- 1Mount Sinai BioDesign, Mount Sinai Medical System, New York, NY, United States
- 2Department of Neurosurgery, Mount Sinai Medical System, New York, NY, United States
- 3Icahn School of Medicine, New York, NY, United States
- 4Rensselaer Polytechnic Institute, Troy, NY, United States
Chronic subdural hematoma is one of the most common neurosurgical pathologies with over 160,000 cases in the United States and Europe each year. The current standard of care involves surgically evacuating the hematoma through a cranial opening, however, varied patient risk profiles, a significant recurrence rate, and increasing financial burden have sparked innovation in the field. This mini-review provides a brief overview of currently used evacuation techniques, including emerging adjuncts such as endoscopic assistance and middle meningeal artery embolization. This review synthesizes the body of available evidence on efficacy and risk profiles for each critical aspect of surgical technique in cSDH evacuation and provides insight into trends in the field and promising new technologies.
Introduction
Chronic subdural hematoma (cSDH) is a collection of fluid, blood, and blood degradation products positioned between the dura mater and the arachnoid linings on the brain surface (1, 2). This condition has a compressive effect on the brain leading to neurological deficits that depend on the size and location of the hematoma. The incidence of cSDH is 8.2–14.0 per 100,000 people annually worldwide and is most common in patients over age 70 (3–11). Due to the aging of the population, the incidence of cSDH is anticipated to double by 2,037 (12, 13).
It was previously postulated that all forms of SDH were caused by venous bleeding from bridging veins (draining from the cortical surface into the dura) leading to the accumulation of blood in the subdural space (14, 15). This acute accumulation leads to clot formation, while persistent clots lead to the formation of fibrous membranes. These membranes form their own microvasculature over time through neo-angiogenesis, and bleeding from these small blood vessels contributes to further expansion, persistence, and recurrence of chronic subdural hematomas (16–18). Like the initial bleeding, rebleeding usually occurs within the inner capillary layer of the dura (15, 19–22). These capillaries are distal branches of the middle meningeal artery (MMA) (17, 23).
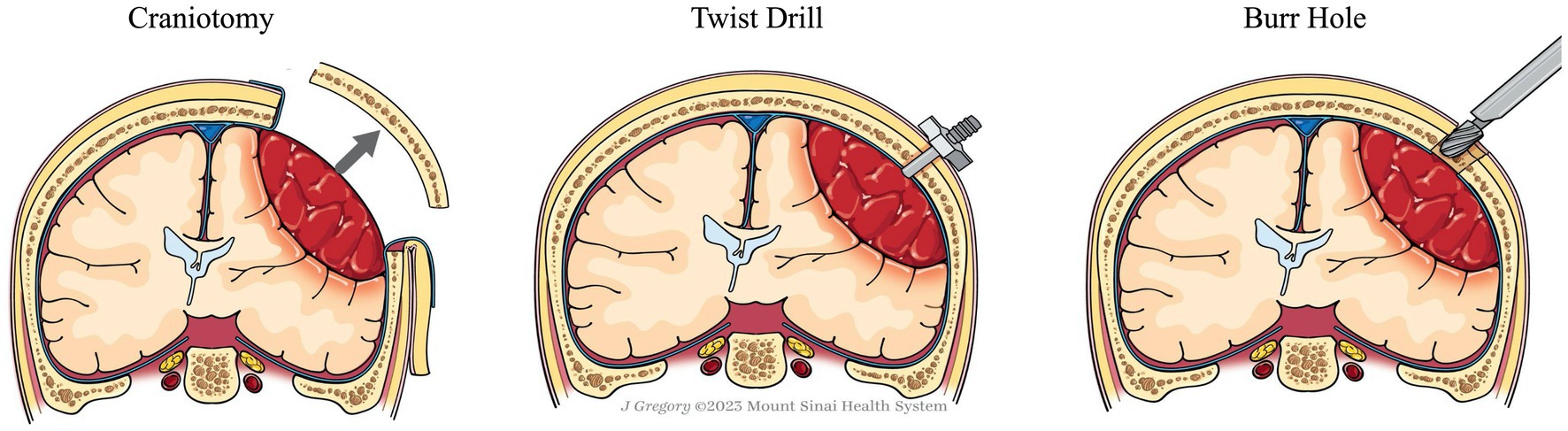
Standard treatment for cSDH involves evacuation of the hematoma to reduce the mass effect and alleviate symptoms (Figure 1) (12). Spontaneous resolution of significant thickness has been reported in a small number of case series, however, it is generally accepted that in the presence of focal symptoms and/or changes in neurologic status, patients should undergo immediate surgical evacuation (12, 24). Effective evacuation improves patient outcomes and reduces the likelihood of recurrence (25).
In the past decade, there has been an increase in the utilization of neuroendoscopy to provide enhanced visualization during hematoma evacuations. Surgical treatment of intracerebral hemorrhage, for example, has seen a recent growth in available tools to enable hematoma evacuation under direct visualization (26, 27). With case studies of neuroendoscopic SDH evacuation now beginning to appear in the literature (28, 29), it is important for the field to stay current on the alternative techniques for cranial access, evacuation, and postoperative drainage, as well as how these techniques may interact with the development and adoption of more modern strategies.
This mini-review aims to concisely summarize historically utilized evacuation techniques for cSDH while contextualizing the modern development of neuroendoscopic SDH evacuation as well as other adjunct procedures.
Methods
A literature search was conducted across three major databases (PubMed, Elsevier, Google Scholar) using the terms “evacuation of chronic subdural hematoma” AND (“minimally invasive” OR “bedside” OR “SEPS” OR “burr hole” OR “craniotomy” OR “twist drill” OR “endoscopy”) AND “elderly.” Additional studies were identified from the references of previously published reviews and included in the analysis.
Publications reporting surgical treatment of acute subdural hematoma (aSDH) were not included given the large difference in pathogenesis, hematoma consistency, and surgical intervention strategies (Figure 1).
