collateral circulation, large vessel occlusion, reperfusion, therapy
Citation: Wang X, Zhang H, Wang Q, Li G, Shen H, Xiao Y, Xu L, Long Y, Chen C, Huang Z and Zhang Y (2023) Effect of intravenous thrombolysis on core growth rate in patients with acute cerebral infarction. Front. Neurol. 14:1096605. doi: 10.3389/fneur.2023.1096605
Received: 12 November 2022; Accepted: 30 January 2023;
Published: 23 February 2023.
Edited by:
Wenbo Zhao, Capital Medical University, ChinaReviewed by:
Song Zhang, Hangzhou Children's Hospital, ChinaPaolo Candelaresi, Ospedale Antonio Cardarelli, Italy
Copyright © 2023 Wang, Zhang, Wang, Li, Shen, Xiao, Xu, Long, Chen, Huang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengyu Huang,  aHVhbmd6eTA3MDZAMTYzLmNvbQ==; Yue Zhang,
aHVhbmd6eTA3MDZAMTYzLmNvbQ==; Yue Zhang,  ZHl1ZXpoYW5nQDEyNi5jb20=
ZHl1ZXpoYW5nQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Xueqi Wang
Xueqi Wang Hao Zhang
Hao Zhang Qi Wang†
Qi Wang† Gang Li
Gang Li Luran Xu
Luran Xu Chen Chen
Chen Chen Yue Zhang
Yue Zhang