
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 02 November 2022
Sec. Neurotrauma
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.995925
This article is part of the Research TopicTraumatic Brain Injury- Novel Strategies Targeting the Secondary Injury: Vol IIView all 4 articles
 Baojie Mao1,2†
Baojie Mao1,2† Lei Feng1,2†
Lei Feng1,2† Dongdong Lin2
Dongdong Lin2 Yanfei Shen3
Yanfei Shen3 Jiangchun Ma2
Jiangchun Ma2 Yuning Lu1,2
Yuning Lu1,2 Rui Zhang1,2
Rui Zhang1,2 Ming Wang2*
Ming Wang2* Shu Wan2*
Shu Wan2*Background: We aimed to evaluate the predictive power of systemic inflammation response index (SIRI), a novel biomarker, to predict all-cause mortality in patients with traumatic brain injury (TBI) in the intensive care unit (ICU).
Methods: Clinical data were retrieved from the Medical Information Mart for Intensive Care-IV (MIMIC-IV) database. Kaplan-Meier (KM) methods and cox proportional hazard models were performed to examine the association between SIRI and all-cause mortality. The predictive power of SIRI was evaluated compared to other leukocyte-related indexes including neutrophils, lymphocytes, monocytes and white blood cells (WBC) by the Receiver Operating Characteristic (ROC)curve for 30-day mortality. In addition, propensity score matching (PSM) was conducted to reduce confounding.
Results: A total of 350 TBI patients were enrolled overall in our study. The optimal cutoff point of SIRI was determined at 11.24 × 109/L. After 1:1 PSM, 66 matched pairs (132 patients) were generated. During the 30-day, in-hospital and 365-day follow-up periods, patients with low SIRI level were associated with improved survival (p < 0.05) compared with patients with high SIRI level. Cox regression analysis identified that higher SIRI values was an independent risk factor for all-cause mortality and results were stable on multiple subgroup analyses. Furthermore, ROC analysis indicated that the area under the curve of SIRI [0.6658 (95% Confidence Interval, 0.5630–0.7687)] was greater than that of neutrophils, monocytes, lymphocytes and WBC. The above results were also observed in the matched cohort.
Conclusion: It was suggested that TBI patients with high SIRI level would suffer from a high risk of 30-day, in-hospital and 365-day mortality. SIRI is a promising inflammatory biomarker for predicting TBI patients' prognosis with relatively better predictive power than other single indicators related to peripheral differential leukocyte counts.
Traumatic brain injury (TBI) has been known as one of the leading causes of disability and death among young adults worldwide, which more than 50 million people suffered from each year (1, 2). The most common external causes of TBI are traffic accidents and falls (3). TBI was commonly categorized into primary and secondary injuries. The primary injury results from mechanical forces that cause direct disruption of the brain tissue. Within several minutes of the primary impact, molecular pathways in the damaged area and surrounding normal tissue are initiated, which play an important role in secondary brain injury (SBI) including excitotoxicity, neuroinflammation and blood-brain barrier (BBB) disruption (4). Rapid CT can be available for identifying almost all forms of TBI and help to make appropriate medical decisions in the shortest time, but little or no information is available by neuroimaging techniques about SBI. Therefore, new biomarkers may be developed for gaining additional information regarding SBI, especially neuroinflammation.
Accumulating evidence have revealed that neuroinflammation plays a critical role in the pathogenesis of SBI. Besides, neutrophils, monocytes and lymphocytes have been thought to be significant working cells in the neuroinflammatory process of traumatic brain injury (2). Therefore, the inflammatory indexes such as neutrophil-to-lymphocyte ratio (NLR) and systemic inflammatory response index (SIRI) are normally thought to represent a crucial predictor of secondary injury. The SIRI is a novel composite indicator with simple detection, strong practicability and low cost. There are reports that SIRI has been shown to have excellent predictive power particularly in cardiovascular disease, infectious diseases and cancer (5–7). In previous studies, it has also been determined to play a great predictive role in prognosis for intracerebral hemorrhage (ICH) and subarachnoid hemorrhage patients (8, 9). However, the association with all-cause death in TBI patients have not been reported yet. This study aimed to evaluate the prognostic significance of SIRI in patients with TBI admitted to the intensive care unit (ICU).
