
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 07 November 2022
Sec. Neuroepidemiology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.997181
Background: The association between shift work, night work, and the risk of dementia are conflicting in the literature.
Objectives: This study was performed to obtain evidence on the potential relationship between shift work, night work, and dementia.
Methods: To investigate the link between shift work, night work, and dementia, we systematically searched PubMed, Embase, and Web of Science from inception to January 1, 2022. The eligibility of the retrieved records and extracted data were independently reviewed by two researchers. The Preferred Reporting Items for Systematic Evaluations and Meta-Analyses (PRISMA) statement was followed. STATA 16.0 was used to conduct the meta-analysis.
Results: A total of four studies involving 103,104 participants were included in this meta-analysis. Pooled results indicated that night shift workers had a 12% increased risk of dementia compared to subjects without night work (HR = 1.12, 95% CI 1.03–1.23, P = 0.094). Shift work was not significantly associated with dementia risk (HR: 1.09, 95% CI: 0.83–1.43, P = 0.546), but the effect of shift work on dementia risk appeared to increase with age, with a correlation observed among workers older than 50 years (HR = 1.31; 95% CI: 1.03–1.68, P = 0.030).
Conclusion: The data presented in our study suggest that night work may be a risk factor for dementia. More prospective studies with objective exposure measurements are required to further confirm this result.
Systematic review registration: https://doi.org/10.37766/inplasy2022.6.0079, identifier: INPLASY202260079.
Shift work includes work schedules that go beyond the typical “9 to 5” working day and often include early starts, compressed work weeks with 12-h shifts, and night work. With the rapid economic growth of modern society, shift and night work is becoming increasingly widespread in various industries such as food production, entertainment, aviation, healthcare, and transportation (1–3). However, the disruption of circadian rhythms caused by shift and night work poses a significant challenge to public health, increasing the risk of cardiovascular disease, cancer, diabetes, and neuropsychiatric disorders (4–7), even causing specific mortality (8). Recent studies have shown that encountering conditions such as night shift work schedules can lead to lack of sleep, poor sleep quality, reduced recovery, and increased physical stress (9, 10).
Since the beginning of the 21st century, population aging has become a common problem in countries around the world (11). With the increase in human life expectancy and the intensification of population aging, neurodegenerative diseases such as Alzheimer's disease, PD, Huntington's disease, and frontotemporal dementia are on the rise every year, posing a serious threat to human health (12). Not only do they cause severe suffering to patients and their families, but they also pose a heavy burden on healthcare services and society as a whole. For example, the annual cost of medical and long-term care for people with Alzheimer's disease in the United States is estimated at USD 259 billion (13). Therefore, it is particularly necessary and urgent to achieve antecedent research and exploration of neurodegenerative diseases, focusing on the earlier stages of cognitive decline. The characteristic early components of cognitive decline have been unclear so far (14); however, recent studies have revealed that the circadian system regulates many physiological processes in the body, such as sleep-wake cycles, metabolism, inflammatory responses, and gene transcription for oxidative stress (15). Animal studies have shown that in the central nervous system, circadian rhythm disturbances can lead to neuroinflammatory responses, oxidative stress, and neuronal death (16), suggesting a strong link between circadian rhythms and central nervous system-related diseases. Some longitudinal studies with long follow-up periods (5–41 years) have similarly reported greater cognitive decline and increased risk of dementia in people with circadian rhythm disorders (CRDs) (including shift work) compared to those without CRDs (17–19). Therefore, we aim at conducting a meta-analysis by summarizing the existing evidence to systematically evaluate the association between shift/night work and risk of dementia.
The Preferred Reporting Items for Systematic Evaluations and Meta-Analyses (PRISMA) statement was followed when performing the systematic evaluation and meta-analysis (20). We comprehensively searched PubMed, Embase, and Web of Science for studies from the build date of each database to January 1, 2022; our search strategy was not restricted by language or year of publication. The following terms were used to construct the search strategy: “night shift work,” “night work,” “shift work,” “shiftwork,” “dementia,” and “Dementias.” The search method was not restricted by publication type. The search strategies are described in Supplementary material. All authors then manually searched references for inclusion in the articles to add further relevant studies.
