
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 18 April 2023
Sec. Neurorehabilitation
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1061003
This article is part of the Research Topic New advances in Rehabilitation of Stroke View all 37 articles
Objective: Pulmonary dysfunction after stroke is increasingly gaining attention from clinical and rehabilitation specialists. However, owing to cognitive and motor dysfunction in patients with stroke, determining the pulmonary function of these patients remains challenging. The present study aimed to devise a simple method for an early evaluation of pulmonary dysfunction in patients with stroke.
Methods: Overall, 41 patients with stroke in the recovery period (stroke group) and 22 matched healthy controls (control group) were included in the study. We first collected data regarding baseline characteristics for all participants. Furthermore, the participants with stroke were examined using additional scales, such as the National Institutes of Health Stroke Scale (NIHSS), Fugl–Meyer assessment scale (FMA), and modified Barthel Index (MBI). Subsequently, we examined the participants with simple pulmonary function detection and diaphragm ultrasound (B-mode). Ultrasound indices calculated were as follows: the thickness of the diaphragm under the position of functional residual capacity (TdiFRC), the thickness of the diaphragm under the position of forced vital capacity (TdiFVC), thickness fraction, and diaphragmatic mobility. Finally, we compared and analyzed all data to identify group differences, the correlation between pulmonary function and diaphragmatic ultrasound indices, and the correlation between pulmonary function and assessment scale scores in patients with stroke, respectively.
Results: Compared with the control group, patients in the stroke group exhibited lower values for indices of pulmonary and diaphragmatic function (p < 0.001), except for TdiFRC (p > 0.05). The majority of the patients with stroke had restrictive ventilatory dysfunction, as indicated by a significantly higher incidence ratio (36 in 41 patients) than that in the control group (0 in 22 patients) (p < 0.001). Moreover, significant correlations were found between pulmonary function and diaphragmatic ultrasound indices (p < 0.05), with the strongest correlation between TdiFVC and pulmonary indices. In the stroke group, pulmonary function indices were negatively correlated with the NIHSS scores (p < 0.001) and positively correlated with the FMA scores (p < 0.001). No (p > 0.05) or weak (p < 0.05) correlation was found between pulmonary function indices and the MBI scores.
Conclusion: We found that patients with stroke had pulmonary dysfunction even in the recovery period. Diaphragmatic ultrasound can be used as a simple and effective tool for detecting pulmonary dysfunction in patients with stroke, with TdiFVC being the most effective index.
Stroke is the second leading cause of death and the third leading cause of disability worldwide (1). Patients who have suffered a stroke frequently complain of motor and speech disorders as well as dysphagia. However, pulmonary dysfunction after stroke is gradually receiving increased attention. Pulmonary dysfunction can lead to a decline in cardiopulmonary function and activity endurance while simultaneously increasing the risk of pulmonary complications, thereby affecting patients’ motor and speech recovery and their quality of life (2). Therefore, the prompt detection of pulmonary dysfunction among patients with stroke will be of great significance to perform respiratory rehabilitation correspondingly.
Although there are several established methods for assessing respiratory function (3), assessment in patients with stroke is often limited owing to deficits in cognitive, speech, or motor domains. To date, simple pulmonary function assessment has been recognized as one of the most commonly used methods. Schermer et al. (4) confirmed that the indicators of simple respiratory function assessment have remarkable reliability and validity. However, this method also requires patients with stroke to have sufficient cognitive abilities to understand the basics of the examination, which even a healthy older adult may find difficult to understand, and it requires them to be able to cooperate throughout the examination. Therefore, it is important to find a convenient and convincing way for the assessment.
Diaphragmatic ultrasound is a commonly used method for measuring diaphragmatic activity. Previous studies have reported a certain relationship between diaphragmatic ultrasound indices and pulmonary function parameters in some diseases, including osteoporosis, vertebral fracture, kyphosis, late-onset Pompe disease, and amyotrophic lateral sclerosis (5–7). To the best of our knowledge, only two studies have reported the correlation between pulmonary function indices and diaphragmatic ultrasound in patients with stroke to date. In one study, Jung et al. (8) reported a statistically significant correlation between diaphragmatic thickness (DT), thickness ratio, and diaphragmatic excursion and between forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), and peak expiratory flow in patients with stroke compared to healthy controls. In another study, Kim et al. (9) reported a positive correlation between the thickening ratio (%), the ratio of diaphragm thickness at end expiration to the thickness at total lung capacity (TCL), and maximal inspiratory pressure (MIP). However, their study measured the ultrasound index for only one diaphragm, and the correlation with the pulmonary function was restricted to only inspiratory muscle function. Thus, further studies are required to identify the relationship between diaphragmatic ultrasound and pulmonary function.