An open and free critical care database, which contained comprehensive clinical data of patients admitted to a tertiary academic medical center in Boston, MA, USA between 2008 and 2019, termed the Medical Information Mart for Intensive Care-IV (MIMIC-IV). The database includes basic patient information, vital signs, laboratory indicators, treatment details and survival data. The information from MIMIC-IV has been approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center (Boston, MA) and Massachusetts Institute of Technology (MIT; Cambridge, MA). As all personal data in this database had been encrypted, informed consent was waived. One author (Mao, Baojie) obtained access to the database and was responsible for data extraction (certification number 46148427).
Those included were patients: (1) first admitted to the ICU during hospital stays; (2) diagnosis of TBI including concussion, cerebral contusion, traumatic epidural hemorrhage, traumatic subdural hemorrhage, traumatic intracerebral hemorrhage and traumatic subarachnoid hemorrhage; (3) age ≥18 years old (Figure 1).
The exclusion criteria were as follows: (1) < 18-year-old; (2) neutrophil counts data, monocyte counts data and lymphocyte counts data at the first day of admission to the ICU were missing; (3) patients with repeated ICU admissions.
The raw data was obtained by using Structure query language (SQL) with Navicat Premium (version 15) from the MIMIC-IV database. The extracted data included which was collected for the first 24 h in the ICU: (1) baseline variable: age, gender, ethnicity, length of stay in hospital (LOS_hospital), length of stay in ICU (LOS_ICU); (2) vital signs: heart rate, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean blood pressure (MBP), respiratory rate (RR) and temperature; (3) comorbidities: congestive heart failure, diabete, malignant cancer, chronic obstructive pulmonary disease (COPD), hyperlipidemia, liver disease, kidney disease; (4) laboratory events: white blood cell counts, neutrophil counts, monocyte counts, lymphocyte counts, red blood cell counts, platelet counts, hemoglobin, serum sodium, serum potassium, serum creatinine, blood urea nitrogen (BUN), red blood cell distribution width (RDW), prothrombin time (PT), partial thromboplastin time (PTT), international normalized ratio (INR); (5) Systemic Inflammatory Response Syndrome Score (SIRS), Glasgow Coma Scale (GCS), Sequential Organ Failure Assessment score (SOFA), Simplified Acute Physiology Score II(SAPS II) and Acute Physiology Score III (APS III); For some variables measured multiple times within 24 h after ICU admission, their averages were used including heart rate, SBP, DBP, MBP, temperature and RR. For our analysis, all-cause mortality within 30 days was the primary endpoint, while in-hospital and 365-day all-cause death was the secondary endpoint. The SIRI was calculated using the following formula: SIRI = neutrophil counts × monocyte counts/lymphocyte counts.
To reduce bias due to missing data, variables with more than 10% missing values were excluded from the study. Correspondingly, few missing values were replaced with overall means.
The Kolmogorov-Smirnov test was used to test for normality distribution. Continuous variables were expressed as mean with standard deviation (for normal distribution) or median with 25–75th percentile (for non-normal distribution), which were analyzed by Student's t-test or Mann-Whitney test, as appropriate. Categorical variables were presented as counts (percentages), compared using the chi-square test.
The optimal SIRI cutoff point was obtained through KM curves using R packages “survival” and “survminer.” KM methods and multivariable cox regressions were used to analyze the effects of the SIRI levels and all-cause mortality. Three multivariate analysis models were established for each end point, and the low SIRI level group (< 11.24 × 109 /L) was set as the reference group. In model 1, the covariates were not adjusted; in model 2, the covariates included age, gender and race; in model 3, Los_hospital, platelet, red blood cell, BUN, RR, INR, PT, PTT, temperature, RDW, GCS, SOFA, APSIII, SAPSII, SIRS were further adjusted, with a P < 0.1 on univariate analysis. We have also performed a subgroup analysis to determine if the association differed for subgroups classified using different variables including gender, age, comorbidities and various physiological scores. ROC analysis was used to examine the association of admission SIRI with 30-day mortality. The areas under the ROC curves were used to compare the SIRI with other inflammatory indicators. Propensity score matching (PSM) analysis was used to minimize the effect of potential confounders. Confounders in model 3 and baseline imbalanced variables were used to evaluate the propensity scores. PSM was performed at a ratio of 1: 1 using a caliper width of 0.2 of the standard deviation of the logit of the propensity score. P < 0.05 were considered statistically significant and all tests were two-tailed. All statistical analyses were performed in R software (version 4.2.0) or STATA software (version 14).