Evacuation techniques
Twist drill craniostomy
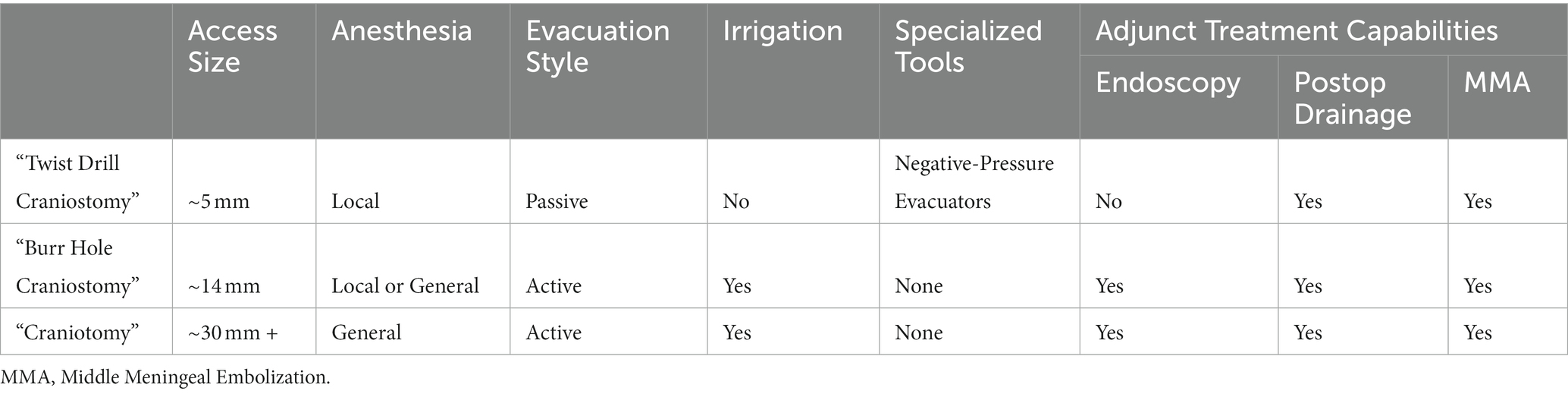
Twist drill craniostomy is the most minimally invasive surgical technique for SDH evacuation, wherein a standard bedside twist drill creates a small (<5 mm) burr hole. The dura is incised and a cannula accesses and passively drains the hematoma. The procedure is commonly performed at the bedside using only local anesthesia (5, 30, 31).
The primary benefits of this technique are reduced invasiveness and the avoidance of general anesthesia, resulting in a lower overall procedural risk, particularly in the elderly or in patients with medical comorbidities (32–34). Reported twist drill craniostomy morbidity ranges from 2.5 to 4.4% and mortality ranges from 2.9–5.1% (12, 30, 35); both significantly lower than other techniques. This lower risk profile comes at the cost of effective treatment, with twist drill craniostomy demonstrating evacuation rates significantly lower than other techniques and resulting in a corresponding higher recurrence rate ranging from 28.1–31.3% (12, 30, 35).
Twist drill craniostomy is the only technique that has seen the development of specialized tools exclusively for the treatment of SDH. These systems, called negative pressure (NPE) evacuation systems, include a stainless steel port that is inserted through the burr hole and connected to a bulb suction reservoir that applies negative pressure and actively drains the hematoma (5, 36–38). Safain et al. compared burr hole and NPE in balanced cohorts of 23 patients and found no significant difference in mortality (4.3% for NPE, 9.1% for burr hole evacuation) or length of stay (11 days for NPE, 9.1 days for burr hole evacuation) between the two groups (39). Furthermore, multiple studies have concluded that the efficacy of NPE is comparable to that of twist drill or burr hole evacuation (9, 30, 40, 41). Since their development, these systems have seen widespread adoption across the field, with Singla et al. reporting that the number of SEPS (Medtronic, United States) devices ordered in the USA increased almost threefold from 2007 to 2011 (42). Their popularity can be attributed to their minimal invasiveness and ability to be performed at the bedside, which is more resource efficient for the hospital system and the patient.
Burr hole craniostomy
Burr hole craniostomy (BHC) is the most common technique for SDH evacuation. BHC begins by drilling one or two 12–14 mm burr holes on the cerebral convexity approximately 5–8 cm apart (40, 43–45). The dura is incised and the hematoma is evacuated using a combination of suction and irrigation (46). Normal saline (NS) and artificial cerebrospinal fluid (ACSF) are commonly used as irrigation solutions, with NS being the most common. Recent studies indicate that ACSF, as an irrigation solution, may improve treatment effectiveness. A study including 234 consecutive patients by Kuwabara et al., found a 23.8% recurrence in patients treated with NS, and a 9.0% recurrence in patients that were treated with ACSF as an irrigation solution (47). Additionally, a retrospective study by Bartley et al. found that irrigating the solution at body temperature results in lower recurrence rates than at room temperature (48). BHC is most commonly performed under general anesthesia, though local anesthesia is a feasible alternative, with one recent study finding local anesthesia evacuation to result in significantly lower complication rates compared to BHC evacuations performed under general anesthesia (49, 50).
Similar to twist drill craniostomies, this technique is preferred in the elderly population, where the increased trauma associated with craniotomy has deleterious effects (51–53). Morbidity and mortality rates in BHC cases remain low, ranging from 4–9.3% and 2.5–3.7%, respectively. Recurrence rate is reported to range from 10.5 to 12.0%, significantly lower than twist drill craniostomy recurrence rates (5, 12, 30, 54).
No specialized tools have yet been developed exclusively for the evacuation of SDH utilizing the burr hole craniostomy technique.
