- 1Department of Neurology, People's Hospital of Wanning, Wanning, Hainan, China
- 2Department of Nursing, People's Hospital of Wanning, Wanning, Hainan, China
- 3College of Life Sciences, Northwest University, Xi'an, Shaanxi, China
- 4Shaanxi Provincial Key Laboratory of Biotechnology, Northwest University, Xi'an, Shaanxi, China
- 5Key Laboratory of Resource Biology and Biotechnology in Western China, Ministry of Education, Northwest University, Xi'an, Shaanxi, China
Background: Stroke is a common cerebrovascular disease. The purpose of this study was to explore the association between LIPC single nucleotide polymorphisms (SNPs) and the risk of stroke in the Chinese population.
Methods: This study recruited 710 stroke patients and 701 healthy controls. The four SNPs (rs690, rs6083, rs3829461, and rs6074) in LIPC were genotyped by the Agena MassARRAY. The correlation between LIPC polymorphisms and stroke risk was measured by odds ratio (OR) and 95% confidence interval (CI). In addition, multifactor dimensionality reduction (MDR) analysis was used to evaluate the impact of SNP–SNP interaction on stroke risk.
Results: Overall analysis showed that rs690 was associated with an increased risk of stroke (T vs. G: OR = 1.19, 95% CI: 1.01–1.40, p = 0.041; additive: OR = 1.20, 95% CI: 1.01–1.42, p = 0.036). The stratified analysis revealed that rs690 was associated with an increased risk of stroke in subjects aged ≤ 64 years, male patients, and smokers, and rs6074 was associated with an increased risk of stroke in subjects aged > 64 years, male patients, drinkers, and non-smokers (p < 0.05). The results of the MDR analysis suggested the four-locus model as the most favorable model for assessing the risk of stroke. The analysis of clinical parameters of stroke patients showed that rs690 was correlated with platelet distribution width (PDW) (p = 0.014) and hematocrit levels (p = 0.004), and rs6074 was correlated with low-density lipoprotein cholesterol (LDL-C) level (p = 0.033). Furthermore, bioinformatics analysis results demonstrated that the expression levels of LIPC and its related genes (APOB, CETP, PNPLA2, and LMF1) were significantly different between the control and stroke groups (p < 0.05), and LIPC-related proteins were mainly related to lipid metabolism.
Conclusion: This study indicated that rs690 and rs6074 in LIPC were significantly associated with increased risk of stroke in the Chinese population, possibly by regulating the levels of PDW, HCT, and LDL-C.
Introduction
Stroke is manifested by neurological deficits caused by impaired blood circulation in the brain. It is mainly described as a brain disease with symptoms such as sudden faintness, hemiplegia, numbness of the limbs, and tilted mouth and tongue. Although medical treatment has made great progress in recent years, the incidence of stroke is still increasing due to factors such as population aging and changes in people's living habits, making it the world's second-leading cause of death and the main cause of disability (1). Notably, China is facing the largest burden of stroke (2) in recent years, with the incidence and prevalence of stroke still increasing, especially in rural areas (3). Deaths from stroke in China account for almost one-third of the total stroke deaths in the world (4). Therefore, research studies on stroke are particularly necessary.
A large number of studies have indicated that genetic factors play an important role in the pathogenesis of stroke (5). Moreover, hypertension, diabetes, atrial fibrillation, smoking, hyperlipidemia, and obesity are also studied to be risk factors for stroke (6). Lipase C (LIPC) is located on the long arm of chromosome 15 in band 21.3 and is mainly responsible for encoding lipase C. The dual function of the LIPC gene, namely, triglyceride hydrolase and ligand/bridging factor, is mainly used for receptor-mediated lipoprotein uptake. Common variants in LIPC have been shown to be associated with plasma lipid levels (7, 8). A previous study has demonstrated an association between the polymorphism (-514 C > T) in LIPC and type 2 diabetes risk, which is modified by the high-density lipoprotein cholesterol (HDL-C) level (9). LIPC variant rs2070895 has been found to be associated with carotid atherosclerosis (10) and hypertension (11). Moreover, LIPC variants (rs2043085 and rs1532085) have been observed to be significantly associated with body mass index (BMI), waist circumference (WC), lipid accumulation product, visceral adiposity index, and triglyceride glucose (TyG) index-related parameters (12). However, there are very few studies on the correlation between LIPC polymorphisms and the risk of stroke.
Therefore, this study was undertaken to detect the association between LIPC polymorphisms and the risk of stroke from 710 patients with stroke and 701 healthy controls in the Chinese population. The results of this study will provide a certain scientific theoretical basis for early screening, prevention, and diagnosis of stroke in the Chinese population.
Materials and methods
Participants
We recruited 710 stroke patients (430 male patients and 280 female patients) from the People's Hospital of Wanning who were admitted to the hospital within 72 h after the onset of symptoms. All patients with focal neurological deficit symptoms persisting for more than 24 h were examined by two professional stroke neurologists and diagnosed by brain computed tomography (CT), magnetic resonance imaging (MRI), cerebrovascular angiography, and lumbar puncture (13). This study excluded patients with transient ischemic attack, lacunar infarction, familial stroke, severe heart, kidney, liver, endocrine, and bone diseases, and cancers. A total of 701 healthy participants (424 male patients and 277 female patients) were selected as the control group from the health examination center of the People's Hospital of Wanning during the same period as the cases. All control individuals had no family history of stroke, severe heart, kidney, liver, endocrine and bone diseases, cancers, and immune-related diseases. The gender and age of the control group were matched with the patients in the case group. The basic information of all subjects, including age, sex, smoking status, drinking status, and clinical test indicators, were collected from questionnaires and clinical data. This study was approved by the Ethics Committee of the People's Hospital of Wanning and conformed to the Declaration of Helsinki. All subjects were informed of the purpose, significance, and experimental process of the study before the experiment. This study was conducted after obtaining informed consent from subjects.
SNP selection and genotyping
The four LIPC polymorphisms (rs690, rs6083, rs3829461, and rs6074) were randomly selected in this study according to previous articles (7, 14, 15). All SNPs had a minor allele frequency (MAF) > 0.05 in the Chinese Han Beijing (CHB) population from the 1000 Genomes Project (http://www.internationalgenome.org/). The functions of candidate SNPs were predicted using the NCBI dbSNP (http://www.ncbi.nlm.nih.gov/projects/SNP) and HaploReg v4.1 databases (https://pubs.broadinstitute.org/mammals/haploreg/haploreg.php) (16). The results showed that the four SNPs in LIPC were both synonymous and coding sequence variants. Peripheral venous blood (5 mL) from each subject was collected for DNA extraction according to the instructions of the whole blood genomic DNA extraction kit (GoldMag Co. Ltd. Xi'an, China) (17). The DNA concentration and purity were determined by spectrometry (NanoDrop 2000 spectrophotometer, Thermo Scientific, USA). In this study, the Agena Bioscience Assay Design Suite was performed (3.1) to design primers (polymerase chain reaction primers and unique base extension primer) for the four SNPs in LIPC. These primers were synthesized by Bioengineering (Shanghai Co., Ltd). The Agena MassARRAY platform (Agena Bioscience, San Diego, CA, USA) was used to genotype the four SNPs. Ultimately, the Agena Typer Software (version 4.0, Agena Bioscience, USA) was used for data analysis and processing (18).