A total of 350 subjects were analyzed in this study. And after PSM, 66 matched pairs (132 patients) were included as the matched cohort (Table 1). The optimal cutoff value of SIRI determined was 11.24 × 109/L (Figure 2). In the Supplementary Figure 1, The best cutoff value was also confirmed in the restricted cubic splines (RCS). We grouped patients by the measurements of SIRI. In the original cohort, compared to patients with low SIRI (< 11.24 × 109 /L), those with high SIRI (≥ 11.24 × 109 /L) were higher proportion of males (p = 0.030), had difference in racial distribution (p = 0.043) and higher level of white blood counts (p = 0.000), serum creatinine (p = 0.012) and BUN (p = 0.000). Moreover, the analysis showed that SIRI was associated with an increased risk for all-cause mortality (30-days, 365-days and in-hospital). After PSM, almost all covariates in the matched cohort were well-balanced (p > 0.050) between two groups except age, LOS_hospital, LOS_ICU.
We generated KM curves for the different groups. Before PSM, 12.0% (42/350) died during the first 30 days, 13.4% (47/350) died during the hospital period, and 14.9% (52/350) died during the 365-day follow-up period. Among the 132 TBI patients included after PSM, 15.9% (21/132) died during the first 30 days, 18.9% (25/132) died during the hospital period, and 18.1% (24/132) died during the 365-day follow-up period (Table 1). As was shown in the Figure 3, patients with low SIRI level were associated with improved survival (p < 0.050) compared with patients with high SIRI level during the 30-day, in-hospital and 365-day follow-up periods both before and after PSM.
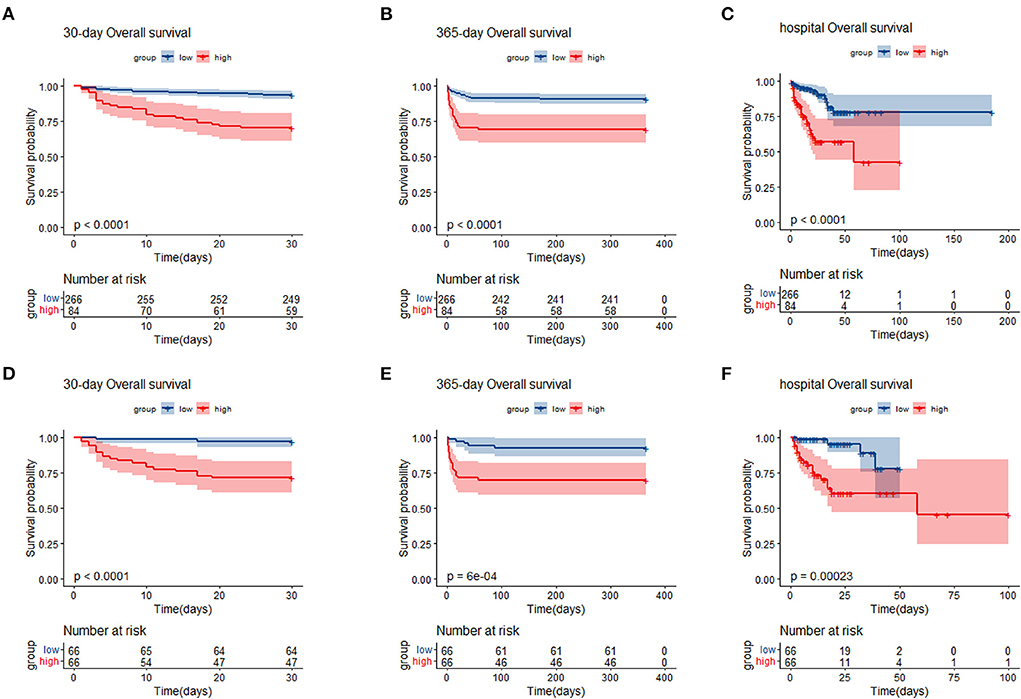
Figure 3. Kaplan–Meier survival analysis plot for 30-day, 365-day survival and in-hospital in patients before PSM (A–C), and patients after PSM (D–F). SIRI, systemic inflammation response index; PSM, propensity score matching; group low (<11.24 × 109/L); group high (≥11.24 × 109/L).