In the present study, we assessed and compared the pulmonary and diaphragm function of patients with stroke by combining a simple respiratory function assessment with diaphragmatic ultrasound detection. We hypothesized that there is a correlation between diaphragmatic ultrasonography and pulmonary function in patients with stroke. Because ultrasound offers the advantage of visually observing morphologic changes in muscle function (10), a convincing correlation with the pulmonary function will be of great help and convenience to physicians in the early detection of pulmonary dysfunction for patients with stroke.
The sample size of the present study was determined based on the sample sizes of previous similar studies (11, 12). Overall, 51 patients with hemiplegia in the convalescent stage of stroke (stroke group) and 22 age-and gender-matched healthy volunteers (control group) were enrolled.
The inclusion criteria for the stroke group were as follows: (1) patients diagnosed with stroke in accordance with the diagnostic criteria by the Chinese classification of cerebrovascular diseases (2015) and confirmed by brain computed tomography or magnetic resonance imaging findings, (2) those aged 18–70 years, (3) those diagnosed with stroke for the first time, with a course of stroke ranging from 1 month to 1 year, (4) those with right hemiplegia, (5) those who were conscious and had stable vital signs with no progression of neurological symptoms, (6) those who had normal cognitive function (mini-mental state examination [MMSE] score > 26) and were able to complete the entire examination, and (7) those who voluntarily participated and provided written informed consent. The exclusion criteria were as follows: (1) patients presenting with a previous history of respiratory diseases, including chronic bronchopneumonia, chronic obstructive pulmonary disease, bronchiectasis, and lung cancer, (2) those with severe skeletal deformities of the thorax or spine, (3) those with neuromuscular junction disease, (4) those with tracheotomy or auxiliary ventilation, (5) those presenting with a history of thoracic or abdominal surgery, (6) those presenting with a history of receiving respiratory training, (7) those presenting with a history of using inhaled corticosteroids or respiratory central stimulants, (8) those whose House–Brackmann assessment revealed facial paralysis of >grade II, (9) those with severe cardiac, lung, kidney, liver, or other organ dysfunctions, (10) those with severe cognitive impairment or mental illness causing inability in cooperation, and (11) pregnant or lactating women.
The inclusion criteria for the control group were as follows: (1) individuals with no previous history of cerebrovascular disease, (2) those aged between 18 and 70 years, and (3) those who were conscious and had stable vital signs and normal cognitive function (MMSE score > 26 points), were cooperative, and who voluntarily participated and signed the informed consent form. The exclusion criteria for the control group were the same as those for the stroke group.
The following were the rejection and shedding criteria: (1) individuals who did not undergo qualified pulmonary function index measurements and (2) those whose ultrasound examination did not reveal clear diaphragmatic images.
This was a prospective cross-sectional study that aimed to assess patients with stroke who were admitted to a rehabilitation unit of Zhujiang Hospital. Moreover, healthy controls were recruited via poster advertisements on local notice boards.
Basic information, including age, gender, height, weight, and smoking history, were collected for all participants. Additional clinical information, including the course of the disease, type of stroke, and brain lesion location, was collected for the patients in the stroke group.
Pulmonary function index values, including FVC, FEV1, MIP, and maximum expiratory pressure (MEP), were collected using a portable pulmonary function detector (Sike x1; Xiamen Saike Medical Equipment Co., Ltd.).
Diaphragmatic function index values, including the thickness of the diaphragm under the position of functional residual capacity (TdiFRC), the maximum thickness of the diaphragm under the position of forced vital capacity (TdiFVC), and diaphragmatic motion (DM) of the right side of the diaphragm, were collected using full digital color Doppler ultrasound (F8000; Shenzhen Dewell Medical Electronics Co., Ltd.).
All participants were placed in the supine position. To observe the region between ribs 8 and 9 and to obtain two-dimensional images, the chest wall was perpendicularly illuminated using a linear transducer. With the ultrasound probe perpendicular to the two ribs, the diaphragm was visualized as a hypoechoic area between the two hyperechoic layers (i.e., diaphragmatic pleura and peritoneum), and DT was measured from this area at the end of inspiration and expiration. The mean value of three measurements was used for the analysis (3).