Craniotomy
Craniotomy is the most invasive, but the most surgically effective technique for evacuating cSDH. A bone flat bone varying from 3 to 5 cm in diameter or larger is elevated over the cSDH and the dura is incised in a cruciate fashion to evacuate the hematoma and allow fluid to drain out of the subdural space. The hematoma membranes can be coagulated and excised to prevent further bleeding. Once the hematoma is completely evacuated, the surgical field is inspected to ensure complete hemostasis, and then the dura can be reapproximated either primarily (using sutures) or with the assistance of dural substitute to facilitate secondary healing. The skull flap is then replaced (51). The procedure is performed in the operating room under general anesthesia (5, 31). Morbidity and mortality associated with this approach were 4–12% and 4.6–12.2%, respectively (5, 12, 30, 35, 54). However, this low morbidity and mortality rate may be due to selection bias, with physicians opting for alternative techniques (such as burr hole evacuation) in the elderly and other fragile populations. The recurrence rate is reported to range from 11 to 19.4% (12, 30).
No specialized tools have yet been developed exclusively for the evacuation of SDH utilizing craniotomy access.
Promising adjunct therapies
Drainage
Following the evacuation, the surgeon has the opportunity to position a surgical drain within the subdural or subgaleal space. Leaving a drain in place for 48 h postoperatively has been found to significantly reduce the risk of symptomatic recurrence and the need for reoperations (36, 46, 55–58). Postoperative drainage can be used in conjunction with any surgical evacuation technique, but it has been most extensively studied in conjunction with burr hole drainage. Several studies have demonstrated that 24–48 h of postoperative drainage leads to improved outcomes relative to no drainage. The optimal duration of postoperative drainage remains an open question, however (30, 40, 59–63). Jensen et al. for example, reported no significant differences in the rate of recurrence or death during 90-day follow-up between groups that received either 48-h or 24-h of passive drainage after burr hole evacuation of cSDH (63).
Optimal drain location remains an open question, with no clear advantage in recurrence rates between subdural or subgaleal drain placement (64). It has been suggested that in the absence of a difference in efficacy, subgaleal drain placement should be preferred on the basis of relative safety over subdural drains (65).
Endoscopic assistance
The use of neuroendoscopy allows for enhanced visualization as the hematoma is evacuated, enabling visualization of trabeculae and septations even when employing burr hole evacuation techniques. This visualization facilitates more complete hematoma evacuation as well as excision of neomembranes and meticulous microscopic hemostasis (66, 67). Neuroendoscopic techniques have been reported in both craniotomy (68) and burr hole evacuations (28, 67, 69–73). Data from early studies suggest that the use of neuroendoscopy in burr hole evacuations results in low complication rates, and reduced recurrence rates (28, 74, 75). Both rigid and flexible endoscopes have been studied for SDH evacuation, with a majority of the studies using rigid endoscopes. Rigid endoscopes are generally preferred due to their availability, image quality, and versatile sheaths with working channels for functions such as irrigation, suction, grasping forceps, and coagulation. In their 25-patient study, Huang et al. mention opting for a rigid endoscope for such reasons (29). No twist drill neuroendoscopic cases were identified, likely due to the access size being too small for current endoscope technologies.
Middle meningeal artery embolization
Middle meningeal artery (MMA) embolization is a modern intervention in which embolization material is delivered to the subdural neomembrane capillaries through catheter-based endovascular techniques. These small vessels are thought to be responsible for expansion and recurrence, and the intent of embolizing them is to restrict blood flow to the subdural neomembranes and thereby inhibit hematoma expansion and recurrence (17, 76–78).
Several cohort studies have suggested that MMA embolization in conjunction with evacuation reduces recurrence rates and is associated with low procedural complication rates (79–89). The embolic agents being studied include the SQUID embolic agent, the ONYX liquid embolic system and, TRUFILL n-BCA. These results have not yet been established in a randomized trial, however, several such trials are ongoing (90–92). The efficacy of MMA embolization as a stand-alone treatment for cSDH without surgical evacuation is similarly being investigated in one arm of the MEMBRANE trial (91).
Ironside et al. found in a meta-analysis of 20 studies comprising 1,416 patients that MMA embolization was performed up-front (as a stand-alone therapy, without evacuation) in 28.4% of patients, as a post-surgical adjunct in 23.2%, and as a rescue therapy following cSDH recurrence in 47.8% (77). Onyinzo et al. described a similar distribution of patients in a 132-patient study.
Since its first description in 2000 by Mandai, evidence has accumulated suggesting that MMA embolization is beneficial when added to standard-of-care treatment for cSDH (80–89, 93). A multicenter study published by Kan et al. evaluated 154 MMA embolizations performed not as adjunct treatment (94). Only 9 patients required a second (salvage) intervention (6.5%) and the thickness of the cSDH was improved in 140 patients (90.9%). General anesthesia was used in 6.1% of these patients.
A meta-analysis by Jumah et al. of 177 patients in 11 studies reported a treatment failure rate of 2.8%, an embolization complication rate of 1.2%, and a surgical rescue rate of 2.7% (95). This demonstrates that MMA embolization can be an effective adjunct therapy alongside surgical evacuation. A 72-patient case series performed by Ban et al. compared outcomes in cSDH patients treated with preoperative MMA embolization as compared with surgical intervention alone (79). Not only was the recurrence rate in the presurgical MMA embolization group significantly lower than the rate following surgery alone (1.4% versus 27.4%), but the embolized group also had a significantly lower rate of surgical complications (0 and 4.3% respectively).
Discussion
Despite widespread experience with conventional surgical treatments and a considerable amount of clinical research, there remains a need for continued innovation in the space of surgical cSDH evacuation. While the minimally invasive techniques of twist drill craniostomy and burr hole craniostomy enable the treatment of more fragile populations, they suffer from higher recurrence rates due to reduced evacuation percentages. The more invasive craniotomy technique has seen great success in younger populations, but due to high morbidity and mortality rates, it is not optimal for many elderly cSDH patients.