Title and abstract screening were carried out by two independent authors' reviews, followed by full-text screening. The studies included in the meta-analysis met all of the following criteria: (a) inclusion in observational case-control studies or cohort studies; (b) the main exposure of study was night/shift work, and the outcome was dementia risk; (c) studies reporting effect estimates with corresponding 95% confidence intervals (CIs) or providing relevant data to calculate crude hazard ratios (HRs) for the association between shift work and dementia. The excluded studies met one of the following criteria:(a) conference abstracts, reviews, or letters; (b) pilot studies; (c) case report, systematic reviews and meta-analyses; (d) duplicate publications; and (e) literature for which full text was not available.
Two authors independently extracted the relevant data from the final included studies, and when differences of opinion arose, the consensus was reached through discussion. If a consensus could not be reached, the disagreement was resolved by consultation with relevant experts. we used pre-designed Microsoft Excel data tables to extract basic information about the selected studies after they had been selected, including first author details such as occupation, country, and age; study year; definition of exposure; and study duration. We also extracted variables impacting the association between night shift work and dementia.
The literature was evaluated by two authors using the New-castle-Ottawa Scale (NOS), a standard for evaluating the quality of literature. The cohort studies consisted of eight items in three areas of study population selection, comparability between groups, and measurement of outcome. The case-control studies included eight components in three areas: research population selection, group comparability, and exposure factor assessment. If the prerequisites were satisfied, a score of one out of nine was given, with a score of seven being deemed high-quality literature (21).
Meta-analysis of effect models was performed using STATA 16.0 software. The hazard ratios (HRs) were pooled to examine the relationship between night/shift work and dementia risk. I2 and Q tests were used to assess heterogeneity. If I2 was ≤50% and P ≥ 0.05 the heterogeneity across the included literature was low, and a fixed-effects model was selected for analysis. If I2 was >50% and P < 0.05, the heterogeneity across the included literature was high, and a random-effects model was used. Sensitivity analyses were conducted using a literature-by-literature exclusion method, and consistent conclusions after literature-by-literature exclusion suggested that the results were robust. Bias was evaluated using the Begg's and Egger's tests, and a P >0.05 indicated the absence of publication bias.
A flow chart of the systematic search and inclusion of studies in this meta-analysis were shown in Figure 1. Initially, 1,286 publications were identified using a complete systematic search of PubMed, Web of Science, and Embase, of which 374 were found to be duplicates. 761 publications were eliminated from the remaining investigations because they were either reviews, systematic reviews, animal trials, or meeting abstracts and did not fit the inclusion requirements. Following a full-text review, 147 items were determined to be unsuitable for recording. Eventually, a total of four articles were included in this meta-analysis (7, 19, 22, 23).
The period of publication of the selected articles was from 2017 to 2020. Of the four included studies, all were prospective cohort studies (19, 22, 23). Three studies were conducted in Denmark (7, 22, 23), and one in Sweden (19). One study included only female nurses (7), and the remaining few included multiple occupations. The included studies used the world health organization's international classification of diseases (ICD) criteria (7, 19, 22, 23). The following ICD codes were used to identify dementia diagnoses collected from National Health Registers using Personal Identification Numbers (PIDs) (7), the Danish psychiatric registry, and the Psychiatric Register (23). The definition of shift and night work varied between studies, with the definition of exposure mainly including start and end times. One study defined night work as work between 23:00–7:00 (7), whereas two did not mention specific times (19, 23), with varying definitions of shifts. None of the cohort studies included participants with a diagnosis of dementia at baseline. Table 1 describes the main characteristics of the included studies.
The four included studies were all considered to be of high quality, with scores ranging from 7 to 9 (Table 2).
Four studies involving 40,130 subjects (7, 19, 22, 23) assessed the association between shift work and the subsequent onset of dementia. Significant heterogeneity was observed (I2 = 78.7%, P [heterogeneity] = 0.003), as the random-effects model was used. There was no statistically significant link between shift work and dementia, according to the pooled result (HR: 1.09, 95% CI: 0.83–1.43, P = 0.546) (Figure 2).