The thickness fraction (TF) of the diaphragm was calculated using the following formula:
TF = (maximum DT at the end of deep inspiration − DT at the end of quiet expiration)/DT at the end of quiet expiration × 100% (13–15).
Diaphragm motion was assessed using ultrasound in M-mode. As described in previous studies (11, 16, 17), the amplitudes were measured with one caliper placed at the baseline of the diaphragm echoic line and the other placed at the apex or the maximum height of the line. At least three measurements were recorded, and the average value of these measurements was used for statistical analysis.
All patients in the stroke group were examined using the National Institutes of Health Stroke Scale (NIHSS), Fugl–Meyer assessment scale (FMA), and modified Barthel Index (MBI) to evaluate their severity of brain damage, motor ability, and activities of daily living (ADL), respectively.
All statistical analyses were performed using Statistical Product and Service Solution version 25.0 statistical software (IBM Corporation, Armonk, NY, United States). The Shapiro–Wilk tests were used to examine normal distribution. The two-sample t-test and nonparametric Mann–Whitney tests were used to compare the two groups in terms of continuous variables, whereas the χ2 test was used for comparison in terms of categorical variables. Pearson correlations were used to measure the relationship between pulmonary and diaphragmatic ultrasound indices and between pulmonary indices and NIHSS, FMA, and MBI scores, respectively. Measurement data were expressed as mean (standard deviation), whereas counting data were expressed as frequencies. The level of statistical significance was set at a p-value of <0.05.
Overall, 73 participants were included in this study and classified into the stroke (n = 51) and control (n = 22) groups. Of the patients in the stroke group, six and four patients were excluded as their qualified pulmonary function index was not measured and a clear diaphragmatic image was not visualized under ultrasound, respectively. Finally, 63 participants were enrolled in the study, including 41 patients with stroke and 22 controls (Figure 1).
No significant differences were identified between the two groups in terms of basic information (p > 0.05). However, compared with individuals in the control group, patients in the stroke group demonstrated significantly lower values for all pulmonary indices (p < 0.001) and most diaphragmatic indices (p < 0.05), except for TdiFRC (p = 0.404). Notably, in the stroke group, 36 patients were diagnosed with restrictive ventilatory disorder. The incidence of restrictive ventilatory disorder in these patients was higher than that in the control group (0 in 22 patients, p < 0.001) (Table 1).
The results of correlation analysis indicated that all pulmonary function indices were moderately correlated with TdiFRC (p < 0.001) (Figure 2) and DM (p < 0.001) (Figure 3) and strongly correlated with TdiFVC (p < 0.001) (Figure 4) and TF (p < 0.001) (Figure 5).
The results of correlation analysis indicated that pulmonary function indices in the stroke group were negatively correlated with NIHSS scores (p < 0.001), positively correlated with FMA scores (p < 0.001), and weakly correlated (FVC and FEV1, p < 0.05) or uncorrelated with MBI scores (MIP and MEP, p > 0.05). Details of the analysis are as follows: First, FVC was negatively correlated with NIHSS scores (r = −0.6339, p < 0.001), positively correlated with FMA scores (r = 0.4954, p < 0.001), and weakly correlated with MBI scores (r = 0.3906, p = 0.0116) (Figure 6A). Second, FEV1 was negatively correlated with NIHSS scores (r = −0.5585, p < 0.001), positively correlated with FMA scores (r = 0.4652, p < 0.001), and weakly correlated with MBI scores (r = 0.3906, p = 0.0116) (Figure 6B). Third, MIP was negatively correlated with NIHSS scores (r = −0.5751, p < 0.001) and positively correlated with FMA scores (r = 0.4885, p < 0.001); however, it was uncorrelated with MBI scores (p > 0.05) (Figure 6C). Fourth, MEP was negatively correlated with NIHSS scores (r = −0.4144, p < 0.001), positively correlated with FMA scores (r = 0.3369, p < 0.05), and weakly correlated with MBI scores (r = 0.2971, p = 0.3604) (Figure 6D).