The development of specialized tools and the potential for future combination therapies is an exciting step toward improved patient care. The use of perioperative drains has demonstrated strong clinical evidence to reduce recurrence rates, however, additional research is needed to optimize placement and length of treatment. Furthermore, the negative pressure systems broadly used with twist drill craniostomy have seen success over the past decade, yet new tools should continue to be developed that utilize modern technological advances. The integration of neuroendoscopy into cSDH evacuation has the potential to improve evacuation rates through minimally invasive cranial access, however significant clinical research and the development of specialized systems are still needed. Similarly, more clinical studies are needed to determine the potential of an evacuation-embolization combination therapy (96). A summary of the different characteristics of each treatment and whether the treatment interfaces with an adjunct technique can be found in Table 1.
cSDH is expected to become one of the most common neurosurgical procedures in the U.S. as the population ages. It is important for the field to continually assess the state of current techniques and the future potential of new technologies to find optimal procedures to maximize hematoma resection while minimizing procedural invasiveness.
Conclusion
The purpose of this mini-review is to present the current treatment options used in the surgical management of chronic subdural hematoma and how they interface with emerging adjunct techniques. Each treatment and technique is briefly described, and associated patient outcomes are presented. The patient data were gathered from large patient studies, well-known RTCs, clinical trials, systematic reviews, and meta-analyzes. The surgical treatments were classified by the size of the access aperture, type of anesthesia, type of evacuation, irrigation or specialized instruments are used, and by the potentially available adjunctive options. Adjunct treatment options currently in development were also summarized. Ultimately, more work must be done to define an evidence-based approach to decide among treatment options in a given case, and to develop tools and techniques to reduce hematoma recurrence while minimizing procedural invasiveness.
Author contributions
BRo primary author, data collection, editing, and figure creation. IM: data collection and editing JV: data collection, editing, and formatting. TW and EH: editing and formatting. JT: Figure creation. TB, BRa, CK, and JB: editing and administrative leadership. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Iliescu, IA. Current diagnosis and treatment of chronic subdural haematomas. J Med Life. (2015) 8:278–84.
2. Kloss, BT, and Lagace, RE. Acute-on-chronic subdural hematoma. Int J Emerg Med. (2010) 3:511–2. doi: 10.1007/s12245-010-0230-8
3. Miranda, LB, Braxton, E, Hobbs, J, and Quigley, MR. Chronic subdural hematoma in the elderly: not a benign disease. J Neurosurg. (2011) 114:72–6. doi: 10.3171/2010.8.JNS10298
4. Stippler, M, Ramirez, P, Berti, A, Macindoe, C, Villalobos, N, and Murray-Krezan, C. Chronic subdural hematoma patients aged 90 years and older. Neurol Res. (2013) 35:243–6. doi: 10.1179/1743132813Y.0000000163
5. Kolias, AG, Chari, A, Santarius, T, and Hutchinson, PJ. Chronic subdural haematoma: modern management and emerging therapies. Nat Rev Neurol. (2014) 10:570–8. doi: 10.1038/nrneurol.2014.163
6. Kumar, AA. Series study of sub acute and chronic subdural haematoma. J Neurol Stroke. (2016) 5:168. doi: 10.15406/jnsk.2016.05.00168
7. Lee, JK, Choi, JH, Kim, CH, Lee, HK, and Moon, JG. Chronic subdural hematomas: a comparative study of three types of operative procedures. J Korean Neurosurg Soc. (2009) 46:210–4. doi: 10.3340/jkns.2009.46.3.210
8. Liu, W, Bakker, NA, and Groen, RJM. Chronic subdural hematoma: a systematic review and meta-analysis of surgical procedures. J Neurosurg. (2014) 121:665–73. doi: 10.3171/2014.5.JNS132715
9. Mori, K, and Maeda, M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases: clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med Chir. (2001) 41:371–81. doi: 10.2176/nmc.41.371
10. Hode, Q. Fatigba, Fanou, Lansdale. Treatment and outcome of chronic subdural hematoma in sub-Saharan Africa and the country of Benin. Iran J Neurosurg. 1:31–5. Available at: https://doaj.org/article/486f93a1cb4140f9b9a3eca5694e71ce
11. Dakurah, TK, Iddrissu, M, Wepeba, G, and Nuamah, I. Chronic subdural haematoma: review of 96 cases attending the Korle Bu teaching hospital, Accra. West Afr J Med. (2005) 24:283–6. doi: 10.4314/wajm.v24i4.28210
12. Ducruet, AF, Grobelny, BT, Zacharia, BE, Hickman, ZL, DeRosa, PL, Anderson, K, et al. The surgical management of chronic subdural hematoma. Neurosurg Rev. (2012) 35:155–69; discussion 169. doi: 10.1007/s10143-011-0349-y
13. Onyinzo, C, Berlis, A, Abel, M, Kudernatsch, M, and Maurer, CJ. Efficacy and mid-term outcome of middle meningeal artery embolization with or without burr hole evacuation for chronic subdural hematoma compared with burr hole evacuation alone. J Neurointerv Surg. (2022) 14:297–300. doi: 10.1136/neurintsurg-2021-017450
14. Miller, JD, and Nader, R. Acute subdural hematoma from bridging vein rupture: a potential mechanism for growth. J Neurosurg. (2014) 120:1378–84. doi: 10.3171/2013.10.JNS13272
15. Edlmann, E, Giorgi-Coll, S, Whitfield, PC, Carpenter, KLH, and Hutchinson, PJ. Pathophysiology of chronic subdural haematoma: inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation. (2017) 14:108. doi: 10.1186/s12974-017-0881-y
16. Weigel, R, Hohenstein, A, and Schilling, L. Vascular endothelial growth factor concentration in chronic subdural hematoma fluid is related to computed tomography appearance and exudation rate. J Neurotrauma. (2014) 31:670–3. doi: 10.1089/neu.2013.2884
17. Tanaka, T, and Kaimori, M. Histological study of vascular structure between the dura mater and the outer membrane in chronic subdural hematoma in an adult. No Shinkei Geka. (1999) 27:431–6.