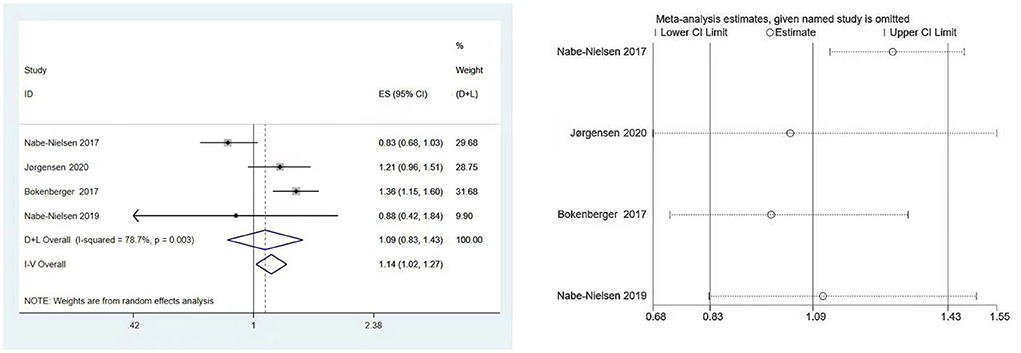
Figure 2. Forest plot and sensitivity analysis of the relationship between shift work and dementia risk.
To see if specific study characteristics influenced results, we performed subgroup analyses by country (Denmark, Sweden), occupation (nurses or mixed), years of shift (<10 vs. >10), age at baseline (<50 vs. >50 years), whether studies were adjusted for pre-existing cardiometabolic disease (yes or no) (Table 3).
When country subgroups were considered, pooled results from Denmark showed that the shift work had no effect on risk of dementia (HR = 0.98; 95% CI: 0.73–1.33, P = 0.908) (7, 22, 23), while the study from Swedish showed a statistically significant association between shift work and dementia (HR = 1.36; 95% CI: 1.15–1.60, P < 0.001) (19).
In terms of the effect of age, Jørgensen et al. (7) found that older (>50 years) shift workers had a dementia risk estimate of 1.31 (HR = 1.31; 95% CI: 1.03–1.68, P = 0.030), while people younger than 50 years was not significant (HR = 0.86; 95% CI: 0.50–1.58, P = 0.607). Nabe-Nielsen et al. (23) found that the incidence of dementia increased by 63% every 5 years with increasing age (HR = 1.63; 95% CI: 1.50–1.78, P < 0.001). Bokenberger et al. and Nabe-Nielsen et al. did not assess the effect of age on the incidence of dementia (19, 22).
In studies that ascertained exposure to occupation, length of sleep, and pre-existing cardio-metabolic disease, no obvious effect on the association between shift work and dementia was observed (7, 19, 22, 23) (Table 3).
The results of Begg's and Egger's test indicated that there was no significant risk of publication bias for the included studies (P = 0.734 and 0.656, respectively). Furthermore, according to sensitivity analysis, the association between shift work and dementia risk was relatively stable (Figure 2).
We performed meta-analyses and calculated pooled effect estimates for three studies (7, 19, 23) that included 63,034 subjects and detected no substantial heterogeneity (I2 = 15.1%, P [Heterogeneity] = 0.308) in the included studies. The fixed-effects models were used to pool multivariate-adjusted HRs across studies to determine the association between night shift work and the risk of dementia (Figure 3). The overall results showed that night shift work may be associated with an increased risk of dementia (HR = 1.12, 95% CI = 1.02–1.23, P = 0.094).