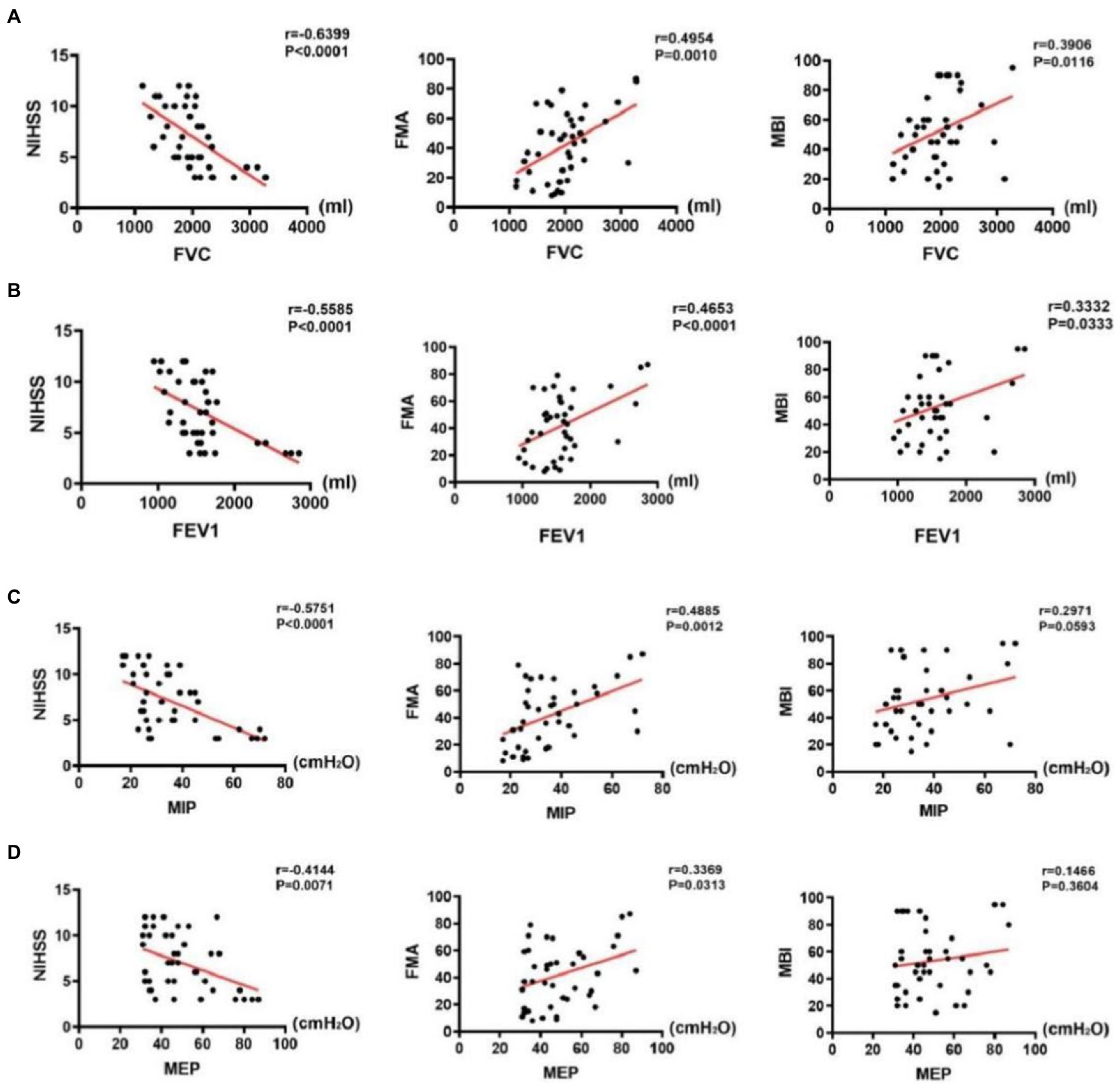
Figure 6. Correlations between pulmonary function indices and assessment scale scores in the stroke group. (A) Correlations between FVC and assessment scale scores, (B) Correlations between FEV1 and assessment scale scores, (C) Correlations between MIP and assessment scale scores, and (D) Correlations between MEP and assessment scale scores.
The results of the present study were consistent with those of previous studies (8, 16, 18) and indicated that the pulmonary function and diaphragmatic ultrasound indices of patients with stroke were decreased compared with those of healthy controls. In previous studies, it has been reported that pulmonary dysfunction in patients with stroke is related to brain injury (19, 20). Changes in the respiratory rate and rhythm as well as weakness of the respiratory muscles may be the direct causes of such injuries (8, 19, 21), and limited thoracic expansion and decreased pulmonary ventilation caused by the weakness of hemiplegic extremities and increased muscle tension may be the indirect causes (22). Furthermore, brain injury directly affects the diaphragmatic muscle on the hemiplegic side, thereby causing muscle weakness, atrophy, and movement reduction (3, 16, 23, 24). Although it has been reported that the nonparalytic diaphragm increases its movement to compensate for the dysfunction of the paralytic diaphragm, the compensation remains insufficient, resulting in a decrease in the vital capacity of patients with stroke (3, 19–21, 25). In addition to the diaphragmatic muscle, other accessory respiratory muscles, such as the sternocleidomastoid, serratus anterior, and intercostal muscles, may be temporarily or permanently deactivated owing to brain damage (5).