Statistical analysis
The differences in gender and drinking and smoking status between cases and controls were assessed using the χ2-test. The differences in age and clinical indicators between the two groups were analyzed by the independent samples t-test. PLINK software (version 1.0.7) was used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) by logistic regression analysis to measure the association between LIPC polymorphisms and stroke risk in the Chinese population under genetic models (allele, genotype, dominant, and recessive and additive models) adjusted by confounding factors. In order to control the influence of confounding factors on the results, we conducted the analyses stratified by age, sex, and smoking and drinking status (19). Data analysis was performed using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). A P-value of < 0.05 was defined as statistical significance. The G*Power 3.1.9.7 software was used to calculate the sample size and statistical power of this study, (20). Parameter is tails = 2, effect size = 0.2, α = 0.05, power = 0.964, and allocation ratio =1. Multifactor dimensionality reduction (MDR) analysis was performed to evaluate the impact of SNP–SNP interaction on the risk of stroke in the Chinese population (21).
Bioinformatics analysis
LIPC-related protein analysis was conducted through the STRING online database (https://cn.string-db.org/). We downloaded the GSE16561 dataset (39 cases and 24 controls) from the GEO database. First, standardize and log the original data. Second, select matching samples (24 cases and 24 controls). Finally, the “Limma” package in R was used to analyze the difference in the expression of LIPC and its related genes in the control and stroke groups. Furthermore, the biological process of LIPC-related protein–protein interaction was analyzed through the Sangerbox database (http://sangerbox.com/home.html).
Results
Subject characteristics
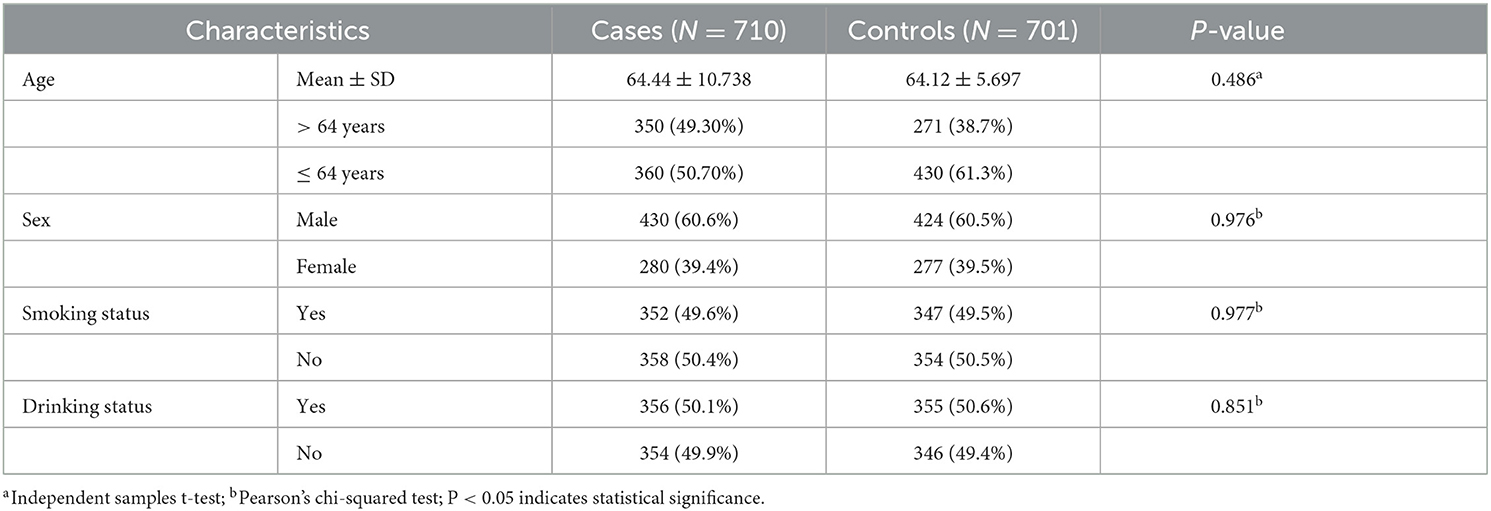
In this study, a total of 710 stroke patients and 701 controls were recruited to explore the correlation between LIPC polymorphisms and the risk of stroke in the Chinese population. In the case group, there were 360 patients older than 64 years and 270 patients younger than or equal to 64 years. In the control group, 271 individuals aged greater than 64 years and 430 individuals aged less than or equal to 64 years. The average ages of cases and controls were 64.44 ± 10.738 years and 64.12 ± 5.697 years, respectively. There were 854 male patients and 557 female patients in all subjects. Detailed information on subjects, such as smoking and drinking status, is shown in Table 1. There were no significant differences in the distribution of age, gender, and drinking and smoking status between the case and control groups (p > 0.05).
