- 1Neuromuscular and Rare Diseases Unit, Department of Neuroscience, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy
- 2Department of Pathophysiology and Transplantation, Dino Ferrari Center, University of Milan, Milan, Italy
- 3Department of Neurology, Leiden University Medical Center, Leiden, Netherlands
- 4Duchenne Center Netherlands, Netherlands
- 5ImagingDMD, University of Florida, Gainesville, FL, United States
- 6Radiology Department, Istituto Auxologico Italiano, IRCCS, Milan, Italy
- 7Department of Radiology, C.J. Gorter MRI Center, Leiden University Medical Center, Leiden, Netherlands
- 8Neurology Unit, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy
- 9Department of Orthopedics, Rehabilitation and Physiotherapy, Leiden University Medical Center, Leiden, Netherlands
- 10OPIS srl, Milan, Italy
- 11Italfarmaco SpA, Milan, Italy
Objective: No treatments are approved for Becker muscular dystrophy (BMD). This study investigated the efficacy and safety of givinostat, a histone deacetylase pan-inhibitor, in adults with BMD.
Methods: Males aged 18–65 years with a diagnosis of BMD confirmed by genetic testing were randomized 2:1 to 12 months treatment with givinostat or placebo. The primary objective was to demonstrate statistical superiority of givinostat over placebo for mean change from baseline in total fibrosis after 12 months. Secondary efficacy endpoints included other histological parameters, magnetic resonance imaging and spectroscopy (MRI and MRS) measures, and functional evaluations.
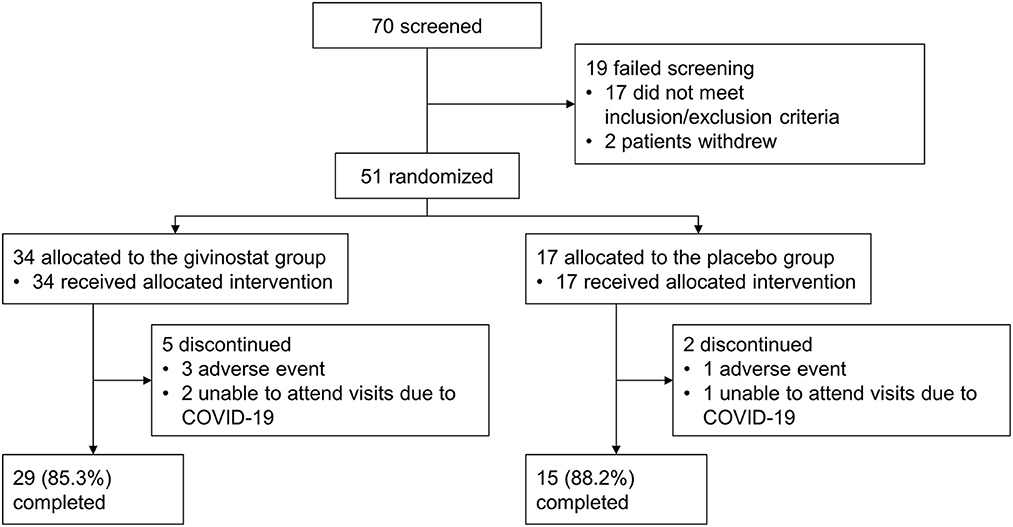
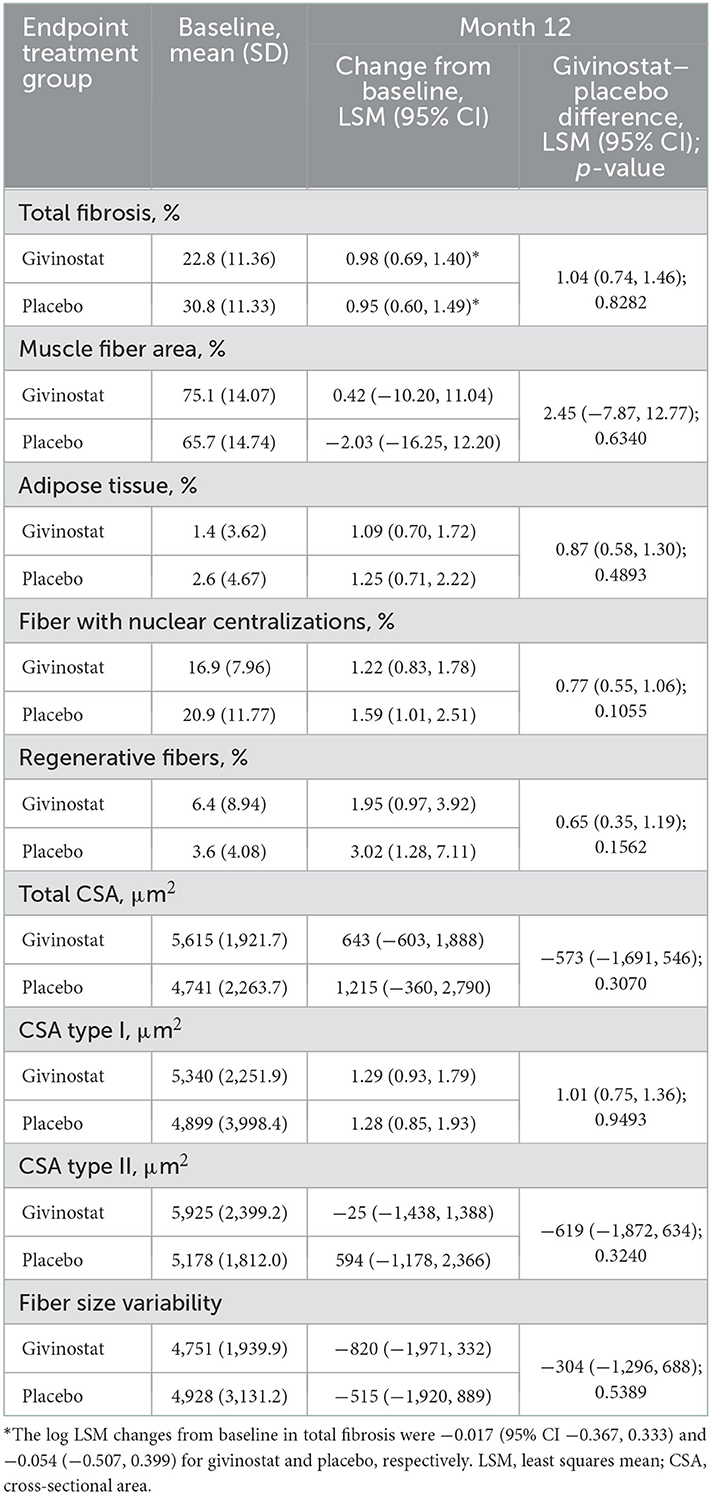
Results: Of 51 patients enrolled, 44 completed treatment. At baseline, there was greater disease involvement in the placebo group than givinostat, based on total fibrosis (mean 30.8 vs. 22.8%) and functional endpoints. Mean total fibrosis did not change from baseline in either group, and the two groups did not differ at Month 12 (least squares mean [LSM] difference 1.04%; p = 0.8282). Secondary histology parameters, MRS, and functional evaluations were consistent with the primary. MRI fat fraction in whole thigh and quadriceps did not change from baseline in the givinostat group, but values increased with placebo, with LSM givinostat–placebo differences at Month 12 of −1.35% (p = 0.0149) and −1.96% (p = 0.0022), respectively. Adverse events, most mild or moderate, were reported by 88.2% and 52.9% patients receiving givinostat and placebo.