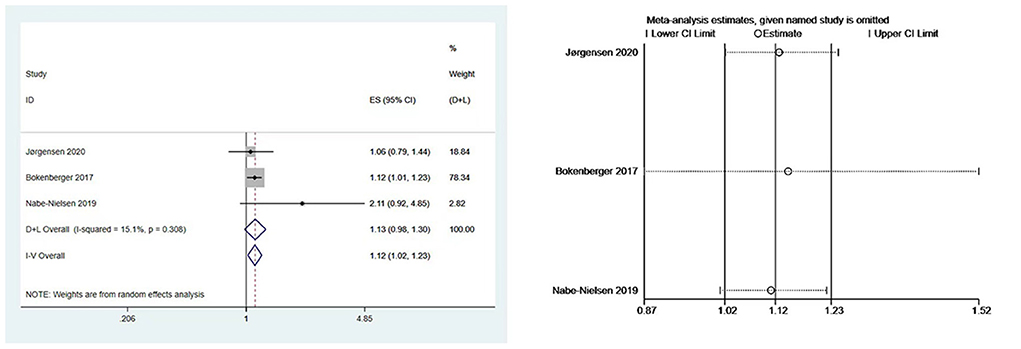
Figure 3. Forest plot and sensitivity analysis of the relationship between night work and dementia risk.
Our subgroup analysis was conducted by country (Denmark and Sweden), occupation type (nurses vs. mixed), and years of night shift (<10 and >10 years). The results are listed in Table 4.
Our subgroup analysis showed that, when grouped by country, the HR of night shift work in Denmark and Sweden was 1.33 (95% CI: 0.71–2.52, P = 0.374) (7, 22, 23) and 1.12 (95% CI: 1.01–1.23; P = 0.024) (19), respectively. When grouped by occupation (7, 19, 22, 23), the pooled HR was 1.06 (95% CI: 0.79–1.14; P = 0.704) for the nurse group and 1.34 (95%CI: 0.77–2.33; P = 0.307) for the mixed occupation group.
Begg's and Egger's tests yielded P values of 1.000 and 0.552, respectively, showing that there was no publication bias. Due to the minimal number of papers covered, funnel plots were not created. Moreover, a sensitivity analysis was carried out by removing one study at a time (Figure 3), the results remained stable.
According to the primary pooled results, we found that there was no statistically significant association between shift work and the risk of dementia (HR = 1.09, 95% CI: 0.83–1.43, P = 0.546), but night shift workers have a 12% increased risk of developing dementia (HR = 1.12, 95% CI: 1.02–1.23, P = 0.094). We conducted a subgroup analysis to assess whether specific study characteristics had an impact on the link between shift/night work and dementia. Despite the small number of studies included, the findings of this analysis may be regarded as a reliable estimate based on high-quality research with over 900,000 participants. These findings add to prior evidence of a potentially detrimental effect of night work on health and longevity.
A systematic review published in 2021 due to the limited number of studies included showed no conclusions can be drawn about the relationship between shift work and dementia (24). Compared to their study, our meta-analysis can not only qualitatively analyze the association between shift work, night shift and dementia, but also quantitatively synthesize the hazard ratio between them, and draw comprehensive conclusions, so as to provide scientific evidence that reduces bias as much as possible and approaches the real risk. The findings in our study regarding overall dementia risk were consistent with theirs, and night shift workers had a 12% increased risk of dementia compared to subjects without night shifts (HR = 1.12, 95% CI 1.02–1.23). Our results do not support that shift work is associated with a risk of dementia. (HR: 1.09, 95% CI: 0.83–1.43). Unfortunately, most studies did not provide the duration and intensity of shift work and night work, so we have insufficient data to conduct a more detailed study. Geographical variation and genetic diversity may play a role in the correlation between shift work, night work and dementia.
The prevalence of dementia is increasing at an alarming prevalence in the aging population. Alzheimer's disease accounts for 60–80% of all cases of dementia and is the most common subtype of dementia (25). It is estimated that the global prevalence of dementia in people aged 65 and over is as high as 7%, with a higher prevalence (8–10%) in developed countries due to the longer life expectancy of the population. (26). Aging, genetic characteristics, and systemic vascular disease are the main risk factors for the development of dementia (27).
There is an established study that confirms that sundowning in dementia and the sleep/wake cycle in delirium patients are influenced by circadian rhythms. Changes in daily routine during night work and shift work can disrupt the body's normal circadian system and lead to a variety of hormonal and metabolic disruptions that can negatively affect physical and mental health (28). One of the pathogenic mechanisms of dementia is the aggregation and accumulation of β-amyloid (Aβ) and tau proteins. Recent evidence from a study by Musiek et al. suggests that sleep rhythm disturbances appear to exacerbate Aβ pathology in mice, and a similar effect may occur in humans as well (29).