The overall analysis of the relationship between LIPC polymorphisms and stroke risk
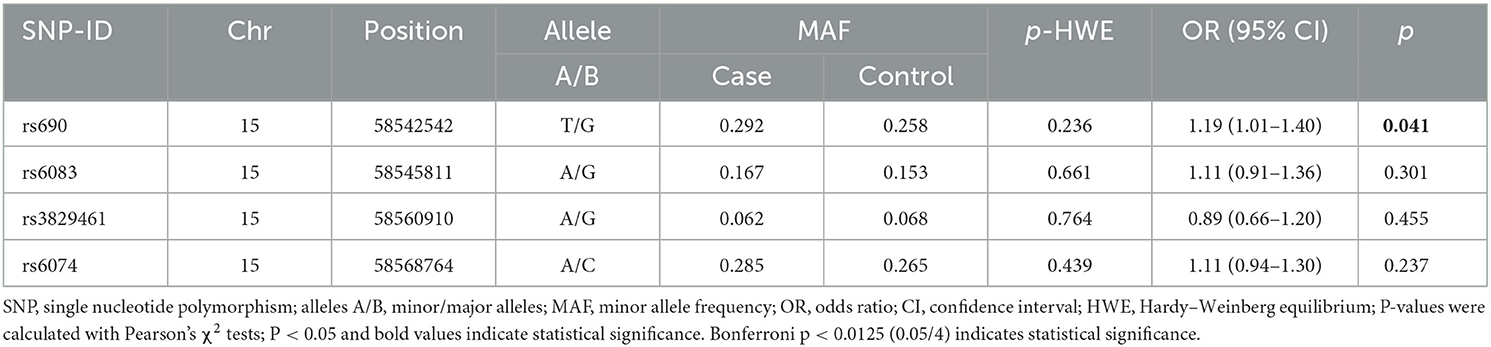
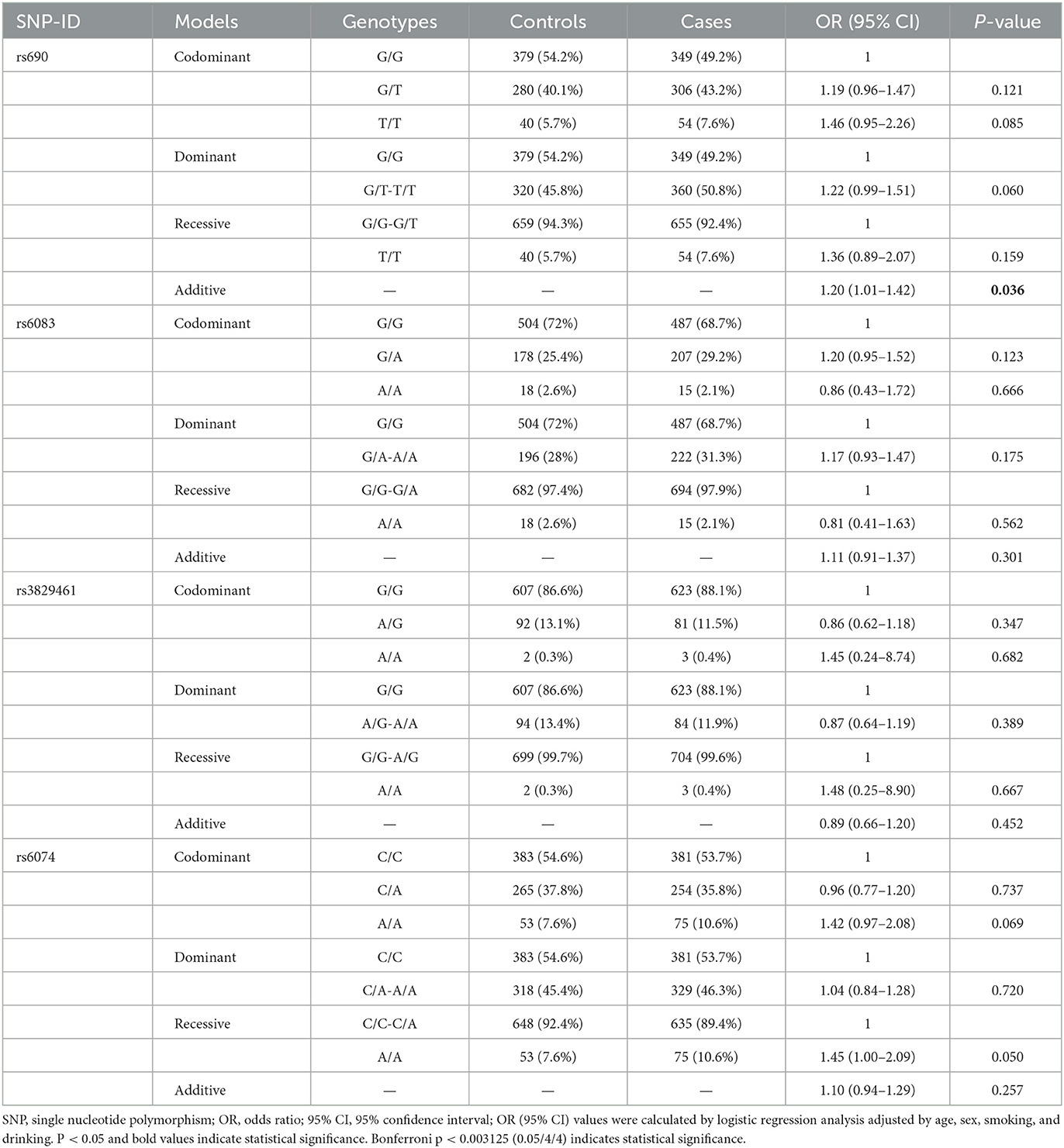
The basic information, including the chromosome number, physical location, and alleles of the candidate SNPs, is presented in Table 2. All genotype distribution of four SNPs in LIPC (rs690, rs6083, rs3829461, and rs6074) met HWE (p > 0.05), indicating that subjects in the study were in a state of genetic balance, and the genotyping results were reliable. The frequency distribution of alleles T and G of rs690 was significantly different between the case and control groups. Compared with the allele G of rs690, allele T was significantly associated with an increased risk of stroke (OR = 1.19, 95% CI: 1.01–1.40, p = 0.041) (Table 2). In the additive model, rs690 was found to be associated with an increased risk of stroke (OR = 1.20, 95% CI: 1.01–1.42, p = 0.036) (Table 3). However, no statistical significance was found between the genotypes of the three SNPs (rs6083, rs3829461, and rs6074) in LIPC and the risk of stroke (p > 0.05).
Stratified analysis of the relationship between LIPC polymorphisms and stroke risk
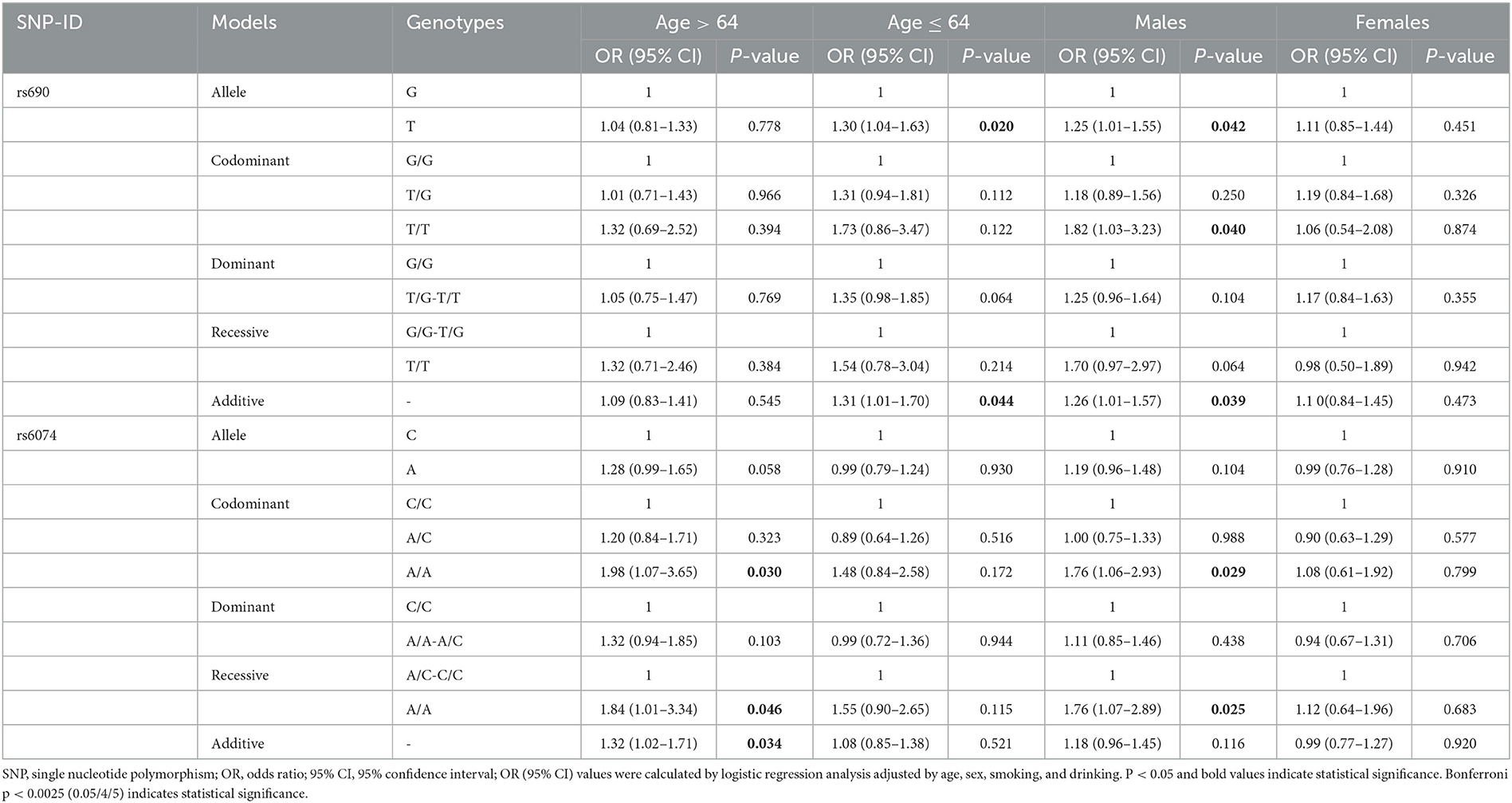
This study further clarified the correlation between LIPC polymorphisms and the risk of stroke in the Chinese population after stratification analysis by age, gender, and smoking and drinking status. The results of the age-stratified analysis showed that rs690 was found to be associated with an increased risk of stroke in subjects aged ≤ 64 years (T vs. G: OR = 1.30, 95% CI: 1.04–1.63, p = 0.020; additive: OR = 1.31, 95% CI: 1.01–1.70, p = 0.044). In the subgroup aged < 64 years, rs6074 was found to be associated with an increased risk of stroke (AA vs. CC: OR = 1.98, 95% CI: 1.07–3.65, p = 0.030 AA vs. AC-CC: OR = 1.84, 95% CI: 1.01–3.34, p = 0.046; additive: OR = 1.32, 95% CI: 1.02–1.71, p = 0.034) (Table 4). Gender-stratified analysis revealed that rs690 was associated with an increased risk of stroke in male patients under the allele (T vs. G: OR = 1.25, 95% CI: 1.01–1.55, p = 0.042), homozygote (TT vs. GG: OR = 1.82, 95% CI: 1.03–3.23, p = 0.040), and additive (OR = 1.26, 95% CI: 1.01–1.57, p = 0.039) models. Compared with genotypes A/C and C/C, male patients with genotype A/A of rs6074 were more susceptible to stroke (OR = 1.76, 95% CI: 1.06–2.93, p = 0.029; recessive: OR = 1.76, 95% CI: 1.07–2.89, p = 0.025) (Table 4). Nevertheless, no association between four SNPs in LIPC and the susceptibility to stroke in female patients was detected.