18. Shono, T, Inamura, T, Morioka, T, Matsumoto, K, Suzuki, SO, Ikezaki, K, et al. Vascular endothelial growth factor in chronic subdural haematomas. J Clin Neurosci. (2001) 8:411–5. doi: 10.1054/jocn.2000.0951
19. Schachenmayr, W, and Friede, RL. The origin of subdural neomembranes. I. Fine structure of the dura-arachnoid interface in man. Am J Pathol. (1978) 92:53–68.
20. Friede, RL, and Schachenmayr, W. The origin ofsubdural neomembranes. II. Fine structural of neomembranes. Am J Pathol. (1978) 92:69–84.
21. Haines, DE, Harkey, HL, and al-Mefty, O. The “subdural” space: a new look at an outdated concept. Neurosurgery. (1993) 32:111–20. doi: 10.1227/00006123-199301000-00017
22. Mack, J, Squier, W, and Eastman, JT. Anatomy and development of the meninges: implications for subdural collections and CSF circulation. Pediatr Radiol. (2009) 39:200–10. doi: 10.1007/s00247-008-1084-6
23. Link, TW, Boddu, S, Marcus, J, Rapoport, BI, Lavi, E, and Knopman, J. Middle meningeal artery embolization as treatment for chronic subdural hematoma: a case series. Oper Neurosurg (Hagerstown). (2018) 14:556–62. doi: 10.1093/ons/opx154
24. Parlato, C, Guarracino, A, and Moraci, A. Spontaneous resolution of chronic subdural hematoma. Surg Neurol. (2000) 53:312–5. doi: 10.1016/S0090-3019(00)00200-7
25. Cai, Q, Guo, Q, Zhang, F, Sun, D, Zhang, W, Ji, B, et al. Evacuation of chronic and subacute subdural hematoma via transcranial neuroendoscopic approach. Neuropsychiatr Dis Treat. (2019) 15:385–90. doi: 10.2147/NDT.S193548
26. Kellner, CP, Chartrain, AG, Nistal, DA, Scaggiante, J, Hom, D, Ghatan, S, et al. The stereotactic Intracerebral hemorrhage underwater blood aspiration (SCUBA) technique for minimally invasive endoscopic intracerebral hemorrhage evacuation. J Neurointerv Surg. (2018) 10:771–6. doi: 10.1136/neurintsurg-2017-013719
27. Kellner, CP, Song, R, Pan, J, Nistal, DA, Scaggiante, J, Chartrain, AG, et al. Long-term functional outcome following minimally invasive endoscopic intracerebral hemorrhage evacuation. J Neurointerv Surg. (2020) 12:489–94. doi: 10.1136/neurintsurg-2019-015528
28. Hellwig, D, Heinze, S, Riegel, T, and Benes, L. Neuroendoscopic treatment of loculated chronic subdural hematoma. Neurosurg Clin N Am. (2000) 11:525–34. doi: 10.1016/S1042-3680(18)30118-9
29. Huang, CJ, Liu, X, Zhou, XT, Qian, W, Li, CH, Wang, JH, et al. Neuroendoscopy-assisted evacuation of chronic subdural hematoma with mixed CT density through a novel small bone flap. J Neurol Surg A Cent Eur Neurosurg. (2020) 81:549–54. doi: 10.1055/s-0040-1715121
30. Weigel, R, Schmiedek, P, and Krauss, JK. Outcome of contemporary surgery for chronic subdural haematoma: evidence based review. J Neurol Neurosurg Psychiatry. (2003) 74:937–43. doi: 10.1136/jnnp.74.7.937
31. Feghali, J, Yang, W, and Huang, J. Updates in chronic subdural hematoma: epidemiology, etiology, pathogenesis, treatment, and outcome. World Neurosurg. (2020) 141:339–45. doi: 10.1016/j.wneu.2020.06.140
32. Mehta, V, Harward, SC, Sankey, EW, Nayar, G, and Codd, PJ. Evidence based diagnosis and management of chronic subdural hematoma: a review of the literature. J Clin Neurosci. (2018) 50:7–15. doi: 10.1016/j.jocn.2018.01.050
33. Ogasawara, K, Koshu, K, Yoshimoto, T, and Ogawa, A. Transient hyperemia immediately after rapid decompression of chronic subdural hematoma. Neurosurgery. (1999) 45:484–8. doi: 10.1097/00006123-199909000-00014
34. Huang, KT, Bi, WL, Abd-el-Barr, M, Yan, SC, Tafel, IJ, Dunn, IF, et al. The Neurocritical and neurosurgical Care of Subdural Hematomas. Neurocrit Care. (2016) 24:294–307. doi: 10.1007/s12028-015-0194-x
35. Lega, BC, Danish, SF, Malhotra, NR, Sonnad, SS, and Stein, SC. Choosing the best operation for chronic subdural hematoma: a decision analysis. J Neurosurg. (2010) 113:615–21. doi: 10.3171/2009.9.JNS08825
36. Flint, AC, Chan, SL, Rao, VA, Efron, AD, Kalani, MA, and Sheridan, WF. Treatment of chronic subdural hematomas with subdural evacuating port system placement in the intensive care unit: evolution of practice and comparison with bur hole evacuation in the operating room. J Neurosurg. (2017) 127:1443–8. doi: 10.3171/2016.9.JNS161166
37. Subdural evacuating port aspiration system. Available at: https://patents.justia.com/patent/7553290
38. Asfora, WT, and Schwebach, L. A modified technique to treat chronic and subacute subdural hematoma: technical note. Surg Neurol. (2003) 59:329–32. doi: 10.1016/S0090-3019(03)00039-9
39. Safain, M, Roguski, M, Antoniou, A, Schirmer, CM, Malek, AM, and Riesenburger, R. A single center’s experience with the bedside subdural evacuating port system: a useful alternative to traditional methods for chronic subdural hematoma evacuation. J Neurosurg. (2013) 118:694–700. doi: 10.3171/2012.11.JNS12689