Different cox proportional hazard regression models were developed to assess the relationship between SIRI and prognosis in TBI patients simultaneously adjusting for possible covariates. The results of these relationships are shown in Table 2. For 30-day all-cause mortality, HR (95% CI) of high SIRI level group (≥11.24 × 109 /L) was 5.31 (2.86, 9.83) in unadjusted model compared with low SIRI level group (< 11.24 × 109 /L). After adjusting for age, gender and race, the association still existed (P < 0.05), and the HR (95% CI) was 5.03 (2.67, 9.44). Further adjustment of possible covariates whose P < 0.1 on univariate analysis showed similar correlation in model 3 (P < 0.05), and the HR (95% CI) was 3.74 (1.87, 7.47). A similar correlation was observed between 365-day and in-hospital all-cause mortality. After PSM, Cox proportional hazard regression analysis revealed that low SIRI level was independently related to better prognosis of TBI patients (Table 3).
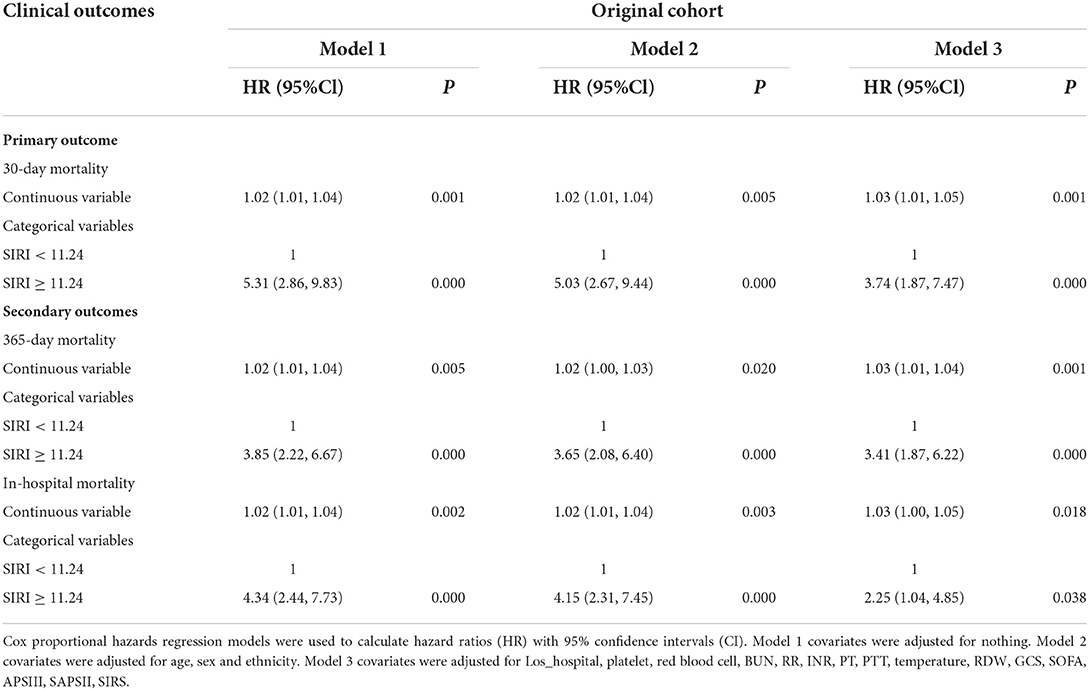
Table 2. Association between SIRI and clinical outcomes of critically ill patients with TBI before PSM.
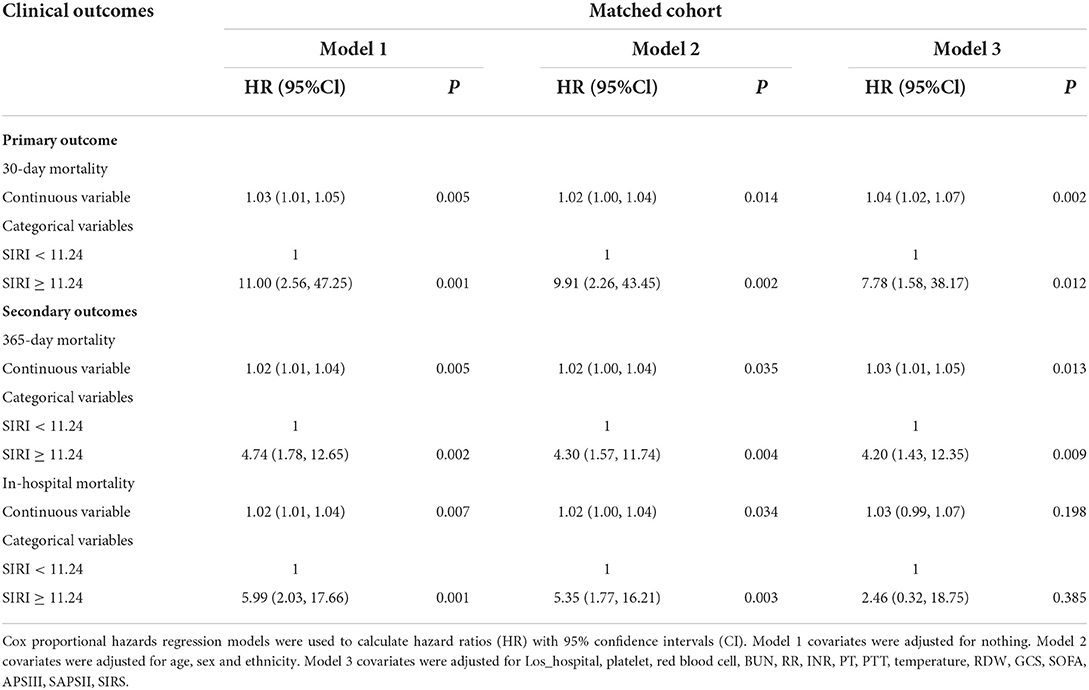
Table 3. Association between SIRI and clinical outcomes of critically ill patients with TBI after PSM.