In the present study, no significant differences in TdiFRC were found between the two groups; however, there were significant differences in TdiFVC, TF, and DM. This finding may be attributed to the differences in the enrolled patient population. In our study, all patients with stroke were in the convalescent stage, with an average NIHSS score of approximately 9 and an MBI score of 53. Accordingly, their brain injury was moderate, and their motor function was partly recovered (26, 27). Moreover, they could perform some ADLs, such as eating, washing, and dressing, which consumes fewer calories and can be completed in eupnea. However, despite the apparently normal pulmonary function in eupnea, the dysfunction of patients with stroke was evident in deep breathing, as indicated by TdiFVC, DM, and TF, which suggested the decompensation of respiratory function. This could be one of the reasons why the incidence of respiratory complications (e.g., lung infection) is high in these patients and why their disease is more likely to progress to a severe condition after a pulmonary attack. Furthermore, this prompts us to be cautious of pulmonary dysfunction in patients with stroke even though they perform ADLs normally.
In the present study, we found that the mean FVC of patients with stroke was approximately 2 L, which was 56.66% of the mean FVC of the normal age group (Table 1). According to the international guidelines (28), 36 out of 41 patients are diagnosed with restrictive ventilatory disorder. In the present study, the incidence of this disorder was significantly higher in the stroke group than that in the control group (p < 0.001). Restrictive ventilatory disorder frequently refers to ventilation dysfunction caused by the limitation of expansion and retraction of the lungs. It is mainly found in the lung tissue; thoracic, cardiac, and mediastinal diseases; and in diaphragmatic paralysis. As previously described, reduced muscle strength of the accessory respiratory muscles, asymmetric activation of the respiratory muscles and movement of the chest wall after stroke (13), abnormal breathing patterns and reduced respiratory flow (6), and a combination of multiple causes limit the patient’s respiratory function, leading to the development of restrictive ventilatory disorders. Moreover, abnormal respiratory patterns and decreased respiratory flow (22) contribute to limited ventilation in patients with stroke. This suggests that respiratory training for patients with stroke should not be limited to the respiratory muscles; rather, general functional training in breathing patterns is crucial. Notably, respiratory rehabilitation has been found to be significant and sufficient. Sutbeyaz (29) reported significant clinical improvements in patients with subacute stroke following inspiratory muscle training, including an increase in lung volume and exercise capacity, the sensation of dyspnea, and quality of life (QOL, 36-Item Short Form Health Survey domains). Furthermore, Dall’Ago (30) reported improvements in patients with cardiac diseases after the training that targeted 30% of MIP compared with the control group with the use of measures for inspiratory strength, functional performance, and QOL.
The diaphragm is the main respiratory muscle as its movement provides approximately two-thirds of the pulmonary capacity. Correlations between pulmonary function and diaphragmatic ultrasound indices have been reported in some studies, including between FEV1, FVC, and TF in patients with osteoporosis, vertebral fracture, and kyphosis (5); between MIP, FVC, TF, and DT in patients with late-onset Pompe disease (6); and between inhalation volume and DM in patients with amyotrophic lateral sclerosis (16). Moreover, other studies of patients with stroke have reported correlations between DT, thickness ratio, and diaphragm excursion and between FVC, FEV1, and peak expiratory flow (8, 9). These findings indicate that a preliminary examination of pulmonary function can be performed through simple diaphragmatic ultrasound detection. Furthermore, we confirmed a positive correlation between pulmonary function and diaphragmatic ultrasound indices (TdiFRC, TdiFVC, DM, and TF) in patients with stroke and healthy controls. Despite the fact that DM is a valuable index, as suggested by other studies (8), we found that TdiFVC is even more significantly correlated with pulmonary function among several indicators of diaphragmatic ultrasound.
It is known that the diaphragm is the main muscle for calm breathing. While contracting during inspiration, it thickens, which causes an increase in the volume of the chest, and more gas rushes into the lungs. However, during forced expiration, the accessory respiratory muscles increase the oxygen demand. Patients with stroke exhibit reduced muscle coordination in the extremities and coordination difficulties in the respiratory muscles. This suggests that the diaphragm plays a more important role in forced expiration, which could be the reason why TdiFVC is more strongly correlated with lung function. In particular, in patients with stroke with seemingly normal pulmonary function under calm breathing, TdiFVC, as an index, can detect underlying diaphragm abnormalities (e.g., insufficient movement under deep breathing) as well as serve as a valuable indicator of pulmonary dysfunction, which is of great significance for pulmonary rehabilitation.