Conclusion: The study failed to achieve the primary endpoint. However, there was a potential signal from the MRI assessments suggesting givinostat could prevent (or slow down) BMD disease progression.
1. Introduction
Becker muscular dystrophy (BMD) is a heterogeneous muscle disease, with substantial variability in age of onset and clinical presentation (1). In the early stages BMD involves active myonecrosis and regeneration; later in the disease course chronic myopathic changes are more likely, including increased skeletal muscle fiber size variability, fibrosis, and fat replacement of muscle tissue (2, 3), with selective muscle hypertrophy (4, 5). Initial symptoms may include cramping and reduced endurance during exercise (6). This is followed by gradual muscle weakness in the hips, pelvis, thighs, and shoulders, leading to walking on toes with lumbar lordosis and early loss of ambulation, although some patients are able to remain ambulatory even into their 60s (6). No treatments are specifically approved for BMD.
BMD is caused by in-frame mutations in the dystrophin gene (although with exceptions), resulting in a reduced amount or truncated size of the dystrophin protein (7). Dystrophin assembles with other proteins to form the dystrophin-associated protein complex (DAPC), which plays a critical role in stabilizing the plasma membrane of striated muscle by linking the actin cytoskeleton to the extracellular matrix. Neuronal nitric oxide synthase (nNOS) is an important component of the DAPC. Nitric oxide produced by nNOS inactivates histone deacetylase (HDAC) 2 via S-nitrosylation of a cysteine residue (8). This mechanism is dysfunctional in dystrophic muscle, leading to aberrantly upregulated HDAC activity (8). A potential target of therapy for BMD is therefore HDAC inhibition. Indeed, in a dystrophin-deficient mouse model inhibition of HDAC activity led to functional and morphological muscle recovery (9).
In this manuscript, we report the results of a study that investigated the efficacy and safety of givinostat, a HDAC pan-inhibitor, in adults with BMD.
2. Materials and methods
This was a Phase II, randomized, double-blind, placebo-controlled study that aimed to evaluate the effects of givinostat on micro- and macroscopic muscle morphology and function. The study was conducted in two centers: University of Milan, Italy, and Leiden University Medical Center, the Netherlands, with the University of Florida (ImagingDMD) serving as the central data management and processing center for magnetic resonance imaging (MRI) and spectroscopy (MRS). Following a four-week screening period, patients were randomized to receive givinostat or placebo for 12 months, attending study visits every 2 weeks for the first 2 months, then every 12 weeks for the remainder of the study.
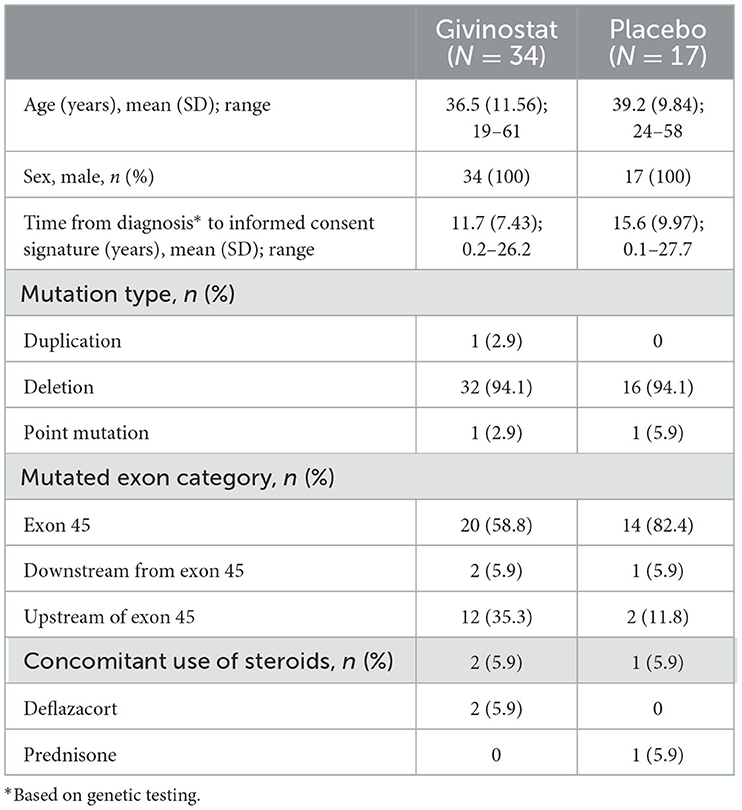
Eligible patients were males aged 18–65 years, inclusive, with a clinical diagnosis of BMD confirmed by genetic testing (based on patient records), and who were able to walk between 200 and 450 m in the 6 min walk test (6MWT). If patients were receiving a systemic corticosteroid, angiotensin converting enzyme inhibitor, or α- or β-adrenergic receptor blocker they were expected to have no significant change in dose or regimen immediately prior to the start of study treatment. Among the reasons for exclusion were: Use of any pharmacologic treatment, other than corticosteroids, or surgery in the 3 months prior to study entry that might have an effect on muscle strength or function; symptomatic cardiomyopathy or heart failure (New York Heart Association Class III or IV) or left ventricular ejection fraction <50% at screening or with heart transplant; and contraindications to muscle biopsy or magnetic resonance scans. All patients provided written informed consent prior to any study-related procedure. Full inclusion and exclusion criteria are listed in the Supplementary material. The study was approved by an independent ethics committee for each institution, and was performed in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization notes for guidance on Good Clinical Practice (ICH/CPMP/135/95). The study was registered at ClinicalTrials.gov (NCT03238235).
During the screening period and after 12 months, chemical shift encoded MRI and MRS of the right lower leg, thigh and gluteus maximus, and an open muscle biopsy of the brachial biceps were performed (see Supplementary methods for detailed methodology and the specific muscles included in each muscle group, and Supplementary Figure 1 for an example MRI image). Patients were randomized on entry to the study, such that 50% had their right arm biopsied at baseline and the left arm at the end of the study; the other 50% had their left arm biopsied at baseline and the right arm at study end. In addition, patients undertook a series of timed-function tests (rise from floor, run/walk 10 m, and climb four standard steps) and a 6MWT (10). They then completed the Motor Function Measure (MFM) (11–13), followed by bilateral strength measures (knee extension and elbow flexion) using hand-held myometry (microFET Dynamometer, Hoggan Scientific LLC, Salt Lake City, UT, USA). The functional and strength assessments were evaluated by qualified functional evaluators (all physiotherapists), with the timed-function tests and 6MWT standardized by a study-specific manual. Safety was assessed throughout the study, in terms of adverse events, hematology, blood chemistry, physical examination and electrocardiogram parameters, and lung function. Selected baseline data, and the correlations between these data, have been published in a previous manuscript (14).