In order to verify the robustness and consistency of our findings, we performed subgroup analyses to assess the association between SIRI and 30-day mortality (Table 4). The result showed the higher SIRI was linked to deteriorative mortality in most strata except in female patients (p = 0.055) and patients with hyperlipidemia (p = 0.058) or high GCS (p = 0.053). Additionally, no statistically significant interactions between SIRI and subgroups were observed. Taken together, the above outcomes prove that results were relatively stable in TBI patients.
ROC curves were plotted to assess the usefulness of SIRI, neutrophils, lymphocytes, monocytes and WBC in predicting mortality in TBI patients. We found that SIRI was relatively more accurate than other single inflammatory markers including neutrophils, lymphocytes, monocytes and WBC (AUC 0.6658 vs. 0.6644; 0.6658 vs. 0.4762; 0.6658 vs. 0.6607; 0.6658 vs. 0.5824, respectively) in the original cohort (Figure 4A and Table 5). Besides, analyses using PSM yielded essentially the same results (AUC 0.7435 vs. 0.5654; 0.7435 vs. 0.6489; 0.7435 vs. 0.6379; 0.7435 vs. 0.6499, respectively) in the Figure 4B, Table 6.
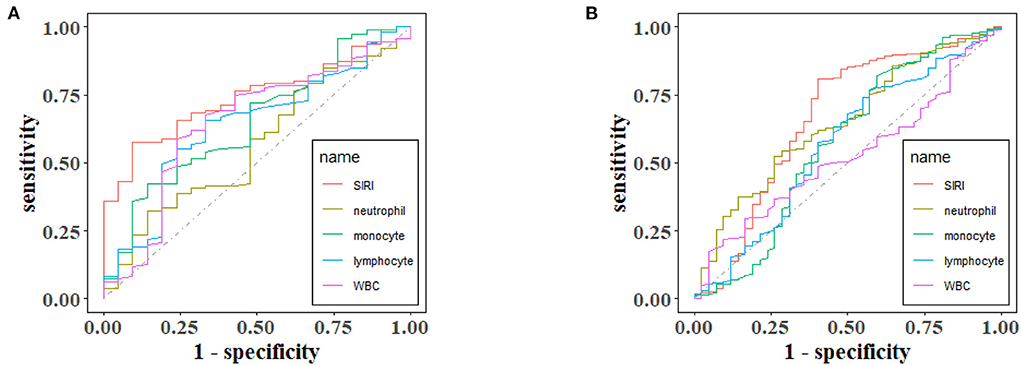
Figure 4. The receiver operating characteristic (ROC) curves of predictive value of inflammatory indexes for 30-day mortality in TBI before (A) and after (B) PSM.
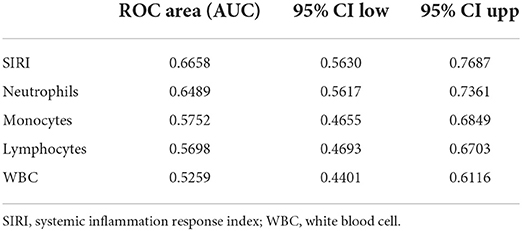
Table 5. Receiver operating curve (ROC) for prediction for 30-day mortality in TBI Patients before PSM.