After stratification by drinking status, the analysis indicated that rs6074 was associated with an increased risk of stroke in drinkers under the recessive model (AA vs. AC-CC: OR = 1.64, 95% CI: 1.00–2.68 p = 0.049). However, no SNP was observed to be related to stroke risk in the non-drinking subgroup (p > 0.05) (Table 5). In the analysis stratified by smoking status, we found that rs690 was associated with an increased risk of stroke in smokers under the allele (OR = 1.29, 95% CI: 1.02–1.63, p = 0.037), dominant (OR = 1.37, 95% CI: 1.02–1.85, p = 0.038), and additive (OR = 1.28, 95% CI: 1.00–1.63, p = 0.048) models. In the non-smoking subgroup, rs6074 was found to be associated with an increased risk of stroke (A vs. C: OR = 1.33, 95% CI: 1.05–1.68, p = 0.016; AA vs. CC: OR = 2.04, 95% CI: 1.20–3.46, p = 0.008; additive: OR = 1.30, 95% CI: 1.04–1.63, p = 0.020).
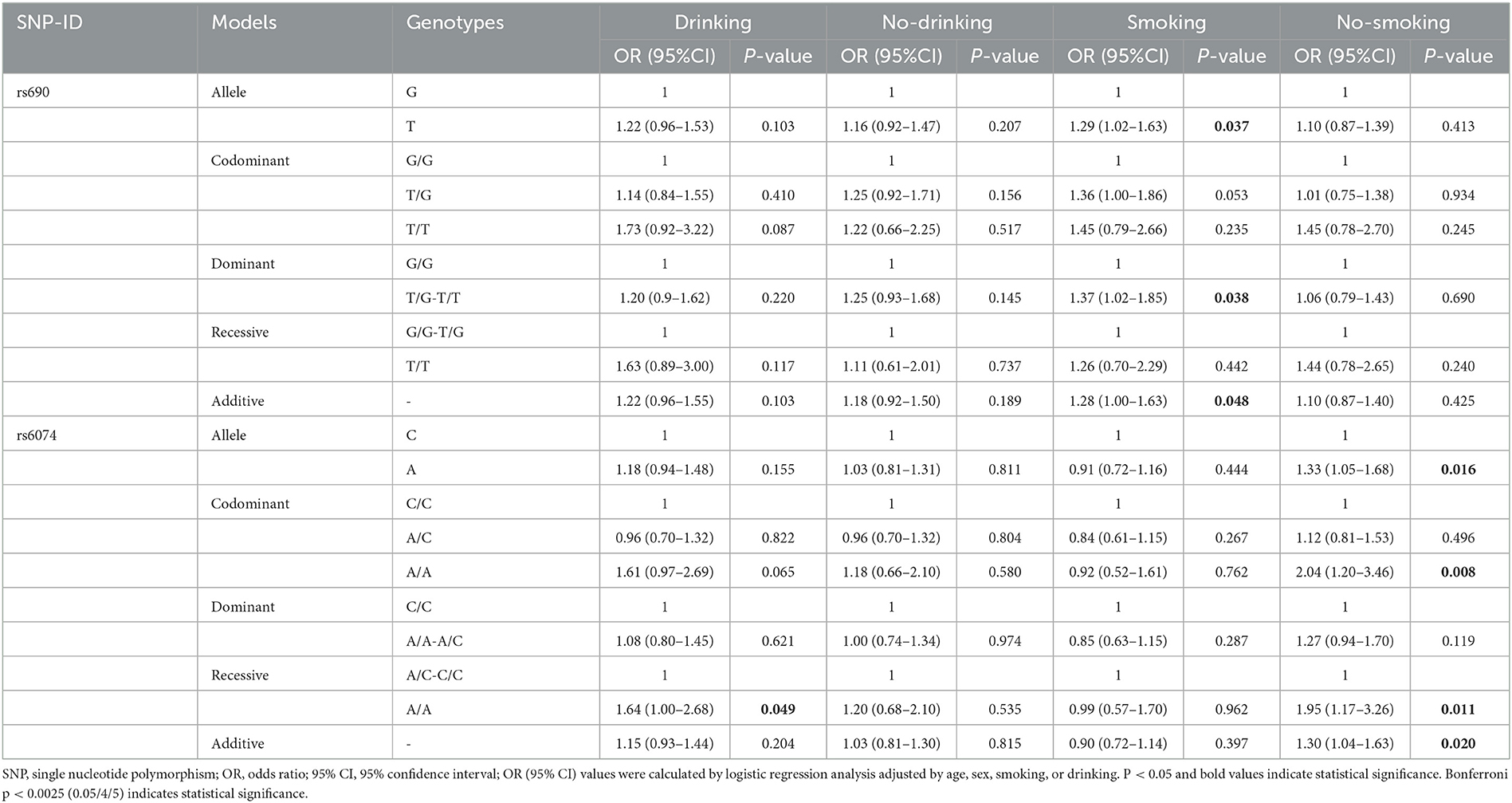
Table 5. Relationships of LIPC gene polymorphisms with the risk of stroke stratified by smoking and drinking.
The relationship between LIPC polymorphisms and clinical indicators of stroke patients
The relationship between LIPC polymorphisms and 42 clinical parameters of stroke patients was also investigated in the study (Supplementary Table 1). We found a significant difference in platelet distribution width (PDW), hematocrit (HCT), and low-density lipoprotein cholesterol (LDL-C) levels between cases and controls (p < 0.05). The TT, TG, and GG genotypes of rs690 were significantly correlated with PDW (p = 0.014) and HCT (p = 0.004) levels (Figure 1). The TT, TG, and GG genotypes of rs6074 were significantly correlated with the level of LDL-C (p = 0.033) (Supplementary Table 1 and Figure 1).