The protocol was amended four times. The main changes in the first amendment were the addition of MRI evaluations of the lower leg and an increase in maximum age from 60 to 65 years. Amendment 2 resulted from a blinded evaluation of data from the first 21 patients, in which 11 patients required dose reduction due to thrombocytopenia, and four patients had high plasma triglyceride levels. The givinostat starting dose was therefore reduced (from the “high dose” to the “low dose,” see Supplementary material), and additional safety dosing rules were introduced (see the “Interventions” Section). Amendment 3 added an interim analysis of baseline characteristics. Amendment 4 changed the primary endpoint as a result of the pre-planned blinded interim analysis to check the sample size (see the sample size Section). The primary endpoint was originally change from baseline in fiber cross-sectional area (CSA) after 12 months of treatment. However, the mean CSA of the brachial biceps fibers in the first 20 baseline biopsies was similar to age-matched CSA in healthy adults. It was therefore deemed unlikely that givinostat could increase fiber CSA. Given fibro-adipose replacement is a hallmark of BMD (2), total fibrosis was considered a more indicative primary outcome measure. The revised sample size calculation increased the required number of patients from 48 to 51.
2.1. Interventions
Patients were randomized 2:1 via an interactive web response system to receive givinostat or matching placebo, stratified by concomitant steroid use at baseline (yes or no). Patients, investigators, and site and sponsor staff were blinded to treatment assignment. Given platelet count reductions are observed after administration of givinostat, personnel who performed the various efficacy analyses were different from those who recorded the safety results.
Givinostat (10 mg/mL) or placebo oral suspension were administered using a graduated dosing syringe as two daily doses (morning and evening) after a meal. The starting dose was selected according to body weight as shown in Supplementary Table 1, and rules were pre-specified for treatment to be permanently discontinued or temporarily interrupted (see Supplementary methods). In the case of treatment interruption, the dose was reduced by 20% once platelets, white blood cells, hemoglobin and/or triglycerides were normal, or diarrhea was mild. In addition, if a patient had at least two consecutive platelet counts ≤ 150 × 109/L that did not meet the stopping criteria, the dosage was reduced by 20% of the current dose.
2.2. Outcomes
The primary objective was to demonstrate statistical superiority of givinostat over placebo in terms of the mean change from baseline in total fibrosis after 12 months of treatment. Secondary efficacy endpoints included change from baseline after 12 months of treatment in: other histological parameters (% muscle fiber area [MFA], % adipose tissue, % fibers with nuclear centralizations, % regenerative fibers, fiber total CSA, fiber size variability, and total dystrophin); MRI measures of muscle fat fraction, MRI CSA and contractile area in the gluteus maximus, thigh and lower leg muscles; MRS fat fraction of the vastus lateralis and soleus; MFM (total and component); timed-function tests (time to climb four standard steps, time to walk/run 10 m, and time to rise from the floor); 6MWT; and muscle strength of the knee extension and elbow flexion measured by hand-held myometry. Additional details on the histology, MRI, MRS, and functional endpoints are in the Supplementary material.
2.3. Sample size and statistical methods
It was calculated that 48 patients with evaluable baseline biopsies (32 and 16 receiving givinostat and placebo, respectively), would provide 80% power to test the null hypothesis of no treatment effect on total fibrosis vs. the alternative hypothesis that the treatment effect was ≥9%, using a two-sided t-test with alpha level of 5% and assuming a common standard deviation (SD) of 10% (based on blinded interim data from the first 20 patients). Allowing for 5% of patients with unevaluable biopsies, the total number of patients to be randomized was 51 (34 and 17, respectively).
Since the blinded data at the time of the sample size re-estimation indicated non-normal distribution of the primary efficacy variable, mean fibrosis at baseline and Month 12 were log transformed prior to analysis, and then back-transformed. An analysis of covariance (ANCOVA) model was fitted to the data with the difference between log Month 12 and log baseline values as the dependent variable, log baseline value as covariate, and treatment and concomitant steroid use at baseline as independent class variables, and the results were presented as least squares means (LSM). The secondary histology, MRI and MRS endpoints were analyzed using a similar method to the primary objective, although without log transformation. The functional endpoint objectives were analyzed using a mixed model for repeated measures, with fixed effect class terms included for treatment, visit, visit by treatment interaction, and concomitant steroid use at baseline, and the baseline value included as a covariate. An unstructured covariance matrix was used to model the within-patient error.
The intent-to-treat (ITT) set was used for all efficacy analyses, and comprised all patients randomized to treatment and who received at least one dose of study medication. The safety set comprised all patients who received at least one dose of study medication, and was used for all safety analyses.
3. Results
3.1. Participants
The study was conducted between January 2018 and March 2021. Of 70 patients screened, 51 were enrolled, 44 of whom completed study treatment (Figure 1). The ITT and safety sets were identical. Patients in the givinostat group were on average slightly younger than those in the placebo group, had a shorter time since diagnosis, and were more likely to have a mutation upstream of exon 45 (Table 1).
3.2. Outcomes
3.2.1. Histology
For the primary endpoint, total fibrosis, the mean value at baseline was higher in the placebo group than the givinostat group, suggesting a greater degree of disease involvement (Table 2). Over the duration of the study, mean values did not change from baseline in either group (with 95% CIs of the log LSM values including 0), and there was no difference between the two groups in the Month 12 assessment. Most of the secondary histology parameters were consistent with the primary endpoint, in that baseline values were generally worse in the placebo group, with either no or minimal change from baseline over the study duration, and no differences between the groups for the Month 12 assessment (Table 2, Supplementary Table 2).