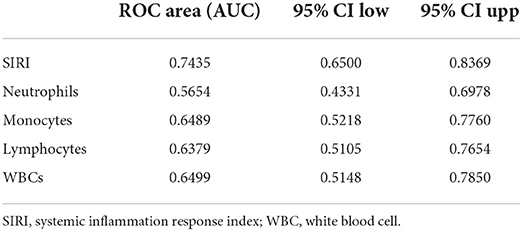
Table 6. Receiver operating curve (ROC) for prediction for 30-day mortality in TBI patients after PSM.
In the current study enrolled 350 TBI patients admitted to the ICU, we found that patients with high SIRI level were associated with a significantly increased risk of all-cause mortality and demonstrated that SIRI was an independent predictor of all-cause in TBI patients after adjusting confounding factors.
As an easily available biomarker, SIRI is an emerging systemic inflammatory marker based on peripheral neutrophils, monocytes, and lymphocytes, which first was developed in 2016 (10). In recent years, scholars have found that SIRI can be used as an indispensable indicator of leukocyte subtypes in neurological diseases such as glioblastoma, acute ischemic stroke and hypoxic ischemic encephalopathy (11–13). Besides, elevated SIRI index could be independent predicting factors for poor outcomes among ICH and aneurysmal subarachnoid hemorrhage patients (8, 9, 14).
To our knowledge, this is the first study to investigate the relationship between SIRI and all-cause mortality in a severe TBI population. First, Analysis of baseline characteristics showed that the mortality of TBI patients was statistically significant be between different SIRI levels (p < 0.05).We found that higher SIRI (≥11.24 × 109/L) was associated with a higher risk of all-cause mortality at 30 days, 365 days, and during the hospital period in our recent study. Then, cox regression analyses revealed that a higher admission SIRI value was an independent risk factor for 30-day, 365-day and in-hospital mortality. In the matched cohort, there is no statistical significance between SIRI and in-hospital mortality in model 3, which may be due to the excessive weight of hospital stays (p = 0.385). Besides, a series of subgroup analyses were performed to verify the robustness of our findings. As shown in subgroup analyses, SIRI maintained its predictive power in most strata. Furthermore, ROC curves revealed that the AUC of admission SIRI was better than those of neutrophils, lymphocytes, monocytes and WBC. After PSM, The AUC of admission SIRI was >0.7, indicating that SIRI has considerable predictive value for 30-day mortality.
More and more studies have demonstrated the correlation of inflammation in the pathogenesis of TBI (2, 15). Secondary damage due to TBI induced by inflammatory cells and inflammatory cascades plays a key role in progression of disease, thus affecting clinical prognosis. Neutrophils are the first responders to tissue injuries in the central nervous system (CNS), followed by monocytes, lymphocytes, and mast cells. On the one hand, neutrophils play a critical role in controlling damage lesions and clearing cellular debris and damaged cells (16). On the other hand, neutrophils are not always neuroprotective and have the ability to break down the BBB and promote neuronal cells death by releasing various inflammatory factors (2). Similarly to neutrophils, monocytes recruitment in circulating and damaged tissues are known to be a characteristic hallmark of inflammation (17). Monocyte-derived macrophages were found to be crucial for the chronic phase that occurred several weeks after brain injury on animal models (2). Previous studies have shown that a higher monocyte count was independently associated with poor outcomes among intracerebral hemorrhage patients (18–20), which supports our research to some extent. When it comes to lymphocytes, the role in patients with brain injury remains to be studied in the future. ROS release can promote T lymphocytes recruitment by activating endothelial barriers (21). In addition, T cells play no significant role in early TBI pathogenesis (22).
It should be noted that our study had some limitations that may prevent the generalizability of findings. First, subgroup analysis was made according to factors which were reported to have strong relationship with SIRI. However, the stability of these results may be limited due to the small sample size. Second, some important data, such as cranial surgery, severity of intracranial pathology on CT, were missing in this database. Aiming to alleviate the impact of disease severity, we have included GCS, SOFA, and APS III in the PSM analysis, which to a certain degree can also reflect the severity of brain injury. However, bias risk due to disease severity still needs to be considered. Third, consistent with previous studies, only the first SIRI on ICU admission was calculated. It is worth noting that the dynamic changes in SIRI may also have a significant correlation with prognosis in TBI, which still needs to be further investigated. Moreover, although a non-linear association trend was found in the RCS analysis, it remains non-significant, especially in high SIRI values, which may be due to the small sample size. Besides, the number of covariates associated with TBI prognosis is very large and under-collected in our study. Finally, patients with missing data were excluded from this analysis, which could have biased the results.