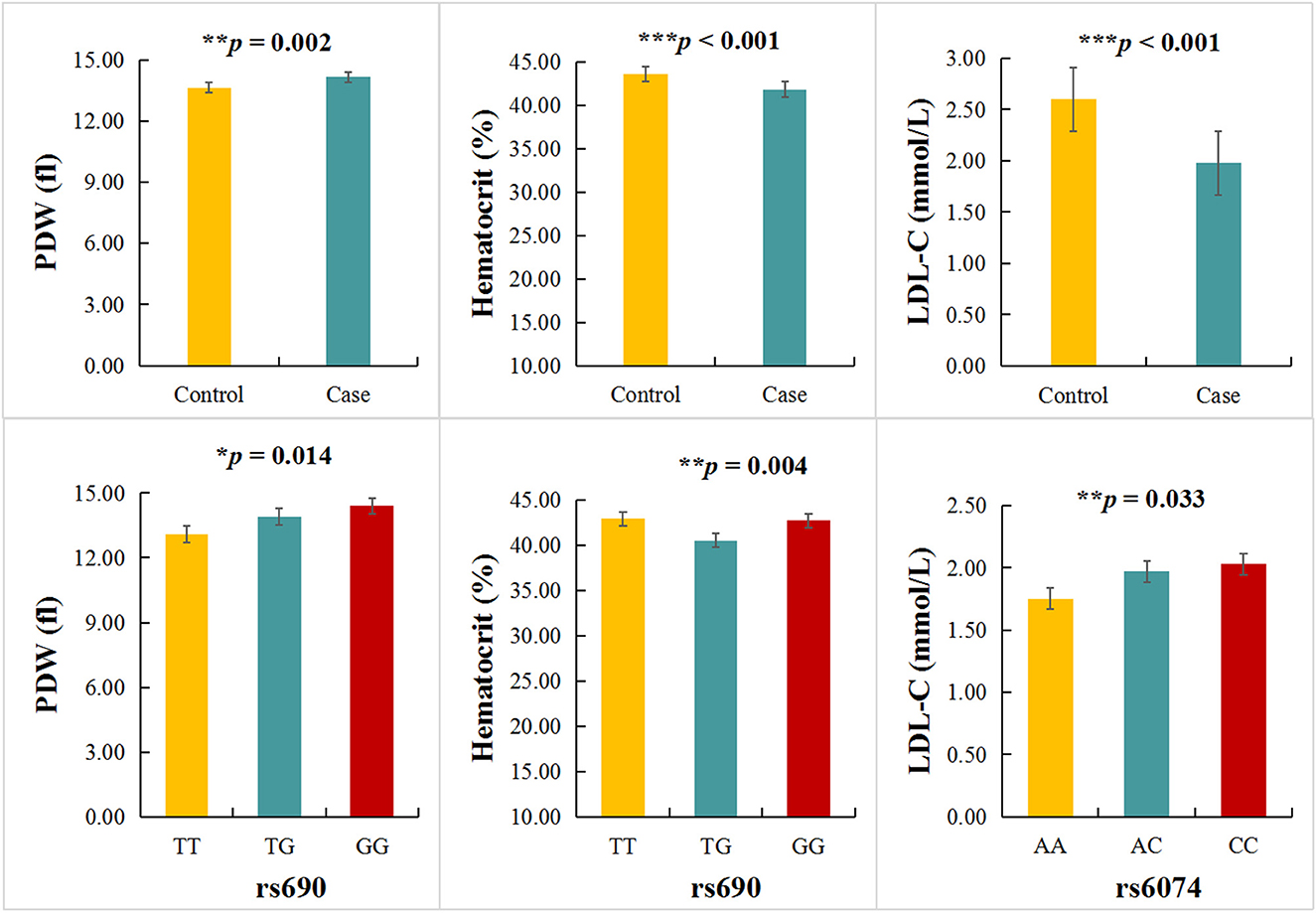
Figure 1. Association between LIPC polymorphisms and clinical indicators. *p < 0.05, **p < 0.01, ***p < 0.001.
MDR analysis of the effect of SNP-SNP interactions on stroke risk
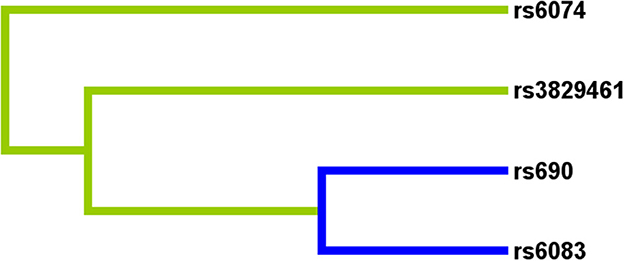
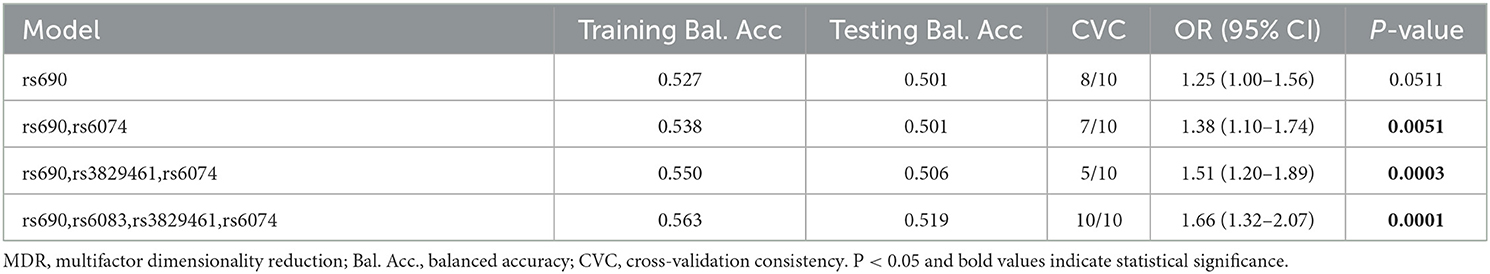
Multifactor dimensionality reduction analysis was performed to further explore the impact of LIPC SNP-SNP interaction on stroke risk. Figure 2 shows that rs690, rs6083, rs3829461, and rs6074 in LIPC had antagonistic effects on stroke risk. In the MDR analysis, by comparing the parameters of cross-validation consistency (CVC), OR, 95% CI, p-value, training balanced accuracy (Bal. Acc.), and testing balanced accuracy, we found that the four-locus model consisting of four SNPs (rs690, rs6083, rs3829461, and rs6074) was the best one to assess the risk of stroke (CVC: 10/10; Testing Bal. Acc = 0.519; OR = 1.66, 95% CI: 1.32–2.07, p = 0.0001, Table 6).