3.2.2. MRI/MRS
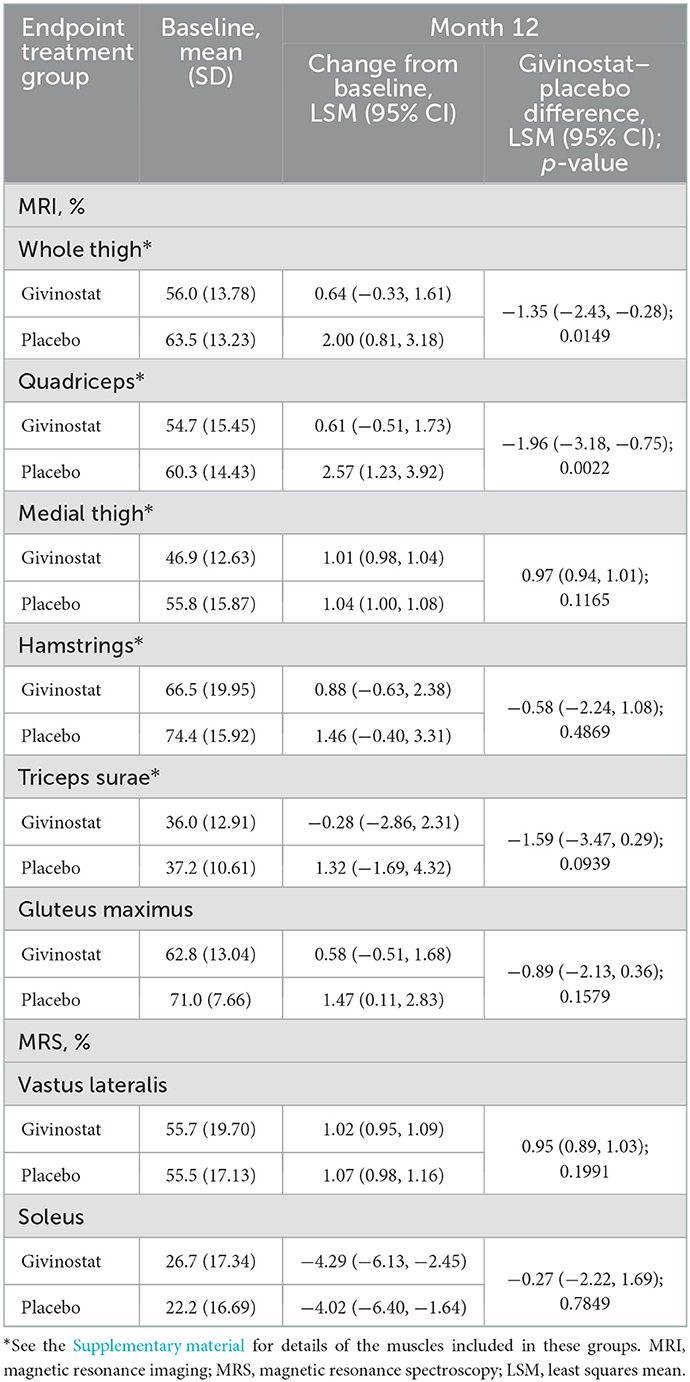
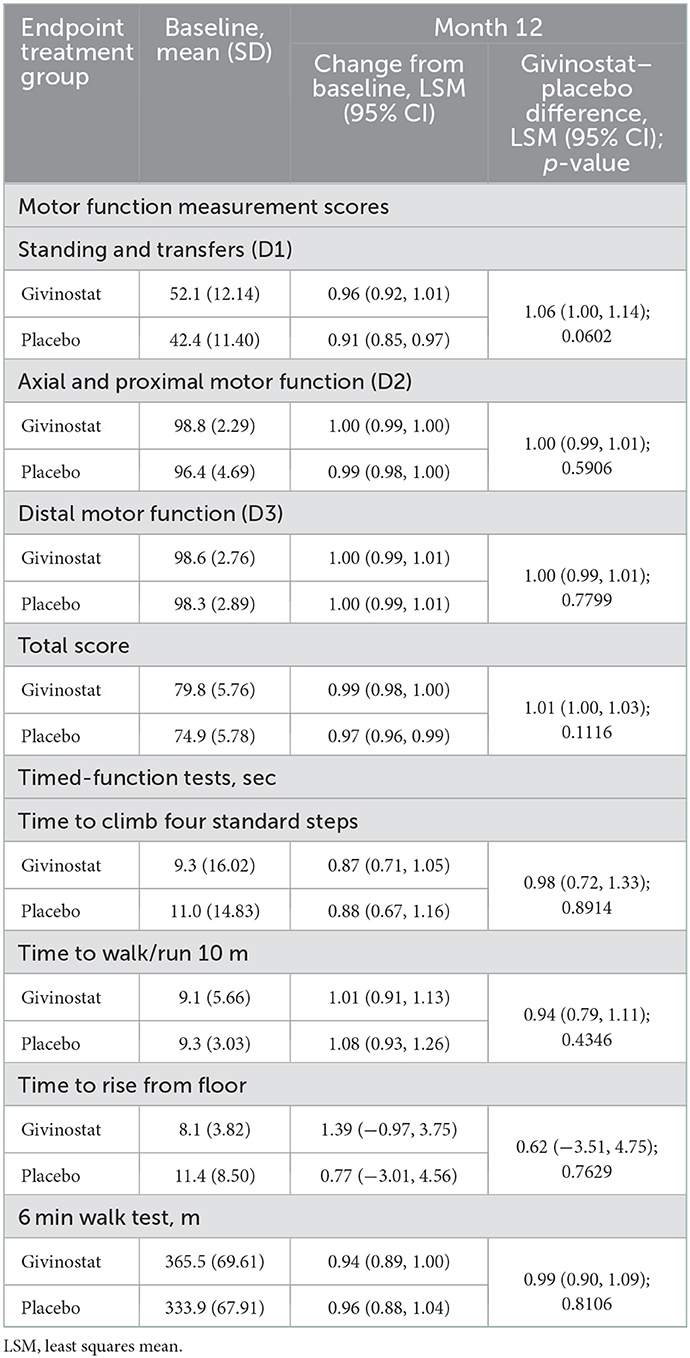
For the MRI fat fraction endpoints, values did not change from baseline over the study duration in the givinostat group, but there were increases (i.e., worsening) from baseline at Month 12 in the placebo group for fat fraction in both the whole thigh and quadriceps, with resultant LSM differences between the two groups of −1.35% (p = 0.0149) and −1.96% (p = 0.0022), respectively (Figure 2, Table 3). The other muscle groups showed similar directions of changes, but there were no givinostat–placebo differences (Table 3).
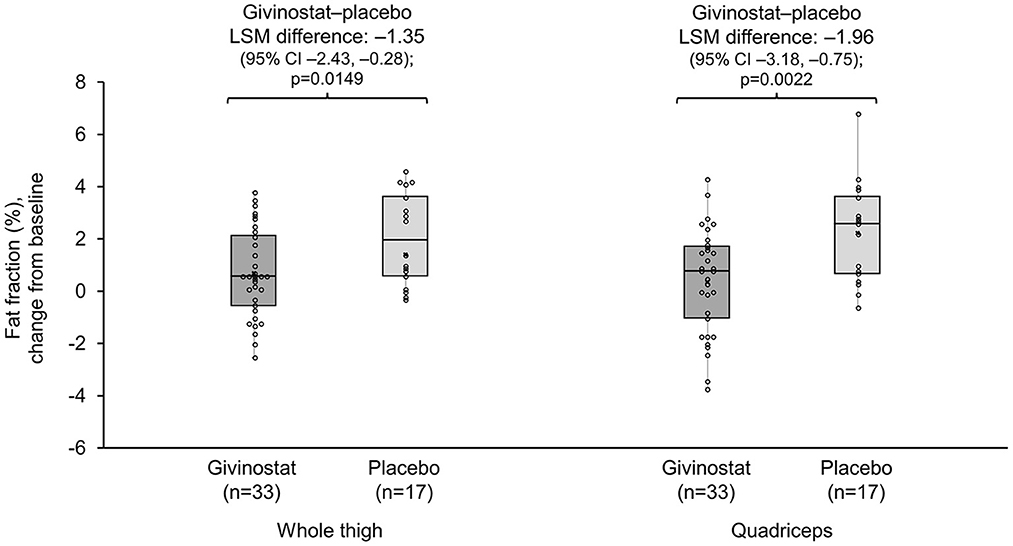
Figure 2. MRI whole thigh and quadriceps fat fractions change from baseline at Month 12. Data plotted are mean change from baseline (mid-point), interquartile range (box), and range (whisker), with individual patient data overlaid. Mean (SD) whole thigh percentages at baseline were 56.0 (13.78) and 63.5 (13.23) for givinostat and placebo, respectively; baseline quadriceps percentages were 54.7 (15.45) and 60.3 (14.43), respectively. MRI, magnetic resonance imaging; LSM, least squares mean.