This study provides an easy-to-get biomarker for predicting prognosis in patients with TBI admitted to the ICU. Patients with high SIRI level would suffer from a high risk of all-cause mortality. SIRI is a promising composite inflammatory biomarker for predicting TBI prognosis with relatively better predictive power than single inflammatory biomarkers—neutrophils, lymphocytes, monocytes and WBC.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Boards of Beth Israel Deaconess Medical Center (Boston, MA) and Massachusetts Institute of Technology (MIT; Cambridge, MA). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
SW and MW designed the work. BM, YS, DL, and JM extracted and analyzed the datasets. BM and LF wrote this paper. BM, RZ, and YL interpreted the results and helped to revise the manuscript. All authors read and approved the final manuscript.
This work was supported by the grants from Medical Health Science and Technology Key Project of Zhejiang Provincial Health Commission (WKJ-ZJ-2014), Key Research and Development Project of Zhejiang Provincial Department of Science and Technology (2021C03105), and Provincial Natural Science Foundation of Zhejiang (Y21H090041).
We would like to thank all the participants and investigators that took part in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.995925/full#supplementary-material
TBI, traumatic brain injury; SIRI, systemic inflammation response index; SBI, secondary brain injury; NLR, neutrophil-to-lymphocyte ratio; ICH, intracerebral hemorrhage; RDW, red blood cell distribution width; SIRS, Systemic Inflammatory Response Syndrome Score; SOFA, Sequential Organ Failure Assessment; SAPS II, Simplified Acute Physiology Score II; GCS, Glasgow Coma Scale; APSIII, Antiphospholipid syndrome; ROC, Receiver Operating Characteristic; SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; COPD, chronic obstructive pulmonary disease; PSM, propensity score matching; HR, hazard ratios; CI, confidence intervals; BBB, blood brain barrier; ICU, intensive care unit; PT, prothrombin time; PTT, partial thromboplastin time; INR, international normalized ratio; BUN, blood urea nitrogen; LOS_hospital, length of stay in hospital; LOS_ICU, length of stay in ICU; RR, respiratory rate; CNS, central nervous system; WBC, white blood cell; RCS, restricted cubic splines, KM, Kaplan-Meier.
1. Jiang JY, Gao GY, Feng JF, Mao Q, Chen LG, Yang XF, et al. Traumatic brain injury in China. Lancet Neurol. (2019) 18:286–95. doi: 10.1016/S1474-4422(18)30469-1
2. Corps KN, Roth TL, McGavern DB. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. (2015) 72:355–62. doi: 10.1001/jamaneurol.2014.3558
3. Brazinova A, Rehorcikova V, Taylor MS, Buckova V, Majdan M, Psota M, et al. Epidemiology of traumatic brain injury in Europe: a living systematic review. J Neurotrauma. (2021) 38:1411–40. doi: 10.1089/neu.2015.4126
4. Greve MW, Zink BJ. Pathophysiology of traumatic brain injury. Mt Sinai J Med. (2009) 76:97–104. doi: 10.1002/msj.20104
5. Jin Z, Wu Q, Chen S, Gao J, Li X, Zhang X, et al. The associations of two novel inflammation indexes, SII and SIRI with the risks for cardiovascular diseases and all-cause mortality: a ten-year follow-up study in 85,154 individuals. J Inflamm Res. (2021) 14:131–40. doi: 10.2147/JIR.S283835
6. Wang L, Zhou Y, Xia S, Lu L, Dai T, Li A, et al. Prognostic value of the systemic inflammation response index (SIRI) before and after surgery in operable breast cancer patients. Cancer Biomark. (2020) 28:537–47. doi: 10.3233/CBM-201682
7. Fois AG, Paliogiannis P, Scano V, Cau S, Babudieri S, Perra R, et al. The systemic inflammation index on admission predicts in-hospital mortality in COVID-19 patients. Molecules. (2020) 25:5725. doi: 10.3390/molecules25235725
8. Li J, Yuan Y, Liao X, Yu Z, Li H, Zheng J. Prognostic significance of admission systemic inflammation response index in patients with spontaneous intracerebral hemorrhage: a propensity score matching analysis. Front Neurol. (2021) 12:718032. doi: 10.3389/fneur.2021.718032
9. Yun S, Yi HJ, Lee DH, Sung JH. Systemic inflammation response index and systemic immune-inflammation index for predicting the prognosis of patients with aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. (2021) 30:105861. doi: 10.1016/j.jstrokecerebrovasdis.2021.105861
10. Qi Q, Zhuang L, Shen Y, Geng Y, Yu S, Chen H, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. (2016) 122:2158–67. doi: 10.1002/cncr.30057
11. Ceran B, Alyamaç Dizdar E, Beşer E, Karaçaglar NB, Sari FN. Diagnostic role of systemic inflammatory indices in infants with moderate-to-severe hypoxic ischemic encephalopathy. Am J Perinatol. (2021). doi: 10.1055/a-1673-1616. [Epub ahead of print].