In addition to measuring pulmonary and diaphragm function in patients with stroke, we performed scale assessments. NIHSS is frequently used to evaluate the severity of brain damage, in which higher scores indicate more severe brain damage. MBI is used to assess the ADL of the patient, wherein higher scores indicate better ADL. It has been reported that reduced FVC in patients with stroke is related to worse ADL (31–33). In the present study, we found a moderately negative correlation between pulmonary function and NIHSS scores; however, there was no or weak correlation between pulmonary function and MBI scores (MIP and MEP, p > 0.05; FVC and FEV1, p < 0.05). This finding may be attributed to the differences in the patient population enrolled. In the present study, patients with stroke had a seemingly normal pulmonary function when performing some ADLs, such as washing, eating, and dressing. Notably, such activities are frequently completed under quiet breathing. Therefore, MBI assessment cannot be used to suspect pulmonary dysfunction in such patients.
This study has some limitations. First, our sample size was comparatively small. Studies with larger sample sizes may provide more positive results. Second, in this study, patients with stroke represented only a population of those with mild-to-moderate neurological impairment after applying our patient screening criteria. Third, as we included patients with a course of stroke ranging from 1 month to 1 year, variations may exist in their assessment. In future, studies may consider narrowing the range of the disease course. Fourth, as the repeatability of ultrasound examination of the left side of the diaphragm is poor owing to limitations in organ anatomy and technology, e.g., the left spleen window is smaller than the right liver window and the measurement on the right side is more feasible than that on the left side (13, 34–36), we included patients with right hemiplegia only for detecting the right side of the diaphragm. Further studies on both sides of the body should be performed considering that the diaphragmatic dysfunction following stroke may be bilateral. Finally, we only examined the diaphragmatic muscles and not the respiratory muscles. Examination of respiratory muscles using other novel techniques, including surface electromyography, optoelectronic plethysmography, and structured light plethysmography, will provide more information about pulmonary dysfunction in patients with stroke.
In conclusion, our results revealed that patients with stroke generally present with pulmonary dysfunction even in the recovery period. Diaphragmatic ultrasound can be used as a simple and effective method for detecting both diaphragmatic function and pulmonary dysfunction in such patients, with TdiFVC being the most effective index.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Institutional Ethics Committee of Zhujiang Hospital of Southern Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This study was supported by the Medical Science and Technology Research Fund Project of Guangdong Province (no. B2020087).
The authors would like to thank the clinical research staff in our department for their assistance during this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Katan, M, and Luft, A. Global burden of stroke. Semin Neurol. (2018) 38:208–11. doi: 10.1055/s-0038-1649503
2. Pelosi, P, and Rocco, PR. The lung and the brain: A dangerous cross-talk. Crit Care. (2011) 15:168. doi: 10.1186/cc10259
3. Kılıçoğlu, MS, Yurdakul, OV, Çelik, Y, and Aydın, T. Investigating the correlation between pulmonary function tests and ultrasonographic diaphragm measurements and the effects of respiratory exercises on these parameters in hemiplegic patients. Top Stroke Rehabil. (2022) 29:218–29. doi: 10.1080/10749357.2021.1911748