Bioinformatics analysis
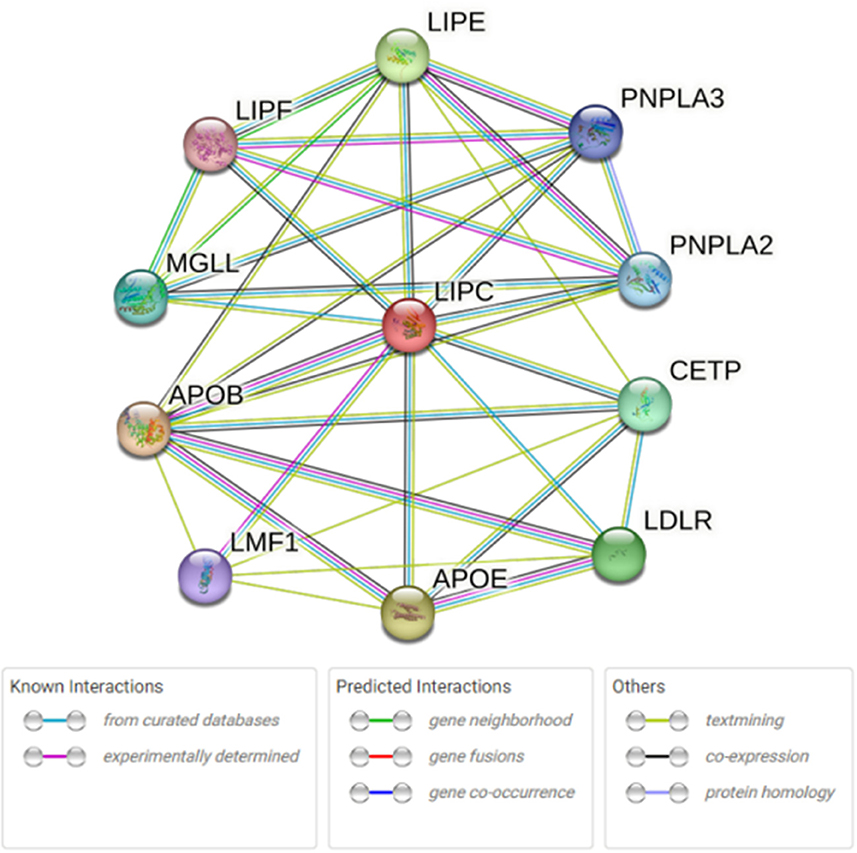
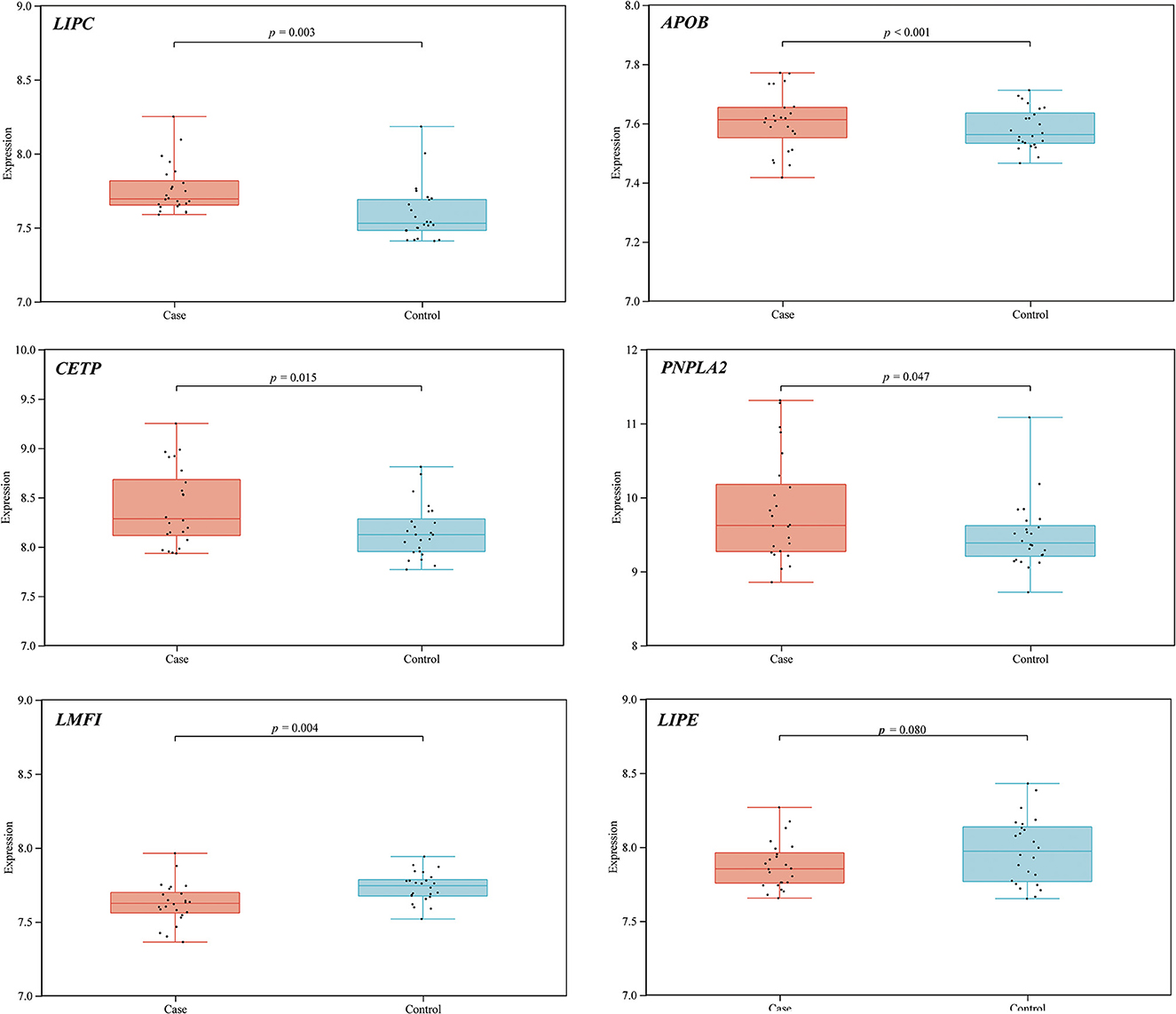
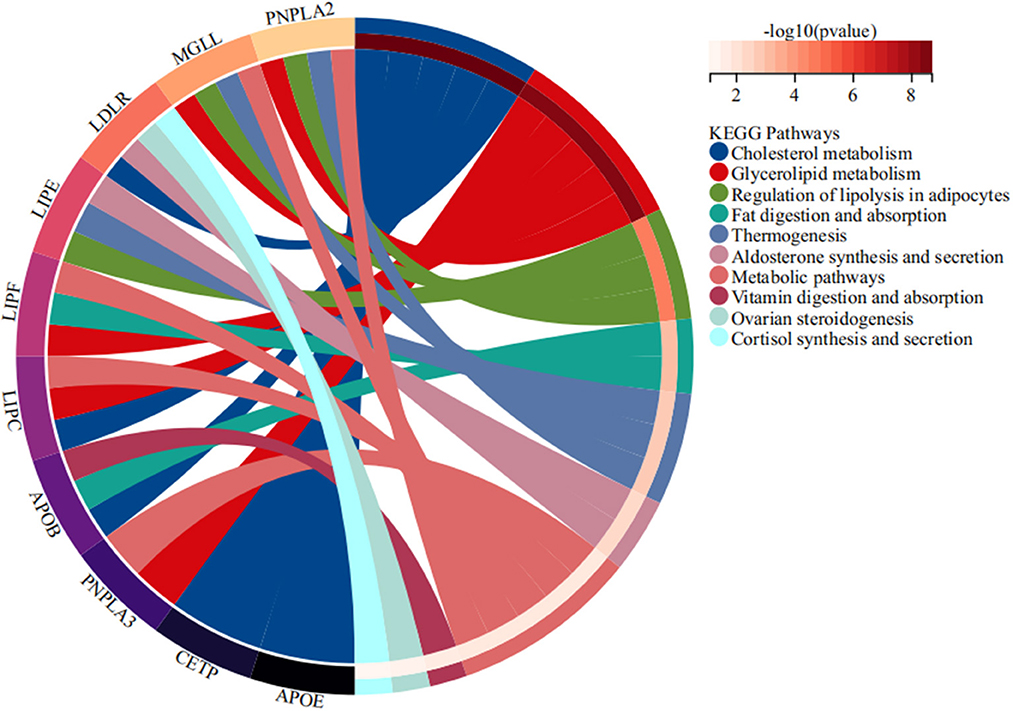
STRING database analysis results showed that there were nine main proteins related to LIPC, namely, APOB, APOE, CETP, LDLR, LIPE, LIPF, MGLL, PNPLA3, PNPLA2, and LMF1 (Figure 3). Based on the Gene Expression Omnibus (GEO) database analysis, significant differences in the expression levels of LIPC, APOB, CETP, PNPLA2, and LMF1 between the control and stroke groups were observed (Supplementary Table 2 and Figure 4). Furthermore, the Sangerbox analysis results showed that the biological processes of the main protein–protein interactions related to LIPC mainly involved the metabolism of cholesterol and glycerolipid pathways (Figure 5).
Discussion
This is the first study to analyze the relationship between LIPC polymorphisms and the risk of stroke in the Chinese population. In the overall analysis, we found that rs690 was associated with an increased risk of stroke in the Chinese population. The stratified analysis revealed that rs690 was associated with an increased risk of stroke in subjects aged >64 years, male subjects, and smokers. In addition, s6074 was associated with an increased risk of stroke in subjects aged ≤ 64 years, male subjects, non-smokers, and drinkers. In addition, we found that rs690 was associated with PDW and HCT levels, and rs6074 is related to the LDL-C level. MDR analysis results demonstrated that the four-locus model was the best model for stroke risk assessment.