12. Wang Z, Li J, Yuan Y, Li T, Zuo M, Liu Y. Prognostic significance of preoperative systemic inflammation response index in newly diagnosed glioblastoma patients underwent gross total resection: a propensity score matching analysis. World J Surg Oncol. (2022) 20:137. doi: 10.1186/s12957-022-02588-0
13. Zhang Y, Xing Z, Zhou K, Jiang S. The predictive role of systemic inflammation response index (SIRI) in the prognosis of stroke patients. Clin Interv Aging. (2021) 16:1997–2007. doi: 10.2147/CIA.S339221
14. Zhang P, Li Y, Zhang H, Wang X, Dong L, Yan Z, et al. Prognostic value of the systemic inflammation response index in patients with aneurismal subarachnoid hemorrhage and a Nomogram model construction. Br J Neurosurg. (2020). doi: 10.1080/02688697.2020.1831438. [Epub ahead of print].
15. Corrigan F, Mander KA, Leonard AV, Vink R. Neurogenic inflammation after traumatic brain injury and its potentiation of classical inflammation. J Neuroinflammation. (2016) 13:264. doi: 10.1186/s12974-016-0738-9
16. McKee CA, Lukens JR. Emerging roles for the immune system in traumatic brain injury. Front Immunol. (2016) 7:556. doi: 10.3389/fimmu.2016.00556
17. Kratofil RM, Kubes P, Deniset JF. Monocyte conversion during inflammation and injury. Arterioscler Thromb Vasc Biol. (2017) 37:35–42. doi: 10.1161/ATVBAHA.116.308198
18. Morotti A, Phuah CL, Anderson CD, Jessel MJ, Schwab K, Ayres AM, et al. Leukocyte count and intracerebral hemorrhage expansion. Stroke. (2016) 47:1473–8. doi: 10.1161/STROKEAHA.116.013176
19. Mackey J, Blatsioris AD, Saha C, Moser EAS, Carter RJL, Cohen-Gadol AA, et al. Higher monocyte count is associated with 30-day case fatality in intracerebral hemorrhage. Neurocrit Care. (2021) 34:456–64. doi: 10.1007/s12028-020-01040-z
20. Walsh KB, Sekar P, Langefeld CD, Moomaw CJ, Elkind MS, Boehme AK, et al. Monocyte count and 30-day case fatality in intracerebral hemorrhage. Stroke. (2015) 46:2302–4. doi: 10.1161/STROKEAHA.115.009880
21. Ling C, Sandor M, Suresh M, Fabry Z. Traumatic injury and the presence of antigen differentially contribute to T-cell recruitment in the CNS. J Neurosci. (2006) 26:731–41. doi: 10.1523/JNEUROSCI.3502-05.2006
Keywords: systemic inflammation response index (SIRI), traumatic brain injury (TBI), all-cause mortality, propensity score matching (PSM), Medical Information Mart for Intensive Care-IV
Citation: Mao B, Feng L, Lin D, Shen Y, Ma J, Lu Y, Zhang R, Wang M and Wan S (2022) The predictive role of systemic inflammation response index in the prognosis of traumatic brain injury: A propensity score matching study. Front. Neurol. 13:995925. doi: 10.3389/fneur.2022.995925
Received: 16 July 2022; Accepted: 18 October 2022;
Published: 02 November 2022.
Edited by:
Yidong Bai, The University of Texas Health Science Center at San Antonio, United StatesReviewed by:
Guangzhi Ning, Tianjin Medical University General Hospital, ChinaCopyright © 2022 Mao, Feng, Lin, Shen, Ma, Lu, Zhang, Wang and Wan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu Wan, d2Fuc2h1QHpqdS5lZHUuY24=; Ming Wang, bWluZ2h1aTA1MDdAemp1LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.