4. Schermer, TR, Jacobs, JE, Chavannes, NH, Hartman, J, Folgering, HT, Bottema, BJ, et al. Validity of spirometric testing in a general practice population of patients with chronic obstructive pulmonary disease (COPD). Thorax. (2003) 58:861–6. doi: 10.1136/thorax.58.10.861
5. Ciloglu, O, Karaali, E, Gorgulu, FF, and Ekiz, T. Diaphragm thickness and stiffness in patients with hyperkyphosis due to osteoporotic vertebral fracture: an ultrasonographic and elastographic study. Pol J Radiol. (2020) 85:575–80. doi: 10.5114/pjr.2020.99751
6. Ruggeri, P, Lo Monaco, L, Musumeci, O, Tavilla, G, Gaeta, M, Caramori, G, et al. Ultrasound assessment of diaphragm function in patients with late-onset Pompe disease. Neurol Sci. (2020) 41:2175–84. doi: 10.1007/s10072-020-04316-6
7. Heiman-Patterson, TD, Khazaal, O, Yu, D, Sherman, ME, Kasarskis, EJ, Jackson, CE, et al. Pulmonary function decline in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. (2021) 22:54–61. doi: 10.1080/21678421.2021.1910713
8. Jung, JH, and Kim, NS. The correlation between diaphragm thickness, diaphragmatic excursion, and pulmonary function in patients with chronic stroke. J Phys Ther Sci. (2017) 29:2176–9. doi: 10.1589/jpts.29.2176
9. Kim, M, Lee, K, Cho, J, and Lee, W. Diaphragm thickness and inspiratory muscle functions in chronic stroke patients. Med Sci Monit. (2017) 23:1247–53. doi: 10.12659/msm.900529
10. Huang, Q, Luo, H, Yang, C, Li, J, Deng, Q, Liu, P, et al. Anatomical prior based vertebra modelling for reappearance of human spines. Neurocomputing. (2022) 500:750–60. doi: 10.1016/j.neucom.2022.05.033
11. Voyvoda, N, Yücel, C, Karatas, G, Oguzülgen, I, and Oktar, S. An evaluation of diaphragmatic movements in hemiplegic patients. Br J Radiol. (2012) 85:411–4. doi: 10.1259/bjr/71968119
12. Jung, KJ, Park, JY, Hwang, DW, Kim, JH, and Kim, JH. Ultrasonographic diaphragmatic motion analysis and its correlation with pulmonary function in hemiplegic stroke patients. Ann Rehabil Med. (2014) 38:29–37. doi: 10.5535/arm.2014.38.1.29
13. Laghi, FA Jr, Saad, M, and Shaikh, H. Ultrasound and non-ultrasound imaging techniques in the assessment of diaphragmatic dysfunction. BMC Pulm Med. (2021) 21:85. doi: 10.1186/s12890-021-01441-6
14. Cardenas, LZ, Santana, PV, Caruso, P, Ribeiro de Carvalho, CR, and Pereira de Albuquerque, AL. Diaphragmatic ultrasound correlates with inspiratory muscle strength and pulmonary function in healthy subjects. Ultrasound Med Biol. (2018) 44:786–93. doi: 10.1016/j.ultrasmedbio.2017.11.020
15. Fantini, R, Mandrioli, J, Zona, S, Antenora, F, Iattoni, A, Monelli, M, et al. Ultrasound assessment of diaphragmatic function in patients with amyotrophic lateral sclerosis. Respirology. (2016) 21:932–8. doi: 10.1111/resp.12759
16. Cohen, E, Mier, A, Heywood, P, Murphy, K, Boultbee, J, and Guz, A. Diaphragmatic movement in hemiplegic patients measured by ultrasonography. Thorax. (1994) 49:890–5. doi: 10.1136/thx.49.9.890
17. Scott, S, Fuld, JP, Carter, R, McEntegart, M, and MacFarlane, NG. Diaphragm ultrasonography as an alternative to whole-body plethysmography in pulmonary function testing. J Ultrasound Med. (2006) 25:225–32. doi: 10.7863/jum.2006.25.2.225
18. Annoni, JM, Ackermann, D, and Kesselring, J. Respiratory function in chronic hemiplegia. Int Disabil Stud. (1990) 12:78–80. doi: 10.3109/03790799009166256
19. Polkey, MI, Green, M, and Moxham, J. Measurement of respiratory muscle strength. Thorax. (1995) 50:1131–5. doi: 10.1136/thx.50.11.1131
20. Teixeira-Salmela, LF, Parreira, VF, Britto, RR, Brant, TC, Inácio, EP, Alcântara, TO, et al. Respiratory pressures and thoracoabdominal motion in community-dwelling chronic stroke survivors. Arch Phys Med Rehabil. (2005) 86:1974–8. doi: 10.1016/j.apmr.2005.03.035
21. Leslie, P, Drinnan, MJ, Ford, GA, and Wilson, JA. Resting respiration in dysphagic patients following acute stroke. Dysphagia. (2002) 17:208–13. doi: 10.1007/s00455-002-0052-9
22. Lanini, B, Bianchi, R, Romagnoli, I, Coli, C, Binazzi, B, Gigliotti, F, et al. Chest wall kinematics in patients with hemiplegia. Am J Respir Crit Care Med. (2003) 168:109–13. doi: 10.1164/rccm.200207-745OC