40. Stanisic, M, Lund-Johansen, M, and Mahesparan, R. Treatment of chronic subdural hematoma by burr-hole craniostomy in adults: influence of some factors on postoperative recurrence. Acta Neurochir. (2005) 147:1249–57. doi: 10.1007/s00701-005-0616-1
41. Horn, EM, Feiz-Erfan, I, Bristol, RE, Spetzler, RF, and Harrington, TR. Bedside twist drill craniostomy for chronic subdural hematoma: a comparative study. Surg Neurol. (2006) 65:150–3. doi: 10.1016/j.surneu.2005.05.030
42. Singla, A, Jacobsen, WP, Yusupov, IR, and Carter, DA. Subdural evacuating port system (SEPS)--minimally invasive approach to the management of chronic/subacute subdural hematomas. Clin Neurol Neurosurg. (2013) 115:425–31. doi: 10.1016/j.clineuro.2012.06.005
43. Ahmed, OEF, El Sawy, A, and El Molla, S. Surgical management of chronic subdural hematomas through single-burr hole craniostomy: is it sufficient? Egypt J Neurol Psychiatr Neurosurg. (2021) 57:1–9. doi: 10.1186/s41983-021-00368-3
44. Markwalder, TM, Steinsiepe, KF, Rohner, M, Reichenbach, W, and Markwalder, H. The course of chronic subdural hematomas after burr-hole craniostomy and closed-system drainage. J Neurosurg. (1981) 55:390–6. doi: 10.3171/jns.1981.55.3.0390
45. Wilson, MH, Wise, D, Davies, G, and Lockey, D. Emergency burr holes: “how to do it”. Scand J Trauma Resusc Emerg Med. (2012) 20:24. doi: 10.1186/1757-7241-20-24
46. Santarius, T, Kirkpatrick, PJ, Ganesan, D, Chia, HL, Jalloh, I, Smielewski, P, et al. Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: a randomised controlled trial. Lancet. (2009) 374:1067–73. doi: 10.1016/S0140-6736(09)61115-6
47. KUWABARA, M, SADATOMO, T, YUKI, K, MIGITA, K, IMADA, Y, SHIMIZU, K, et al. The effect of irrigation solutions on recurrence of chronic subdural hematoma: a consecutive cohort study of 234 patients. Neurol Med Chir. (2017) 57:210–6. doi: 10.2176/nmc.oa.2016-0228
48. Bartley, A, Bartek, J Jr, Jakola, AS, Sundblom, J, Fält, M, Förander, P, et al. Effect of irrigation fluid temperature on recurrence in the evacuation of chronic subdural hematoma: a randomized clinical trial. JAMA Neurol. (2023) 80:58–63. doi: 10.1001/jamaneurol.2022.4133
49. Zhuang, Z, Chen, Z, Chen, H, Chen, B, Zhou, J, Liu, A, et al. Using local anesthesia for Burr hole surgery of chronic subdural hematoma reduces postoperative complications, length of stay, and hospitalization cost: a retrospective cohort study from a single center. Front Surg. (2022) 9:783885. doi: 10.3389/fsurg.2022.783885
50. Duerinck, J, van der Veken, J, Schuind, S, van Calenbergh, F, van Loon, J, du Four, S, et al. Randomized trial comparing Burr hole Craniostomy, Minicraniotomy, and twist Drill Craniostomy for treatment of chronic subdural hematoma. Neurosurgery. (2022) 91:304–11. doi: 10.1227/neu.0000000000001997
51. Chen, JW, Xu, JC, Malkasian, D, Perez-Rosendahl, MA, and Tran, DK. The Mini-craniotomy for cSDH revisited: new perspectives. Front Neurol. (2021) 12:660885. doi: 10.3389/fneur.2021.660885
52. Bartek, J Jr, Sjåvik, K, Kristiansson, H, Ståhl, F, Fornebo, I, Förander, P, et al. Predictors of recurrence and complications after chronic subdural hematoma surgery: a population-based study. World Neurosurg. (2017) 106:609–14. doi: 10.1016/j.wneu.2017.07.044
53. Regan, JM, Worley, E, Shelburne, C, Pullarkat, R, and Watson, JC. Burr hole washout versus craniotomy for chronic subdural hematoma: patient outcome and cost analysis. PLoS One. (2015) 10:e0115085. doi: 10.1371/journal.pone.0115085
54. Almenawer, SA, Farrokhyar, F, Hong, C, Alhazzani, W, Manoranjan, B, Yarascavitch, B, et al. Chronic subdural hematoma management: a systematic review and meta-analysis of 34,829 patients. Ann Surg. (2014) 259:449–57. doi: 10.1097/SLA.0000000000000255
55. Raghavan, A, Smith, G, Onyewadume, L, Peck, MR, Herring, E, Pace, J, et al. Morbidity and mortality after Burr hole Craniostomy versus craniotomy for chronic subdural hematoma evacuation: a single-center experience. World Neurosurg. (2020) 134:e196–203. doi: 10.1016/j.wneu.2019.10.023
56. Guilfoyle, MR, Hutchinson, PJA, and Santarius, T. Improved long-term survival with subdural drains following evacuation of chronic subdural haematoma. Acta Neurochir. (2017) 159:903–5. doi: 10.1007/s00701-017-3095-2
57. Alcalá-Cerra, G, Young, AMH, Moscote-Salazar, LR, and Paternina-Caicedo, A. Efficacy and safety of subdural drains after burr-hole evacuation of chronic subdural hematomas: systematic review and meta-analysis of randomized controlled trials. World Neurosurg. (2014) 82:1148–57. doi: 10.1016/j.wneu.2014.08.012
58. Ramnarayan, R, Arulmurugan, B, Wilson, PM, and Nayar, R. Twist drill craniostomy with closed drainage for chronic subdural haematoma in the elderly: an effective method. Clin Neurol Neurosurg. (2008) 110:774–8. doi: 10.1016/j.clineuro.2008.04.013