LIPC encodes the hepatic lipase. A whole-exome sequencing study has revealed that the abnormal combination of the AA genotype of rs17269397 in LIPC and cholesterol levels can affect the risk of coronary artery disease (CAD) (22). A study using the meta-analysis approach has shown that rs2070895 in LIPC was associated with an increased risk of hypertension (11). Previous studies have demonstrated that LIPC plays a vital role in the pathogenesis of cardiovascular and cerebrovascular diseases. In addition, it has been elucidated that LIPC exhibits a gene-level association with myocardial infarction (23). This study was the first to reveal that LIPC polymorphisms were associated with the risk of stroke in the Chinese population.
It has been reported that age, gender, alcohol consumption, and smoking are important risk factors for stroke (24). More precisely, the main driver for increased stroke prevalence is the aging of the population (25, 26). The incidence of stroke is higher in male patients, and also male patients have their first stroke earlier than female patients. However, female patients have more severe strokes than male patients (27, 28). Alcohol consumption has complex effects on cardiovascular health, and views on the associations between alcohol consumption and stroke risk remain controversial (29, 30). Cigarette smoking is a well-established risk factor for all forms of stroke. Continued smoking after a stroke is associated with a high risk of stroke recurrence and other cardiovascular diseases (31). Our research has shown that rs690 and rs6074 in LIPC were associated with an increased risk of stroke in male patients. These two loci were also associated with an increased risk of stroke in different age subgroups ( ≤ 64 years and < 64 years, respectively). Furthermore, the T allele of rs690 was associated with an increased risk of stroke in smokers. The allele A of rs6074 was associated with an increased risk of stroke in non-smokers and alcohol drinkers. Hence, we speculated that the impacts of age, gender, smoking, and drinking on the susceptibility to stroke were stronger than the combination of rs690 and rs6074.
Studies have shown that the combination of rs690 in LIPC and other SNPs in LIPC can affect the bioavailability of dietary cholesterol in healthy adult male patients (32) and is associated with the HDL-C level (7). Our research also showed that rs690 was associated with the PDW and HCT levels of stroke patients instead of HDL-C. PDW was an important risk factor for stroke in atrial fibrillation patients (33). Several large-scale observational studies have pointed out an increased risk of stroke in individuals with elevated HCT (34). Therefore, we speculated that rs690 in LIPC modified the risk of stroke by modulating PDW and/or HCT levels. Among South Indian subjects without diabetes, the CA genotype of rs6074 has been found to be associated with a low level of HDL-C (35), suggesting that rs6074 is related to the LDL-C level rather than HDL-C. Previous study has found that a very low LDL-C level is associated with high risks of all-cause and stroke mortality (36), thus, the AA genotype of rs6074 was associated with an increased risk of stroke by regulating the LDL-C level. LIPC polymorphism rs6083 has been found to be associated with cholesterol, triglyceride, LDL-C (14), and HDL-C levels (7). However, little is known about the association between rs6083 and the clinical parameters of stroke patients.
As genetic polymorphisms worked jointly on disease risk, MDR analysis was conducted in this study to further explore the impact of SNP–SNP interactions on the risk of stroke, indicating that SNP–SNP interactions demonstrate an antagonistic effect on the risk of stroke, and the combination of the four sites is the best risk assessment model for stroke. A study has exhibited an association between abnormal lipid metabolism and stroke (37). Our bioinformatics analysis results indicated that the expression levels of LIPC and its related genes (APOB, CETP, PNPLA2, and LMF1) showed significant differences between stroke and control groups. The main LIPC-related protein–protein interactions were connected with lipid metabolism, which is expected to provide a new direction for investigating the effect of LIPC on stroke risk.
There are several limitations to this study. First, this study is the first to explore the relationship between LIPC polymorphisms and stroke susceptibility in the Chinese population. To further verify the results, a large sample will be collected in the following studies. Second, the subjects in this study came from the same hospital during the same period, and the geographic limitations in sample selection should not be overlooked. Third, we only predicted the signal pathway that LIPC might participate in through the databases, and the impact of SNPs on the LIPC gene mRNA level and the function of LIPC in stroke have not been explored in this study.
Conclusion
These findings revealed that LIPC polymorphisms (rs690 and rs6074) were associated with an increased risk of stroke in the Chinese population, which may modify stroke risk by regulating PDW, HCT, and LDL-C levels, respectively. These results provide a novel direction for the early screening and prevention of stroke in a high-risk population in China.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of People's Hospital of Wanning and in compliance with the Declaration of Helsinki. The patients/participants provided their written informed consent to participate in this study.
Author contributions
SW and TJ: conceived and designed the experiments and drafted the article and/or revised it critically for important content. JP and QZ: performed the experiments. XC and XH: analyzed the data. SS: responsible for reagents, materials, and analysis tools. QY and JL: prepared the figures and/or tables. All authors have read and approved the manuscript.
Acknowledgments
The authors are grateful to the individuals for their participation in this study. The authors would also like to thank the clinicians and hospital staff who contributed to the sample and data collection for this study. The authors would also like to thank all participants for this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1095282/full#supplementary-material
Abbreviations
LIPC, lipase C; MDR, multifactor dimensionality reduction; SNP, single nucleotide polymorphism; HWE, Hardy-Weinberg equilibrium; OR, odds ratio; CI, confidence intervals; CT, computed tomography; MAF, minor allele frequency; MRI, magnetic resonance imaging; PDW, platelet distribution width; HCT, hematocrit; LDL-C, low-density lipoprotein; CVC, cross-validation consistency; Bal. Acc., training balanced accuracy.
References
1. Katan M, Luft A. Global Burden of Stroke. Semin Neurol. (2018) 38:208–11. doi: 10.1055/s-0038-1649503
2. Wu S, Wu B, Liu M, Chen Z, Wang W, Anderson CS, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurology. (2019)18:394–405. doi: 10.1016/S1474-4422
3. Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, et al. Prevalence, incidence, and mortality of stroke in china: results from a nationwide population-based survey of 480 687 adults. Circulation. (2017) 135:759–71. doi: 10.1161/CIRCULATIONAHA.116.025250
4. Gao Y, Jiang B, Sun H, Ru X, Sun D, Wang L, et al. The burden of stroke in China: results from a nationwide population-based epidemiological survey. PLoS ONE. (2018) 13:e0208398. doi: 10.1371/journal.pone.0208398
5. Bersano A, Kraemer M, Burlina A, Mancuso M, Finsterer J, Sacco S, et al. Heritable and non-heritable uncommon causes of stroke. J Neurol. (2021) 268:2780–807. doi: 10.1007/s00415-020-09836-x
6. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2014) 45:2160–236. doi: 10.1161/STR.0000000000000024
7. Pirim D, Bunker CH, Hokanson JE, Hamman RF, Demirci FY, Kamboh MI. Hepatic lipase (LIPC) sequencing in individuals with extremely high and low high-density lipoprotein cholesterol levels. PLoS One. (2020) 15:e0243919. doi: 10.1371/journal.pone.0243919
8. Khounphinith E, Yin RX, Qiu L, Zhang FH, Yan RQ, Lu L, et al. Association of the LIPC rs1532085 SNP and serum lipid traits in the Chinese Maonan and Han populations. Int J Clin Exp Pathol. (2018) 11:2038–52.