A Swedish study (19) showed that the HR for dementia was associated with night work and shift work, and longer exposure to shift/night work appears to be associated with a higher incidence of dementia. In addition, Bokenberger et al. conducted a partial study on the apolipoprotein E (APOE e4) genotype, illustrating that the increased risk of dementia in individuals exposed to shift work and night work lasts 20 years longer in APOE e4 carriers compared to day workers. The incidence of dementia is influenced by genotype; however, other studies (7, 19, 22) have failed to consider genotype-related effects, which may be a factor contributing to the differences in our pooled results.
Jørgensen et al. found (7) that night and shift work did not affect the incidence of dementia compared with fixed day work when any duration of the shift was not considered. They did, however, find a strong relationship between permanent night shift employment for >6 years and the risk of dementia, suggesting that persistent night shift work may increase dementia risk. However, this estimate is based on a tiny number of occurrences. Nonetheless, the increased risk of dementia observed in long-term night shift workers are consistent with the evidence of putative biochemical pathways between sleep and dementia. The findings of this study, which show that night-shift workers have higher dementia risk, are similar to those of a Danish study by Nabe-Nielsen et al. (23), in which higher risk of dementia were observed in permanent night shift workers in the Danish Work Environment Cohort Study (HR = 3.25; 95% CI: 1.35–7.83), consistent with the estimates of Jørgensen et al. (HR = 2.32; 95% CI: 1.32–4.09). In a research of men in Copenhagen, no evidence of a link between midlife shift employment (defined as shift work or regular night work) and dementia incidence over 40 years was discovered (22). The disparities in results between Jørgensen et al. and the Copenhagen study could be explained by major variances in the study populations, including birth cohort and occupational characteristics.
In our age subgroup analysis, the Swedish study found (23) that age may be an important influencing factor in the association of shift and night work with dementia, with older subjects having a higher incidence of dementia than younger subjects and that the differences in the association between shift work and dementia across regions may also be attributed to older age of the Swedish subgroup of participants in our study. Overall, it is generally viewed that the psychological stress induced by the disruption in the circadian rhythm due to night shift work has been suggested to have an adverse impact on brain structures involved in higher cognitive functions (30–33). Shift work may include some non-night shifts as the exposure (34), which may be one of the explanations for this non-significant association for shift work with risk of dementia in current study. Furthermore, putative explanations might be that, most often, permanent night shift workers keep a desynchronized circadian rhythm, with awakening during normal sleeping periods (35). This desynchronized circadian rhythm might affect sleep quality involving chronical disrupted sleep (36, 37). Indeed, day sleeps have typically been found to be considerably shorter than normal night sleeps, because of multiple reasons including increased environmental noise, light levels, conflicting social, and family demands (38). In a word, permanent night shifts might be the poor schedule for workers in terms of dementia.
However, more comprehensive and detailed studies are required to validate our findings. Notably, our pooled risk estimates were heavily influenced by the Swedish cohort study (23), which did not adjust for alcohol consumption, body mass index, or tobacco use, and all potentially relevant confounders consistently varied within each cohort in exposed and unexposed subjects. Especially, shift and night workers may have different lifestyles and behaviors (smoking and/or obesity) (39, 40), and dementia prevalence may vary depending on region and heredity (41, 42).
Despite the originality of our results, there are some limitations to our study. First, in the case of shift night work, there is insufficient data to conduct a more detailed study if only the present shift work schedule is considered, rather than the regularity/irregularity of the schedule, as well as the duration and intensity. Second, with the exception of Bokenberger et al. who did not give an explicit definition of shift night work, the authors of the other studies defined shift night work differently because of different study populations (e. g., specific groups of workers or general working populations), different methods of exposure measurement (e. g., job exposure matrices or questionnaires) and different definitions of exposure and non-exposure. This shortcoming may give rise to heterogeneity between studies and hinder the identification of reliable risk factors. Thirdly, there may still be confounding factors that cannot be controlled, such as the quality of the time worked or differences in rest periods during shifts. This may have an impact on the results as the number and type of confounding factors considered in each study differ. Finally, the original studies included in this paper are mostly from the Nordic region and are underrepresented. The results obtained in this review must be interpreted with caution due to the lack of studies from other parts of the world.