59. Chon, KH, Lee, JM, Koh, EJ, and Choi, HY. Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir. (2012) 154:1541–8. doi: 10.1007/s00701-012-1399-9
60. Yamamoto, H, Hirashima, Y, Hamada, H, Hayashi, N, Origasa, H, and Endo, S. Independent predictors of recurrence of chronic subdural hematoma: results of multivariate analysis performed using a logistic regression model. J Neurosurg. (2003) 98:1217–21. doi: 10.3171/jns.2003.98.6.1217
61. Ernestus, RI, Beldzinski, P, Lanfermann, H, and Klug, N. Chronic subdural hematoma: surgical treatment and outcome in 104 patients. Surg Neurol. (1997) 48:220–5. doi: 10.1016/S0090-3019(97)80031-6
62. Torihashi, K, Sadamasa, N, Yoshida, K, Narumi, O, Chin, M, and Yamagata, S. Independent predictors for recurrence of chronic subdural hematoma: a review of 343 consecutive surgical cases. Neurosurgery. (2008) 63:1125–9. doi: 10.1227/01.NEU.0000335782.60059.17
63. Jensen, TSR, Haldrup, M, Hjortdal Grønhøj, M, Miscov, R, Larsen, CC, Debrabant, B, et al. National randomized clinical trial on subdural drainage time after chronic subdural hematoma evacuation. J Neurosurg. (2021) 137:799–806. doi: 10.3171/2021.10.JNS211608
64. Glancz, LJ, Poon, MTC, Coulter, IC, Hutchinson, PJ, Kolias, AG, and Brennan, PM. Does drain position and duration influence outcomes in patients undergoing Burr-hole evacuation of chronic subdural hematoma? Lessons from a UK multicenter prospective cohort study. Neurosurgery. (2019) 85:486–93. doi: 10.1093/neuros/nyy366
65. Oral, S, Borklu, RE, Kucuk, A, Ulutabanca, H, and Selcuklu, A. Comparison of subgaleal and subdural closed drainage system in the surgical treatment of chronic subdural hematoma. North Clin Istanb. (2015) 2:115–21. doi: 10.14744/nci.2015.06977
66. Boyaci, S, Gumustas, OG, Korkmaz, S, and Aksoy, K. Endoscopic evacuation of subdural collections. Turk Neurosurg. (2016) 26:871–7. doi: 10.5137/1019-5149.JTN.14113-15.2
67. Májovský, M, Masopust, V, Netuka, D, and Beneš, V. Flexible endoscope-assisted evacuation of chronic subdural hematomas. Acta Neurochir. (2016) 158:1987–92. doi: 10.1007/s00701-016-2902-5
68. Ichimura, S, Takahara, K, Nakaya, M, Yoshida, K, and Fujii, K. Neuroendoscopic technique for recurrent chronic subdural hematoma with small craniotomy. Turk Neurosurg. (2020) 30:701–6. doi: 10.5137/1019-5149.JTN.27918-19.4
69. Rodziewicz, GS, and Chuang, WC. Endoscopic removal of organized chronic subdural hematoma. Surg Neurol. (1995) 43:569–72. doi: 10.1016/0090-3019(95)00005-4
70. Yan, K, Gao, H, Wang, Q, Xu, X, Wu, W, Zhou, X, et al. Endoscopic surgery to chronic subdural hematoma with neovessel septation: technical notes and literature review. Neurol Res. (2016) 38:467–76. doi: 10.1080/01616412.2016.1139772
71. Zhang, J, Liu, X, Fan, X, Fu, K, Xu, C, Hu, Q, et al. The use of endoscopic-assisted burr-hole craniostomy for septated chronic subdural haematoma: a retrospective cohort comparison study. Brain Res. (2018) 1678:245–53. doi: 10.1016/j.brainres.2017.10.017
72. Singh, H, Patir, R, Vaishya, S, Miglani, R, Gupta, A, and Kaur, A. Endoscopic evacuation of septated chronic subdural hemorrhage - technical considerations, results, and outcome. Surg Neurol Int. (2022) 13:8. doi: 10.25259/SNI_963_2021
73. Ichimura, S, Takahara, K, Nakaya, M, Yoshida, K, Mochizuki, Y, Fukuchi, M, et al. Neuroendoscopic hematoma removal with a small craniotomy for acute subdural hematoma. J Clin Neurosci. (2019) 61:311–4. doi: 10.1016/j.jocn.2018.11.043
74. Gruber, DP, and Crone, KR. Endoscopic washout: a new technique for treating chronic subdural hematomas in infants. Pediatr Neurosurg. (1997) 27:292–5. doi: 10.1159/000121273
75. Mobbs, R, and Khong, P. Endoscopic-assisted evacuation of subdural collections. J Clin Neurosci. (2009) 16:701–4. doi: 10.1016/j.jocn.2008.06.023
76. Gandhoke, GS, Kaif, M, Choi, L, Williamson, RW, and Nakaji, P. Histopathological features of the outer membrane of chronic subdural hematoma and correlation with clinical and radiological features. J Clin Neurosci. (2013) 20:1398–401. doi: 10.1016/j.jocn.2013.01.010
77. Ironside, N, Nguyen, C, do, Q, Ugiliweneza, B, Chen, CJ, Sieg, EP, et al. Middle meningeal artery embolization for chronic subdural hematoma: a systematic review and meta-analysis. J Neurointerv Surg. (2021) 13:951–7. doi: 10.1136/neurintsurg-2021-017352
78. Fiorella, D, and Arthur, AS. Middle meningeal artery embolization for the management of chronic subdural hematoma. J Neurointerv Surg. (2019) 11:912–5. doi: 10.1136/neurintsurg-2019-014730
79. Ban, SP, Hwang, G, Byoun, HS, Kim, T, Lee, SU, Bang, JS, et al. Middle meningeal artery embolization for chronic subdural hematoma. Radiology. (2018) 286:992–9. doi: 10.1148/radiol.2017170053