23. Park, GY, Kim, SR, Kim, YW, Jo, KW, Lee, EJ, Kim, YM, et al. Decreased diaphragm excursion in stroke patients with dysphagia as assessed by M-mode sonography. Arch Phys Med Rehabil. (2015) 96:114–21. doi: 10.1016/j.apmr.2014.08.019
24. de Almeida, IC, Clementino, AC, Rocha, EH, Brandão, DC, and Dornelas de Andrade, A. Effects of hemiplegy on pulmonary function and diaphragmatic dome displacement. Respir Physiol Neurobiol. (2011) 178:196–201. doi: 10.1016/j.resp.2011.05.017
25. Kim, J, Park, JH, and Yim, J. Effects of respiratory muscle and endurance training using an individualized training device on the pulmonary function and exercise capacity in stroke patients. Med Sci Monit. (2014) 20:2543–9. doi: 10.12659/MSM.891112
26. Kwah, LK, and Diong, J. National Institutes of Health stroke scale (NIHSS). J Physiother. (2014) 60:61. doi: 10.1016/j.jphys.2013.12.012
27. Liu, F, Tsang, RC, Zhou, J, Zhou, M, Zha, F, Long, J, et al. Relationship of Barthel index and its short form with the modified Rankin scale in acute stroke patients. J Stroke Cerebrovasc Dis. (2020) 29:105033. doi: 10.1016/j.jstrokecerebrovasdis.2020.105033
28. Pellegrino, R, Viegi, G, Brusasco, V, Crapo, RO, Burgos, F, Casaburi, R, et al. Interpretative strategies for lung function tests. Eur Respir J. (2005) 26:948–68. doi: 10.1183/09031936.05.00035205
29. Sutbeyaz, ST, Koseoglu, F, Inan, L, and Coskun, O. Respiratory muscle training improves cardiopulmonary function and exercise tolerance in subjects with subacute stroke: A randomized controlled trial. Clin Rehabil. (2010) 24:240–50. doi: 10.1177/0269215509358932
30. Dall’Ago, P, Chiappa, GR, Guths, H, Stein, R, and Ribeiro, JP. Inspiratory muscle training in patients with heart failure and inspiratory muscle weakness: A randomized trial. J Am Coll Cardiol. (2006) 47:757–63. doi: 10.1016/j.jacc.2005.09.052
31. Yoon, J, Park, J, Lee, D, and Roh, H. Comparisons of respiratory function and activities of daily living between spinal cord injury and stroke patients and normal elderly people. J Phys Ther Sci. (2012) 24:465–9. doi: 10.1589/jpts.24.465
32. Lee, RN, Graydon, JE, and Ross, E. Effects of psychological well-being, physical status, and social support on oxygen-dependent COPD patients’ level of functioning. Res Nurs Health. (1991) 14:323–8. doi: 10.1002/nur.4770140503
33. Ryu, SR, Shin, AY, Han, JY, Choi, IS, Kim, JH, and Lee, SG. The effectiveness of pulmonary rehabilitation program on functional improvement in patients with spinal cord injury. J Korean Acad Rehab Med. (2008) 32:32–7.
34. Noh, DK, Koh, JH, and You, JS. Inter-and intratester reliability values of ultrasound imaging measurements of diaphragm movement in the thoracic and thoracolumbar curves in adolescent idiopathic scoliosis. Physiother Theor Pract. (2016) 32:139–43. doi: 10.3109/09593985.2015.1091871
35. Bobbia, X, Clément, A, Claret, PG, Bastide, S, Alonso, S, Wagner, P, et al. Diaphragmatic excursion measurement in emergency patients with acute dyspnea: Toward a new diagnostic tool? Am J Emerg Med. (2016) 34:1653–7. doi: 10.1016/j.ajem.2016.05.055
Keywords: stroke, pulmonary function, diaphragmatic ultrasound, evaluation, motor function
Citation: Chen Y, Zhou S, Liao L, He J, Tang D, Wu W and Wang K (2023) Diaphragmatic ultrasound can help evaluate pulmonary dysfunction in patients with stroke. Front. Neurol. 14:1061003. doi: 10.3389/fneur.2023.1061003
Received: 04 October 2022; Accepted: 16 March 2023;
Published: 18 April 2023.
Edited by:
Sheng Li, University of Texas Health Science Center at Houston, United StatesReviewed by:
Zengyong Li, National Research Center for Rehabilitation Technical Aids, ChinaCopyright © 2023 Chen, Zhou, Liao, He, Tang, Wu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen Wu, d3V3ZW42NkAxNjMuY29t; Kangling Wang, d2thbmdsaW5nQDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.