The MRI contractile area endpoints were broadly consistent with the MRI fat fraction endpoints, with trends to decreases (i.e., worsening) from baseline in the placebo group, but no change with givinostat (Supplementary Table 3). The two groups differed in terms of the whole thigh contractile area assessment (LSM difference 1.37 cm2; p = 0.0375), and approached a difference in the quadriceps assessment (0.63 cm2; p = 0.0528). There were no differences between groups for the MRI CSA endpoints (Supplementary Table 3), or for the two MRS fat fraction endpoints (Table 3).
3.2.3. Functional endpoints
As with the histology endpoints, baseline functional endpoint values were consistent with greater disease involvement in the placebo group, with lower MFM score, longer times required to complete the timed-function tests, a shorter distance covered in the 6MWT, and lower values in the hand-held myometry assessments (Table 4, Supplementary Table 4). Changes over the duration of the study in these parameters were small, and there were no differences between the two groups at Month 12.
3.3. Safety
A total of 17 patients commenced the study on high-dose givinostat, 17 on low-dose givinostat, and 17 on placebo. Subsequently, 26 patients (82.4%) in the givinostat group had their dose reduced due to an adverse event (either decreased platelet count or hypertriglyceridemia). Three patients (8.8%) in the givinostat group and one patient (5.9%) in the placebo group permanently discontinued treatment due to adverse events, one as a result of decreased platelet count (in the givinostat group) and three for hypertriglyceridemia (two in the givinostat group, and one in the placebo group).
All of the patients receiving high-dose givinostat experienced at least one adverse event, most commonly decreased platelet counts and diarrhea (Table 5). However, the majority were mild or moderate in severity, with none either serious or fatal. A lower proportion of patients receiving low-dose givinostat experienced adverse events (76.5%)—and again the majority were mild or moderate in severity, with none either serious or fatal.
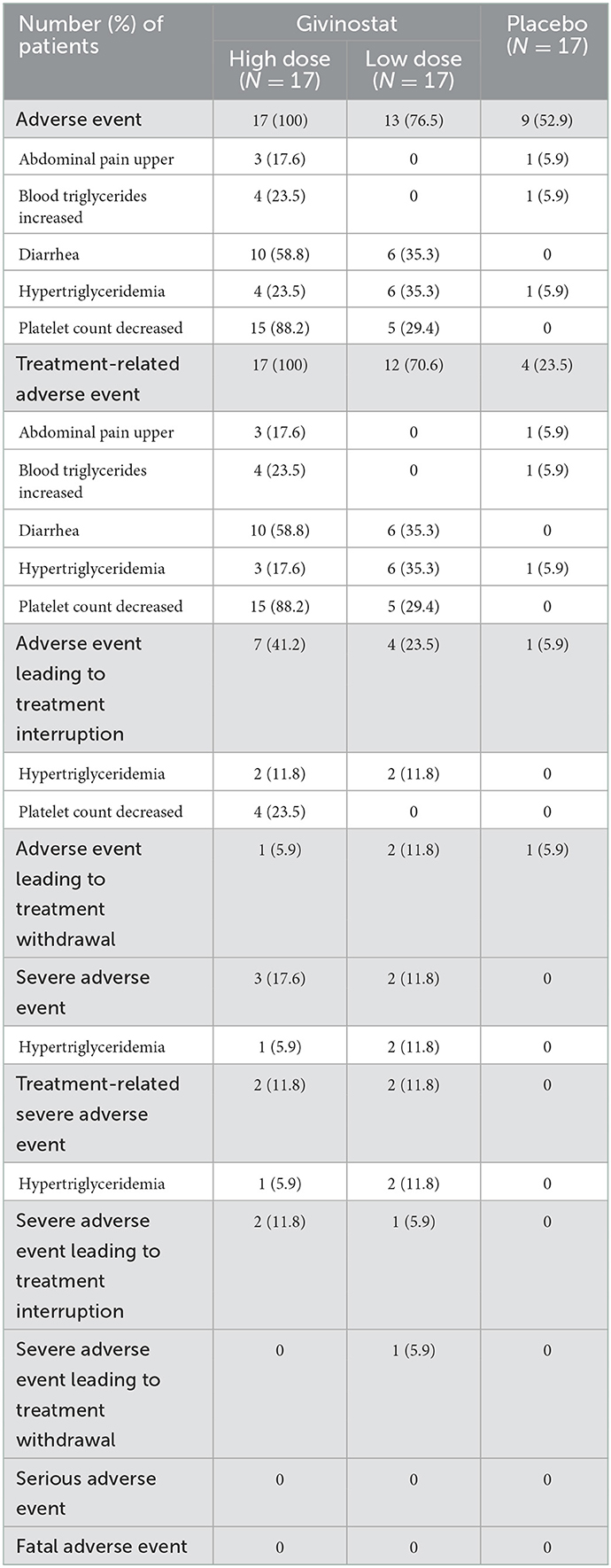
Table 5. Adverse events by treatment received at baseline, overall and most common preferred terms (≥3 patients in any group for adverse events and treatment-related adverse events; ≥2 patients in any group for adverse events leading to treatment interruption or withdrawal, and severe adverse events).
The mean platelet count was lower at baseline in the givinostat group than the placebo group (Supplementary Table 5). There was a reduction from baseline in the mean value at Month 12 in the givinostat group, and no change in the placebo group (although with high variability around the mean in both groups), with 36.4% of patients in the givinostat group having a shift from normal to low platelet counts during the study (compared with none in the placebo group); 52.9% of patients in the givinostat group required a reduction in dose due to decreased platelet count. Mean triglyceride levels increased from baseline to Month 12 in the givinostat group, with no change in the placebo group; 50.0% of patients in the givinostat group had a shift from normal levels at baseline to high levels at some point during the study (Supplementary Table 5). There were only minor changes in other hematology and blood chemistry values, with no relevant changes in physical examination or electrocardiogram parameters or lung function.
4. Discussion
This was the first clinical trial to evaluate the effects of givinostat in BMD. The study aimed to evaluate givinostat effects on muscle morphology (by histology and magnetic resonance), and on muscle function and strength, as well as evaluating tolerability. The primary objective was to demonstrate superiority of givinostat over placebo in terms of the mean change from baseline in total fibrosis after 12 months of treatment. This was not met. This was also true for the other histological parameters. In contrast, whole thigh and quadriceps MRI measures showed muscle deterioration in the placebo group (increased fat replacement and decline in contractile area), but no change from baseline over the 12 month follow-up period in the givinostat group. This resulted in differences between the two groups at Month 12, providing a potential signal that the use of givinostat was associated with stabilization of disease progression.