80. Okuma, Y, Hirotsune, N, Sato, Y, Tanabe, T, Muraoka, K, and Nishino, S. Midterm follow-up of patients with middle meningeal artery embolization in intractable chronic subdural hematoma. World Neurosurg. (2019) 126:e671–8. doi: 10.1016/j.wneu.2019.02.121
81. Link, TW, Boddu, S, Paine, SM, Kamel, H, and Knopman, J. Middle meningeal artery embolization for chronic subdural hematoma: a series of 60 cases. Neurosurgery. (2019) 85:801–7. doi: 10.1093/neuros/nyy521
82. Tempaku, A, Yamauchi, S, Ikeda, H, Tsubota, N, Furukawa, H, Maeda, D, et al. Usefulness of interventional embolization of the middle meningeal artery for recurrent chronic subdural hematoma: five cases and a review of the literature. Interv Neuroradiol. (2015) 21:366–71. doi: 10.1177/1591019915583224
83. Matsumoto, H, Hanayama, H, Okada, T, Sakurai, Y, Minami, H, Masuda, A, et al. Which surgical procedure is effective for refractory chronic subdural hematoma? Analysis of our surgical procedures and literature review. J Clin Neurosci. (2018) 49:40–7. doi: 10.1016/j.jocn.2017.11.009
84. Kim, E. Embolization therapy for refractory hemorrhage in patients with chronic subdural hematomas. World Neurosurg. (2017) 101:520–7. doi: 10.1016/j.wneu.2017.02.070
85. Hashimoto, T, Ohashi, T, Watanabe, D, Koyama, S, Namatame, H, Izawa, H, et al. Usefulness of embolization of the middle meningeal artery for refractory chronic subdural hematomas. Surg Neurol Int. (2013) 4:104. doi: 10.4103/2152-7806.116679
86. Mino, M, Nishimura, S, Hori, E, Kohama, M, Yonezawa, S, Midorikawa, H, et al. Efficacy of middle meningeal artery embolization in the treatment of refractory chronic subdural hematoma. Surg Neurol Int. (2010) 1:78. doi: 10.4103/2152-7806.73801
87. Ishihara, H, Ishihara, S, Kohyama, S, Yamane, F, Ogawa, M, Sato, A, et al. Experience in endovascular treatment of recurrent chronic subdural hematoma. Interv Neuroradiol. (2007) 13:141–4. doi: 10.1177/15910199070130S121
88. Chihara, H, Imamura, H, Ogura, T, Adachi, H, Imai, Y, and Sakai, N. Recurrence of a refractory chronic subdural hematoma after middle meningeal artery embolization that required craniotomy. NMC Case Rep J. (2014) 1:1–5. doi: 10.2176/nmccrj.2013-0343
89. Srivatsan, A, Mohanty, A, Nascimento, FA, Hafeez, MU, Srinivasan, VM, Thomas, A, et al. Middle meningeal artery embolization for chronic subdural hematoma: Meta-analysis and systematic review. World Neurosurg. (2019) 122:613–9. doi: 10.1016/j.wneu.2018.11.167
90. Embolization of the Middle Meningeal Artery With ONYX™ Liquid Embolic System for Subacute and Chronic Subdural Hematoma - Full Text View - ClinicalTrials.Gov. Available at: https://clinicaltrials.gov/ct2/show/NCT04402632
91. Middle Meningeal Artery Embolization for the Treatment of Subdural Hematomas With TRUFILL® n-BCA - Full Text View - ClinicalTrials.Gov. Available at: https://clinicaltrials.gov/ct2/show/NCT04816591
92. The SQUID Trial for the Embolization of the Middle Meningeal Artery for Treatment of Chronic Subdural Hematoma (STEM) - Full Text View - Clinicaltrials.gov. Available at: https://clinicaltrials.gov/ct2/show/NCT04410146
93. Mandai, S, Sakurai, M, and Matsumoto, Y. Middle meningeal artery embolization for refractory chronic subdural hematoma. Case Report J Neurosurg. (2000) 93:686–8. doi: 10.3171/jns.2000.93.4.0686
94. Kan, P, Maragkos, GA, Srivatsan, A, Srinivasan, V, Johnson, J, Burkhardt, JK, et al. Middle meningeal artery embolization for chronic subdural hematoma: a multi-center experience of 154 consecutive Embolizations. Neurosurgery. (2021) 88:268–77. doi: 10.1093/neuros/nyaa379
95. Jumah, F, Osama, M, Islim, AI, Jumah, A, Patra, DP, Kosty, J, et al. Efficacy and safety of middle meningeal artery embolization in the management of refractory or chronic subdural hematomas: a systematic review and meta-analysis. Acta Neurochir. (2020) 162:499–507. doi: 10.1007/s00701-019-04161-3
Keywords: surgical techinque, burr hole and drainage, endoscope assistance, MMA embolization, twist drill craniostomy, craniotomy, subdural evacuating port system (SEPS)
Citation: Rodriguez B, Morgan I, Young T, Vlastos J, Williams T, Hrabarchuk EI, Tepper J, Baker T, Kellner CP, Bederson J and Rapoport BI (2023) Surgical techniques for evacuation of chronic subdural hematoma: a mini-review. Front. Neurol. 14:1086645. doi: 10.3389/fneur.2023.1086645
Edited by:
Ruben Dammers, Erasmus Medical Center, NetherlandsReviewed by:
Hiroaki Matsumoto, Yoshida Hospital, JapanCopyright © 2023 Rodriguez, Morgan, Young, Vlastos, Williams, Hrabarchuk, Tepper, Baker, Kellner, Bederson and Rapoport. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benjamin Rodriguez, QmVuamFtaW4ucm9kcmlndWV6QGljYWhuLm1zc20uZWR1
 Benjamin Rodriguez
Benjamin Rodriguez Isabella Morgan
Isabella Morgan Tirone Young1,2,3
Tirone Young1,2,3 Eugene I. Hrabarchuk
Eugene I. Hrabarchuk Christopher P. Kellner
Christopher P. Kellner