In conclusion, the findings of our investigation revealed that night work may be a risk factor for dementia, with no statistically significant association between shift work and the risk of dementia. Due to the absence of detailed data on duration and intensity of shift and night work included in this meta-analysis, these findings should be regarded with care. More high-quality prospective cohort studies are needed to obtain valid and reliable data. Furthermore, detailed assessments of lifetime work schedules are warranted.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Z-ZW and ZS developed the protocol, participated in the literature search, extracted data, and drafted the manuscript. M-LZ was responsible for the analysis and interpretation of the data. KX contributed to statistical expertise. FZ supervised. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (81873387) and the National Key Talents Project of Traditional Chinese Medicine (2019). This study was also supported by the foundation of the training Project for Young and Middle-aged Science and Technology leaders in Xianyang (Major Scientific and Technological Innovation Project) (2019k01-52) and the training Program of Shaanxi University of Traditional Chinese Medicine (2017SZKY-018). The funding agents play no role in study design, data collection, and data analyses.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.997181/full#supplementary-material
PRISMA, Preferred Reporting Items for Systematic Evaluations and Meta-Analyses; CI, confidence interval; NOS, Newcastle-Ottawa scale; DA, dopamine; HR, hazard ratio; ICD, International Classification of Diseases; ATC, Anatomical Therapeutic Chemical Classification System; NPR, National Patient Register.
1. McEwen B. Stress individual. Mechanisms leading to disease. Arch Intern Med. (1993) 153:2093–101. doi: 10.1001/archinte.153.18.2093
2. Matheson A, O'Brien L, Reid JA. The impact of shiftwork on health: a literature review. J Clin Nurs. (2014) 23:3309–20. doi: 10.1111/jocn.12524
3. Liu R, Jacobs DI, Hansen J, Fu A, Stevens RG, Zhu Y. Aberrant methylation of miR-34b is associated with long-term shiftwork: a potential mechanism for increased breast cancer susceptibility. Cancer Causes Control. (2015) 26:171–8. doi: 10.1007/s10552-014-0494-z
4. Johnson CY, Tanz LJ, Lawson CC, Schernhammer ES, Vetter C, Rich-Edwards JW. Night shift work and cardiovascular disease biomarkers in female nurses. Am J Ind Med. (2020) 63:240–8. doi: 10.1002/ajim.23079
5. Hansen J. Night shift work and risk of breast cancer. Curr Environ Health Rep. (2017) 4:325–39. doi: 10.1007/s40572-017-0155-y
6. Vetter C, Dashti HS, Lane JM, Anderson SG, Schernhammer ES, Rutter MK, et al. Night shift work, genetic risk, and type 2 diabetes in the UK biobank. Diabetes Care. (2018) 41:762–9. doi: 10.2337/dc17-1933
7. Jørgensen JT, Hansen J, Westendorp RGJ, Nabe-Nielsen K, Stayner LT, Simonsen MK, et al. Shift work and incidence of dementia: a Danish Nurse Cohort study. Alzheimers Dement. (2020) 16:1268–79. doi: 10.1002/alz.12126
8. Jørgensen JT, Karlsen S, Stayner L, Hansen J, Andersen ZJ. Shift work and overall and cause-specific mortality in the Danish nurse cohort. Scand J Work Environ Health. (2017) 43:117–26. doi: 10.5271/sjweh.3612
9. Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A. (2016) 113: E1402–11. doi: 10.1073/pnas.1516953113