The contrasting study results, with no difference between the two groups in the histological and functional assessments but differences in the whole thigh and quadriceps MRI assessments may, of course, indicate that givinostat is not effective in this BMD population. However, givinostat has previously demonstrated efficacy in mouse DMD models (15, 16), and has also been shown to increase the fraction of muscle tissue and to reduce the amount of fibrotic tissue in boys with DMD (17). It is generally assumed that if a molecule is effective in DMD it will also be effective in BMD, although there is no specific preclinical model of BMD, and no molecules have so far been approved in both DMD and BMD. Furthermore, although corticosteroids are standard of care in the management of DMD (18), efficacy is less clear in BMD (6). Alternative explanations of these results are the imbalance at baseline between groups in histological parameters, including fibrosis, as well as the stability of the histological parameters over time. Given that givinostat is more likely to work by slowing down muscle deterioration, the selected patient population, who did not show muscle histological deterioration, was possibly not suited to show an effect of the drug. Furthermore, the histological and functional measures may not be sensitive enough in the BMD population as endpoints, as indicated by the lack of change over 12 months. In addition, whereas MRI assesses disease involvement across a large area, biopsy captures involvement in a small fraction of the muscle. Moreover, the muscles in the upper extremities (the site of the biopsies) are less likely to show pathology in BMD than the proximal lower extremity muscles (the location of the MRI). It would therefore have been interesting to conduct MRI of the upper extremity muscles in the current study to allow a direct comparison with the biopsy results.
A number of previous studies have used MRI to evaluate muscle involvement and disease progression in BMD (19–31), with skeletal muscle fat fractions correlating with motor function (19, 21), and changes predicting functional deterioration (19). In one of these, MRI-assessed muscle fat fraction increased significantly over 24 months in the thigh [median +1.9% (−0.7–5.4), p = 0.01] (21). This is consistent with the change that we observed in the placebo group in our study. For a patient with BMD, the fat fraction increase over time can be described by a sigmoidal curve, similar to that which has been shown in DMD (32–34), and the baseline value can therefore predict future fat fractions (23, 35). Given these various findings, it may be useful not only to stratify patients into future studies by their functional status (the 6MWT may not be the best instrument for this), but, if MRI is to be used as an endpoint, according to their MRI fat fraction (22).
Whereas, givinostat had an effect on some of the MRI endpoints, this was not the case with either of the MRS endpoints. This is likely attributable to the large degree of tissue heterogeneity in BMD, which, as we reported previously (14), is difficult to reliably capture using single voxel MRS. In contrast, the analysis of MRI images follows the contour of the muscles and reflects a larger region of muscle tissue (14). MRS results may therefore not be representative of the whole muscle in BMD (in contrast with DMD), and the quantification of fat fraction in this population may necessitate using an image-based MRI.
Givinostat and placebo also did not differ in terms of the strength assessments, timed-function tests or 6MWT, although there was a trend to stabilization of the MFM D1 and total score. Similar to the histology results, baseline imbalances between groups, and lack of deterioration over time in the placebo group, may explain these findings. In a previous manuscript, we used baseline data from the current study to assess correlations between various parameters (14). Despite substantial heterogeneity between patients, MRI fat fractions in the whole thigh and quadriceps correlated significantly with the functional endpoints. This, together with the observations from the current study that the MRI assessments could detect progression in fat replacement in patients receiving placebo, suggests that givinostat effects on functional endpoints could be observed in a study with longer duration and with a patient population that is in a more rapid disease decline phase.
The safety profile of givinostat in the current study was consistent with previous studies in other diseases, including JAK2 positive chronic myeloproliferative neoplasms such as polycythemia vera (36–38), with adverse events being predominantly mild to moderate in severity and more frequent at the higher dose. The exception was hypertriglyceridemia, which was not tested in previous studies. This was therefore the first study to include detailed post-baseline evaluations of triglyceride levels, and it is of note that 41.2% of patients receiving placebo had high triglyceride levels at some point during the study, one of whom discontinued study treatment. Further work is therefore needed to determine the clinical relevance of these findings.
The main limitations of this study are associated with the unexpected difference in disease involvement at baseline between the two patient groups. This, together with the relatively small sample size (although appropriate for this type of study) and high inter-patient variability in the various outcome measures (typical of BMD), suggests caution in the interpretation of the overall findings. In addition, it is clear that total fibrosis is not the best primary endpoint for a study in BMD, given the minimal changes over the follow-up period (suggesting that a longer follow-up would be required to detect any changes, and even then perhaps only in patients with later-stage disease), and other measures such as MRI-based quantification of fat fraction may be better suited. Furthermore, a large number of patients did not meet the eligibility criteria (39), mainly due to the ambulatory criteria and the high ejection fraction. This will be important to consider for future protocols. The heterogeneity of BMD also contributes to the challenges of conducting studies in this disease—a range of BMD phenotypes were eligible for inclusion in the study. The key strengths of the study are that it was conducted at just two clinical sites, both of which are experienced at conducting these evaluations, with all MRS/MRI data evaluated in a single laboratory (University of Florida).
Overall, the efficacy results from the current study highlight the challenges of conducting a study in BMD, a relatively slowly-progressing disorder where the main aim is to prevent (or at least delay) disease progression, rather than cure. Although the study failed to achieve the primary endpoint, there was a potential signal from the MRI assessments that suggests givinostat could prevent (or at least slow down) disease progression in BMD, slowing fat replacement in the whole thigh and quadriceps muscles. This study also provides additional support to the use of MRI as an assessment tool in future BMD studies.
Data availability statement
The raw data supporting the conclusions of this article are available on reasonable request from the sponsor, following submission of a valid research protocol to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by an Independent Ethics Committee for each institution. The patients/participants provided their written informed consent to participate in this study.
Author contributions
GC, EN, KV, RW, and MR: conceptualization, methodology, investigation, writing—review and editing, and visualization. CC, HK, DV, FM, JB, NV, SN, and LA: methodology, investigation, writing—review and editing, and visualization. SC and PB: conceptualization, methodology, supervision, writing—review and editing, and visualization. All authors approved the submitted version of the manuscript.
Funding
This study was funded by Italfarmaco SpA and Regione Lombardia, Grant 231836, as part of the European Regional Development Fund (ERDF) of the Regional Operational Program (ROP) 2014–2020. This work was generated within the European Reference Network for Neuromuscular Diseases (ERN–NMD). Several of the authors are members of the European Reference Network for Rare Neuromuscular Diseases [ERN EURO-NMD]. Dutch authors are member of the Netherlands Neuromuscular Center. University of Florida authors are part of the ImagingDMD Consortium (funded by RO1AR056973).
Acknowledgments
Writing support was provided by David Young of Young Medical Communications and Consulting Ltd. This support was funded by Italfarmaco SpA. The following members of the ImagingDMD Consortium are acknowledged for their contributions: Glenn Walter, Sean Forbes, and William Triplett. In addition, contribution to the study is acknowledged from Drs Stojan Peric, Gabriele Siciliano, Elena Pegoraro, and Luca Bello.