9. Guerra-García MT, Moreno-Macías H, Ochoa-Guzmán A, Ordoñez-Sánchez ML, Rodríguez-Guillen R, Vázquez-Cárdenas P, et al. The−514C>T polymorphism in the LIPC gene modifies type 2 diabetes risk through modulation of HDL-cholesterol levels in Mexicans. J Endocrinol Invest. (2021) 44:557–65. doi: 10.1007/s40618-020-01346-x
10. Zago VHS, Parra ES, Virgínio VWM, Vendrame F, Gomes É IL, Scherrer DZ, et al. Lipase C, Hepatic Type−250A/G (rs2070895) variant enhances carotid atherosclerosis in normolipidemic and asymptomatic individuals from Brazil. Lipids. (2020) 55:225–37. doi: 10.1002/lipd.12232
11. Zhao X, Ren Y, Li H, Wu Y. Association of LIPC−250G/A and−514C/T polymorphisms and hypertension: a systematic review and meta-analysis. Lipids Health Dis. (2018) 17:238. doi: 10.1186/s12944-018-0884-4
12. Teng MS, Wu S, Er LK, Hsu LA, Chou HH, Ko YL, et al. variants as genetic determinants of adiposity status, visceral adiposity indicators, and triglyceride-glucose (TyG) index-related parameters mediated by serum triglyceride levels. Diabetol Metab Syndr. (2018) 10:79. doi: 10.1186/s13098-018-0383-9
14. Long T, Lu S, Li H, Lin R, Qin Y, Li L, et al. Association of APOB and LIPC polymorphisms with type 2 diabetes in Chinese Han population. Gene. (2018) 672:150–5. doi: 10.1016/j.gene.2018.06.010
15. Montazeri Z, Li X, Nyiraneza C, Ma X, Timofeeva M, Svinti V, et al. Systematic meta-analyses, field synopsis and global assessment of the evidence of genetic association studies in colorectal cancer. Gut. (2020) 69:1460–71. doi: 10.1136/gutjnl-2019-319313
16. Ward LD, Kellis M. HaploReg v4: systematic mining of putative causal variants, cell types, regulators and target genes for human complex traits and disease. Nucleic Acids Res. (2016) 44:D877–881. doi: 10.1093/nar/gkv1340
17. Zhou L, Dong S, Deng Y, Yang P, Zheng Y, Yao L, et al. GOLGA7 rs11337, a Polymorphism at the MicroRNA binding site, is associated with glioma prognosis. Mol. Ther. Nucleic acids. (2019) 18:56–65. doi: 10.1016/j.omtn.2019.08.006
18. Giannoudis A, Sartori A, Eastoe L, Zakaria R, Charlton C, Hickson N, et al. Genomic profiling using the UltraSEEK panel identifies discordancy between paired primary and breast cancer brain metastases and an association with brain metastasis-free survival. Breast Cancer Res Treat. (2021) 190:241–53. doi: 10.1007/s10549-021-06364-8
19. Clarke GM, Anderson CA, Pettersson FH, Cardon LR, Morris AP, Zondervan KT. Basic statistical analysis in genetic case-control studies. Nat Protoc. (2011) 6:121–33. doi: 10.1038/nprot.2010.182
20. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. (2007) 39:175–91. doi: 10.3758/BF03193146
21. Gola D, Mahachie John JM, van Steen K, König IR, A. roadmap to multifactor dimensionality reduction methods. Brief Bioinform. (2016) 17:293–308. doi: 10.1093/bib/bbv038
22. Park HS, Kim IJ, Kim EG Ryu CS, Lee JY, Ko EJ, et al. A study of associations between CUBN, HNF1A, and LIPC gene polymorphisms and coronary artery disease. Sci Rep. (2020) 10:16294. doi: 10.1038/s41598-020-73048-6
23. Hindorff LA, Lemaitre RN, Smith NL, Bis JC, Marciante KD, Rice KM, et al. Common genetic variation in six lipid-related and statin-related genes, statin use and risk of incident nonfatal myocardial infarction and stroke. Pharmacogenet Genomics. (2008) 18:677–82. doi: 10.1097/FPC.0b013e3283033528
24. Boehme AK, Esenwa C, Elkind MS. stroke risk factors, genetics, and prevention. Circ Res. (2017) 120:472–95. doi: 10.1161/CIRCRESAHA.116.308398
25. Béjot Y. Aging population and stroke: Red flag. Rev Neurol. (2020) 176:751–3. doi: 10.1016/j.neurol.2020.07.008
26. Yousufuddin M, Young N. Aging and ischemic stroke. Aging. (2019) 11:2542–4. doi: 10.18632/aging.101931
27. Appelros P, Åsberg S. Sex differences in stroke. Handb Clin Neurol. (2020) 175:299–312. doi: 10.1016/B978-0-444-64123-6.00021-7
28. Rexrode KM, Madsen TE Yu AYX, Carcel C, Lichtman JH, Miller EC. The Impact of Sex and Gender on Stroke. Circ Res. (2022) 130:512–28. doi: 10.1161/CIRCRESAHA.121.319915
29. Duan Y, Wang A, Wang Y, Wang X, Chen S, Zhao Q, et al. Cumulative alcohol consumption and stroke risk in men. J Neurol. (2019) 266:2112–9. doi: 10.1007/s00415-019-09361-6
30. Jeong SM, Lee HR, Han K, Jeon KH, Kim D, Yoo JE, et al. Association of change in alcohol consumption with risk of ischemic Stroke. Stroke. (2022) 53:2488–96. doi: 10.1161/STROKEAHA.121.037590
31. Parikh NS, Parasram M, White H, Merkler AE, Navi BB, Kamel H. Smoking cessation in stroke survivors in the united states: a nationwide analysis. Stroke. (2022) 53:1285–91. doi: 10.1161/STROKEAHA.121.036941
32. Desmarchelier C, Wolff E, Defoort C, Nowicki M, Morange PE, Alessi MC, et al. A combination of single nucleotide polymorphisms is associated with the interindividual variability of cholesterol bioavailability in healthy adult males. Mol Nutr Food Res. (2020) 64:e2000480. doi: 10.1002/mnfr.202000480
33. Lyu QS, Liu B, Huang C, Huang YQ. The association between platelet distribution width and stroke in atrial fibrillation patients. Ann Clin Lab Sci. (2019) 49:143–7.
34. Stavropoulos K, Imprialos KP, Bouloukou S, Boutari C, Doumas M. Hematocrit and stroke: a forgotten and neglected link? Semin Thromb Hemost. (2017) 43:591–8. doi: 10.1055/s-0037-1602663
35. Ayyappa KA, Ghosh S, Mohan V, Radha V. Association of hepatic lipase gene polymorphisms with hypertriglyceridemia and low high-density lipoprotein-cholesterol levels among South Indian subjects without diabetes. Diabetes Technol Ther. (2013) 15:503–12. doi: 10.1089/dia.2012.0302
36. Rong S, Li B, Chen L, Sun Y, Du Y, Liu B, et al. Association of low-density lipoprotein cholesterol levels with more than 20-year risk of cardiovascular and all-cause mortality in the general population. J Am Heart Assoc. (2022) 11:e023690. doi: 10.1161/JAHA.121.023690
Keywords: LIPC, polymorphisms, stoke, susceptibility, Chinese population, case-control study
Citation: Pan J, Zhuo Q, Chen X, Huang X, Shen S, Yang Q, Luo J, Wang S and Jin T (2023) Association of LIPC polymorphisms with stroke risk in the Chinese population. Front. Neurol. 14:1095282. doi: 10.3389/fneur.2023.1095282
Received: 11 November 2022; Accepted: 18 April 2023;
Published: 18 May 2023.
Edited by:
Tatjana Rundek, University of Miami, United StatesReviewed by:
Xingli Xu, Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital, ChinaYonggang Hao, Graduate School, Zhejiang University, China
Copyright © 2023 Pan, Zhuo, Chen, Huang, Shen, Yang, Luo, Wang and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suiyan Wang, Mjc5MzI3NTY1QHFxLmNvbQ==; Tianbo Jin, amludGlhbmJvQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Jiaxing Pan1†
Jiaxing Pan1† Suiyan Wang
Suiyan Wang