10. Sharma A, Laurenti MC, Dalla Man C, Varghese RT, Cobelli C, Rizza RA, et al. Glucose metabolism during rotational shift-work in healthcare workers. Diabetologia. (2017) 60:1483–90. doi: 10.1007/s00125-017-4317-0
11. Beard JR, Officer A, de Carvalho IA, Sadana R, Pot AM, et al. The World report on aging and health: a policy framework for healthy aging. Lancet. (2016) 387:2145–54. doi: 10.1016/S0140-6736(15)00516-4
12. Kwon MJ, Kim JH, Kim T, Lee SB. Pharmacological intervention of early neuropathy in neurodegenerative diseases. Pharmacol Res. (2017) 119:169–77. doi: 10.1016/j.phrs.2017.02.003
13. Alzheimer's Association. Alzheimer's disease facts and figures. Alzheimer's Dement. (2016) 12:459–509. doi: 10.1016/j.jalz.2016.03.001
14. Raji CA, Eyre H, Wei SH, Bredesen DE, Moylan S, Law M, et al. Hot topics in research: preventive neuroradiology in brain aging and cognitive decline. AJNR Am J Neuroradiol. (2015) 36:1803–9. doi: 10.3174/ajnr.A4409
15. Harvey JRM, Plante AE, Meredith AL. Ion channels controlling circadian rhythms in suprachiasmatic nucleus excitability. Physiol Rev. (2020) 100:1415–54. doi: 10.1152/physrev.00027.2019
16. Segal JP, Tresidder KA, Bhatt C, Gilron I, Ghasemlou N. Circadian control of pain and neuroinflammation. J Neurosci Res. (2018) 96:1002–20. doi: 10.1002/jnr.24150
17. Tranah GJ, Blackwell T, Stone KL, Ancoli-Israel S, Paudel ML, Ensrud KE, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. (2011) 70:722–32. doi: 10.1002/ana.22468
18. Schlosser Covell GE, Dhawan PS, Lee Iannotti JK, Hoffman-Snyder CR, Wellik KE, Caselli RJ, et al. Disrupted daytime activity and altered sleep-wake patterns may predict transition to mild cognitive impairment or dementia: a critically appraised topic. Neurologist. (2012) 18:426–9. doi: 10.1097/NRL.0b013e318272f7ef
19. Bokenberger K, Sjolander A, Dahl Aslan AK, Karlsson IK, Akerstedt T, Pedersen NL. Shift work and risk of incident dementia: a study of two population-based cohorts. Eur J Epidemiol. (2018) 33:977–87. doi: 10.1007/s10654-018-0430-8
20. Liberati A, Altman DG, Tetzlaff J, Mulrow C, GÃtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. (2009) 151:W−65. doi: 10.7326/0003-4819-151-4-200908180-00136
21. Kotepui KU, Kotepui M. Prevalence of and risk factors for Plasmodium spp. coinfection with hepatitis B virus: a systematic review and meta-analysis. Malar J. (2020) 19:368. doi: 10.1186/s12936-020-03428-w
22. Nabe-Nielsen K, Hansen ÅM, Ishtiak-Ahmed K, Grynderup MB, Gyntelberg F, Islamoska S, et al. Night shift work, long working hours and dementia: a longitudinal study of the Danish Work Environment Cohort Study. BMJ Open. (2019) 9:e027027. doi: 10.1136/bmjopen-2018-027027
23. Nabe-Nielsen K, Garde AH, Ishtiak-Ahmed K, Gyntelberg F, Mortensen EL, Phung TKT, et al. Shift work, long working hours, and later risk of dementia: a long-term follow-up of the Copenhagen Male Study. Scand J Work Environ Health. (2017) 43:569–77. doi: 10.5271/sjweh.3660
24. Leso V, Caturano A, Vetrani I, Iavicoli I. Shift or night shift work and dementia risk: a systematic review. Eur Rev Med Pharmacol Sci. (2021) 25:222–32. doi: 10.26355/eurrev_202101_24388
25. Erkkinen MG, Kim MO, Geschwind MD. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb Perspect Biol. (2018) 10:a033118. doi: 10.1101/cshperspect.a033118
26. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: A systematic review and meta-analysis. Alzheimer's Dement. (2013) 9:75.e2. doi: 10.1016/j.jalz.2012.11.007
27. Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimer's Dement. (2015) 11:718–26 doi: 10.1016/j.jalz.2015.05.016
28. Boivin DB, Boudreau P. Impacts of shift work on sleep and circadian rhythms. Pathol Biol. (Paris). (2014) 62:292–301. doi: 10.1016/j.patbio.2014.08.001
29. Musiek ES, Xiong DD, Holtzman DM. Sleep, circadian rhythms, and the pathogenesis of Alzheimer's disease. Exp Mol Med. (2015) 47:e148. doi: 10.1038/emm.2014.121
30. Kecklund G, Axelsson J. Health consequences of shift work and insufficient sleep. BMJ. (2016) 355:i5210. doi: 10.1136/bmj.i5210
31. Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. (2009) 10:434–45. doi: 10.1038/nrn2639
32. Li L, Wu M, Liao Y, Ouyang L, Du M, Lei D, et al. Grey matter reduction associated with posttraumatic stress disorder and traumatic stress. Neurosci Biobehav Rev. (2014) 43:163–72. doi: 10.1016/j.neubiorev.2014.04.003
33. Zheng C, Quan M, Yang Z, Zhang T. Directionality index of neural information flow as a measure of synaptic plasticity in chronic unpredictable stress rats. Neurosci Lett. (2011) 490:52–6. doi: 10.1016/j.neulet.2010.12.024
34. Kervezee L, Kosmadopoulos A, Boivin DB. Metabolic and cardiovascular consequences of shift work: the role of circadian disruption and sleep disturbances Eur J Neurosci. (2020) 51:396–412. doi: 10.1111/ejn.14216
35. Eastman CI, Boulos Z, Terman M, Campbell SS, Dijk D-J. Lewy Light treatment for sleep disorders: consensus report: VI. Shift work. J Biol Rhythm. (1995) 10:157–64, 10.1177/074873049501000208 doi: 10.1177/074873049501000208
36. Monk TH, Buysse DJ, Billy BD, Fletcher ME, Kennedy KS, Begley AE, Schlarb JE. Beach Shift workers report worse sleep than day workers, even in retirement. J Sleep Res. (2013) 22:201–8. doi: 10.1111/jsr.12003
37. Murali NS, Svatikova AVK. Somers Cardiovascular physiology and sleep. Front. Biosci. (2003) 8:s636–52. doi: 10.2741/1105
38. Folkard S, Barton J. Does the ‘forbidden zone' for sleep onset influence morning shift sleep duration? Ergonomics. (1993) 36:85–91, 10.1080/00140139308967858 doi: 10.1080/00140139308967858
39. Pepłońska B, Burdelak W, Krysicka J, Bukowska A, Marcinkiewicz A, Sobala W, et al. Night shift work and modifiable lifestyle factors. Int J Occup Med Environ Health. (2014) 27:693–706. doi: 10.2478/s13382-014-0298-0
40. Buchvold HV, Pallesen S, Øyane NM, Bjorvatn B. Associations between night work and BMI, alcohol, smoking, caffeine, and exercise–a cross-sectional study. BMC Public Health. (2015) 15:1112. doi: 10.1186/s12889-015-2470-2
41. Ferencz B, Gerritsen L. Genetics and underlying pathology of dementia. Neuropsychol Rev. (2015) 25:113–24. doi: 10.1007/s11065-014-9276-3
Keywords: shift work, night work, dementia, risk factor, systematic evaluation, meta-analysis
Citation: Wang Z-Z, Sun Z, Zhang M-L, Xiong K and Zhou F (2022) Relationship between shift work, night work, and subsequent dementia: A systematic evaluation and meta-analysis. Front. Neurol. 13:997181. doi: 10.3389/fneur.2022.997181
Received: 22 July 2022; Accepted: 07 October 2022;
Published: 07 November 2022.
Edited by:
Steve Simpson-Yap, The University of Melbourne, AustraliaReviewed by:
Depu Wang, Xi'an Jiaotong University, ChinaCopyright © 2022 Wang, Sun, Zhang, Xiong and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Zhou, MzYyODExNzE1QHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.