Conflict of interest
GC declares the receipt of a grant from Regione Lombardia, Italy [ITF-Becker: nuovo approccio terapeutico alla distrofia muscolare di Becker (DMB) Project ID 231836], and that he has participated in the Scientific Advisory board of PTC Therapeutics, Sarepta, and Roche. In addition, GC is the President of the Italian Association of Myology. EN reports that he has worked as an investigator on the Italfarmaco SpA clinical trial described in this manuscript. Outside the submitted work, he reports research grants from the Duchenne Parent Project, Spieren voor Spieren, the Parent Project Muscular Dystrophy, the Dutch Research Council, and Prinses Beatrix Spierfonds, ad hoc consultancy fees from Epirium Bio, Edgewise, Regenxbio, and Momenta Therapeutics, and membership of the scientific board of the Association Francaise contre les Myopathies. In addition, he worked as investigator in clinical trials for Italfarmaco, Fibrogen, NS Pharma, Sarepta, and Reveragen. All reimbursements were received by the LUMC. KV reports a research service agreement with Italfarmaco SpA, grants from the National Institutes of Health, and research service support from Sarepta Therapeutics, Catabasis Pharmaceuticals, PTC Therapeutics, Summit Therapeutics, Astellas Pharma, ML Bio/VCU, Edgewise Therapeutics, all directed to the University of Florida. HK reports research support from Philips, trial support, and scientific advisory board membership from ImagingDMD, grants and scientific advisory board membership from Prinses Beatrix Spierfonds, grants from the Dutch Research Council (NWO), and an external review member of TREAT-NMD Advisory Committee for Therapeutics (TACT). No personal fees were collected, with all payments going to the LUMC. RW declares grants to her institution from the US Department of Defense. DV reports consulting fees from Italfarmaco SpA, the sponsor of the study. SC and PB are employees of Italfarmaco SpA. LA is an employee of OPIS srl.
The authors declare that this study received funding from Italfarmaco SpA. The employees of the funder had the following involvement in the study: study design and analysis, interpretation of data, the writing of this article and the decision to submit it for publication.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1095121/full#supplementary-material
References
1. Clarke A, Johnson M, Harris JB. Improved diagnosis of becker muscular dystrophy by dystrophin testing. Neurology. (1989) 39:1011–7. doi: 10.1212/WNL.39.8.1011
2. Wilson K, Faelan C, Patterson-Kane JC, Rudmann DG, Moore SA, Frank D, et al. Duchenne and Becker muscular dystrophies: a review of animal models, clinical end points, and biomarker quantification. Toxicol Pathol. (2017) 45:961–76. doi: 10.1177/0192623317734823
3. Ripolone M, Velardo D, Mondello S, Zanotti S, Magri F, Minuti E, et al. Muscle histological changes in a large cohort of patients affected with Becker muscular dystrophy. Acta Neuropathol Commun. (2022) 10:48. doi: 10.1186/s40478-022-01354-3
4. Kornegay JN, Childers MK, Bogan DJ, Bogan JR, Nghiem P, Wang J, et al. The paradox of muscle hypertrophy in muscular dystrophy. Phys Med Rehabil Clin N Am. (2012) 23:149–72. doi: 10.1016/j.pmr.2011.11.014
5. Monforte M, Mercuri E, Laschena F, Ricci E, Tasca G. Calf muscle involvement in Becker muscular dystrophy: when size does not matter. J Neurol Sci. (2014) 347:301–4. doi: 10.1016/j.jns.2014.10.030
6. Darras BT, Urion DK, Ghosh PS. Dystrophinopathies. In:Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJ, Stephens K, Amemiya A, , editors. GeneReviews. Seattle, WA: University of Washington (2022). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK1119/ (accessed October 19, 2022).
7. Ikeda T, Fujinaka H, Goto K, Nakajima T, Ozawa T. Becker muscular dystrophy caused by exon 2-truncating mutation of DMD. Hum Genome Var. (2019) 6:52. doi: 10.1038/s41439-019-0083-5
8. Colussi C, Mozzetta C, Gurtner A, Illi B, Rosati J, Straino S, et al. HDAC2 blockade by nitric oxide and histone deacetylase inhibitors reveals a common target in duchenne muscular dystrophy treatment. Proc Natl Acad Sci USA. (2008) 105:19183–7. doi: 10.1073/pnas.0805514105
9. Minetti GC, Colussi C, Adami R, Serra C, Mozzetta C, Parente V, et al. Functional and morphological recovery of dystrophic muscles in mice treated with deacetylase inhibitors. Nat Med. (2006) 12:1147–50. doi: 10.1038/nm1479
10. McDonald CM, Henricson EK, Han JJ, Abresch RT, Nicorici A, Atkinson L, et al. The 6-minute walk test in Duchenne/Becker muscular dystrophy: longitudinal observations. Muscle Nerve. (2010) 42:966–74. doi: 10.1002/mus.21808
11. Bérard C, Payan C, Hodgkinson I, Fermanian J. A motor function measure scale for neuromuscular diseases. Construction and validation study. Neuromuscul Disord. (2005) 15:463–70. doi: 10.1016/j.nmd.2005.03.004
12. Fischer D, Hafner P, Rubino D, Schmid M, Neuhaus C, Jung H, et al. The 6-minute walk test, motor function measure and quantitative thigh muscle MRI in Becker muscular dystrophy: a cross-sectional study. Neuromuscul Disord. (2016) 26:414–22. doi: 10.1016/j.nmd.2016.04.009
13. Vuillerot C, Girardot F, Payan C, Fermanian J, Iwaz J, De lattre C, et al. Monitoring changes and predicting loss of ambulation in Duchenne muscular dystrophy with the motor function measure. Dev Med Child Neurol. (2010) 52:60–5. doi: 10.1111/j.1469-8749.2009.03316.x
14. Comi GP, Niks EH, Cinnante CM, Kan HE, Vandenborne K, Willcocks RJ, et al. Characterization of patients with Becker muscular dystrophy by histology, magnetic resonance imaging, function, and strength assessments. Muscle Nerve. (2022) 65:326–33. doi: 10.1002/mus.27475
15. Consalvi S, Mozzetta C, Bettica P, Germani M, Fiorentini F, Del Bene F, et al. Preclinical studies in the mdx mouse model of Duchenne muscular dystrophy with the histone deacetylase inhibitor givinostat. Mol Med. (2013) 19:79–87. doi: 10.2119/molmed.2013.00011
16. Licandro SA, Crippa L, Pomarico R, Perego R, Fossati G, Leoni F, et al. The pan HDAC inhibitor givinostat improves muscle function and histological parameters in two Duchenne muscular dystrophy murine models expressing different haplotypes of the LTBP4 gene. Skelet Muscle. (2021) 11:19. doi: 10.1186/s13395-021-00273-6
17. Bettica P, Petrini S, D'Oria V, D'Amico A, Catteruccia M, Pane M, et al. Histological effects of givinostat in boys with Duchenne muscular dystrophy. Neuromuscul Disord. (2016) 26:643–9. doi: 10.1016/j.nmd.2016.07.002
18. Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, Brumbaugh D, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. (2018) 17:251–67. doi: 10.1016/S1474-4422(18)30024-3
19. Barp A, Bello L, Caumo L, Campadello P, Semplicini C, Lazzarotto A, et al. Muscle MRI and functional outcome measures in Becker muscular dystrophy. Sci Rep. (2017) 7:16060. doi: 10.1038/s41598-017-16170-2
20. Faridian-Aragh N, Wagner KR, Leung DG, Carrino JA. Magnetic resonance imaging phenotyping of Becker muscular dystrophy. Muscle and Nerve. (2014) 50:962–7. doi: 10.1002/mus.24246
21. van de Velde NM, Hooijmans MT, Sardjoe Mishre ASD, Keene KR, Koeks Z, Veeger TTJ, et al. Selection approach to identify the optimal biomarker using quantitative muscle MRI and functional assessments in Becker muscular dystrophy. Neurology. (2021) 97:e513–22. doi: 10.1212/WNL.0000000000012233
22. Sheikh AM, Rudolf K, Witting N, Vissing J. Quantitative muscle MRI as outcome measure in patients with Becker muscular dystrophy - a 1-year follow-up study. Front Neurol. (2021) 11:613489. doi: 10.3389/fneur.2020.613489
23. Veeger TTJ, van de Velde NM, Keene KR, Niks EH, Hooijmans MT, Webb AG, et al. Baseline fat fraction is a strong predictor of disease progression in Becker muscular dystrophy. NMR Biomed. (2022) 35:e4691. doi: 10.1002/nbm.4691
24. Tasca G, Iannaccone E, Monforte M, Masciullo M, Bianco F, Laschena F, et al. Muscle MRI in Becker muscular dystrophy. Neuromuscul Disord. (2012) 22:S100–6. doi: 10.1016/j.nmd.2012.05.015
25. Hooijmans MT, Froeling M, Koeks Z, Verschuuren JJGM, Webb A, Niks EH, et al. Multi-parametric MR in Becker muscular dystrophy patients. NMR Biomed. (2020) 33:e4385. doi: 10.1002/nbm.4385
26. Wokke BH, Hooijmans MT, van den Bergen JC, Webb AG, Verschuuren JJ, Kan HE. Muscle MRS detects elevated PDE/ATP ratios prior to fatty infiltration in Becker muscular dystrophy. NMR Biomed. (2014) 27:1371–7. doi: 10.1002/nbm.3199
27. Van Den Bergen JC, Wokke BH, Janson AA, Van Duinen SG, Hulsker MA, Ginjaar HB, et al. Dystrophin levels and clinical severity in Becker muscular dystrophy patients. J Neurol Neurosurg Psychiatry. (2014) 85:747–53. doi: 10.1136/jnnp-2013-306350
28. Løkken N, Hedermann G, Thomsen C, Vissing J. Contractile properties are disrupted in Becker muscular dystrophy, but not in limb girdle type 2I. Ann Neurol. (2016) 80:466–71. doi: 10.1002/ana.24743
29. Loughran T, Higgins DM, McCallum M, Coombs A, Straub V, Hollingsworth KG. Improving highly accelerated fat fraction measurements for clinical trials in muscular dystrophy: origin and quantitative effect of R2* changes. Radiology. (2015) 275:570–8. doi: 10.1148/radiol.14141191
30. Hollingsworth KG, Higgins DM, McCallum M, Ward L, Coombs A, Straub V. Investigating the quantitative fidelity of prospectively undersampled chemical shift imaging in muscular dystrophy with compressed sensing and parallel imaging reconstruction. Magn Reson Med. (2014) 72:1610–9. doi: 10.1002/mrm.25072
31. Bonati U, Schmid M, Hafner P, Haas T, Bieri O, Gloor M, et al. Longitudinal 2-point dixon muscle magnetic resonance imaging in Becker muscular dystrophy. Muscle Nerve. (2015) 51:918–21. doi: 10.1002/mus.24629
32. Rooney WD, Berlow YA, Triplett WT, Forbes SC, Willcocks RJ, Wang DJ, et al. Modeling disease trajectory in Duchenne muscular dystrophy. Neurology. (2020) 94:e1622–33. doi: 10.1212/WNL.0000000000009244
33. Naarding KJ, Reyngoudt H, van Zwet EW, Hooijmans MT, Tian C, Rybalsky I, et al. MRI vastus lateralis fat fraction predicts loss of ambulation in Duchenne muscular dystrophy. Neurology. (2020) 94:e1386–94. doi: 10.1212/WNL.0000000000008939
34. Naarding KJ, van der Holst M, van Zwet EW, van de Velde NM, de Groot IJM, Verschuuren JJGM, et al. Association of elbow flexor MRI fat fraction with loss of hand-to-mouth movement in patients with Duchenne muscular dystrophy. Neurology. (2021) 97:e1737–42. doi: 10.1212/WNL.0000000000012724
35. Barnard AM, Willcocks RJ, Triplett WT, Forbes SC, Daniels MJ, Chakraborty S, et al. MR biomarkers predict clinical function in Duchenne muscular dystrophy. Neurology. (2020) 94:e897–909. doi: 10.1212/WNL.0000000000009012
36. Rambaldi A, Iurlo A, Vannucchi AM, Noble R, von Bubnoff N, Guarini A, et al. A two-part study of givinostat in patients with polycythemia vera: the maximum tolerated dose selection and the proof of concept final results. Blood. (2017) 130:253. doi: 10.1182/blood.V130.Suppl_1.253.253
37. Rambaldi A, Iurlo A, Vannucchi AM, Noble R, von Bubnoff N, Guarini A, et al. Safety and efficacy of the maximum tolerated dose of givinostat in polycythemia vera: a two-part Phase Ib/II study. Leukemia. (2020) 34:2234–7. doi: 10.1038/s41375-020-0735-y
38. Rambaldi A, Dellacasa CM, Finazzi G, Carobbio A, Ferrari ML, Guglielmelli P, et al. A pilot study of the histone-deacetylase inhibitor givinostat in patients with JAK2V617F positive chronic myeloproliferative neoplasms. Br J Haematol. (2010) 150:446–55. doi: 10.1111/j.1365-2141.2010.08266.x
Keywords: Becker muscular dystrophy, therapy, disease progression, fibrosis, magnetic resonance imaging (MRI)
Citation: Comi GP, Niks EH, Vandenborne K, Cinnante CM, Kan HE, Willcocks RJ, Velardo D, Magri F, Ripolone M, van Benthem JJ, van de Velde NM, Nava S, Ambrosoli L, Cazzaniga S and Bettica PU (2023) Givinostat for Becker muscular dystrophy: A randomized, placebo-controlled, double-blind study. Front. Neurol. 14:1095121. doi: 10.3389/fneur.2023.1095121
Received: 10 November 2022; Accepted: 09 January 2023;
Published: 30 January 2023.
Edited by:
Rosanna Cardani, IRCCS San Donato Polyclinic, ItalyReviewed by:
David Bendahan, UMR7339 Centre de Résonance Magnétique Biologique et Médicale (CRMBM), FranceYi Dai, Peking Union Medical College Hospital (CAMS), China
Susan T. Iannaccone, University of Texas Southwestern Medical Center, United States
Copyright © 2023 Comi, Niks, Vandenborne, Cinnante, Kan, Willcocks, Velardo, Magri, Ripolone, van Benthem, van de Velde, Nava, Ambrosoli, Cazzaniga and Bettica. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giacomo P. Comi,  Z2lhY29tby5jb21pQHVuaW1pLml0
Z2lhY29tby5jb21pQHVuaW1pLml0
†ORCID: Giacomo P. Comi orcid.org/0000-0002-1383-5248
Erik H. Niks orcid.org/0000-0001-5892-5143
Claudia M. Cinnante orcid.org/0000-0002-8388-2074
Hermien E. Kan orcid.org/0000-0002-5772-7177
Michela Ripolone orcid.org/0000-0001-9293-6823
 Giacomo P. Comi
Giacomo P. Comi Erik H. Niks
Erik H. Niks Krista Vandenborne5
Krista Vandenborne5 Hermien E. Kan
Hermien E. Kan Francesca Magri
Francesca Magri Michela Ripolone
Michela Ripolone