
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Neurol., 01 September 2022
Sec. Multiple Sclerosis and Neuroimmunology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.995690
The literature on the effect of multiple sclerosis disease-modifying treatments (DMTs) on multiple sclerosis-related cognitive dysfunction has grown exponentially over the last few years. A detailed analysis of this topic can be found in the comprehensive systematic review and meta-analysis of Landmeyer et al. (1). Although this review rightly highlighted important weaknesses of the literature reviewed, it was not able to delve into some aspects of the field that should not be overlooked and deserve much more in-depth reflection.
For instance, some research shows that DMTs greatly benefit processing speed outcomes (Table 1). As an example of how significant these effects can be, 60% of alemtuzumab-treated patients had clinically meaningful improvement in SDMT score just after the second course of the treatment (2), and 62.2% of ocrelizumab-treated patients achieved clinically meaningful improvements over 96 weeks, a percentage that was even higher (72%) in those patients with mild impairment at baseline (3). We consider that these results, along with other aspects detailed throughout this opinion article, should be questioned from the viewpoint of biological plausibility and should make us rethink whether the current research regarding the effects of DMTs on longitudinal cognitive performance is adequate.
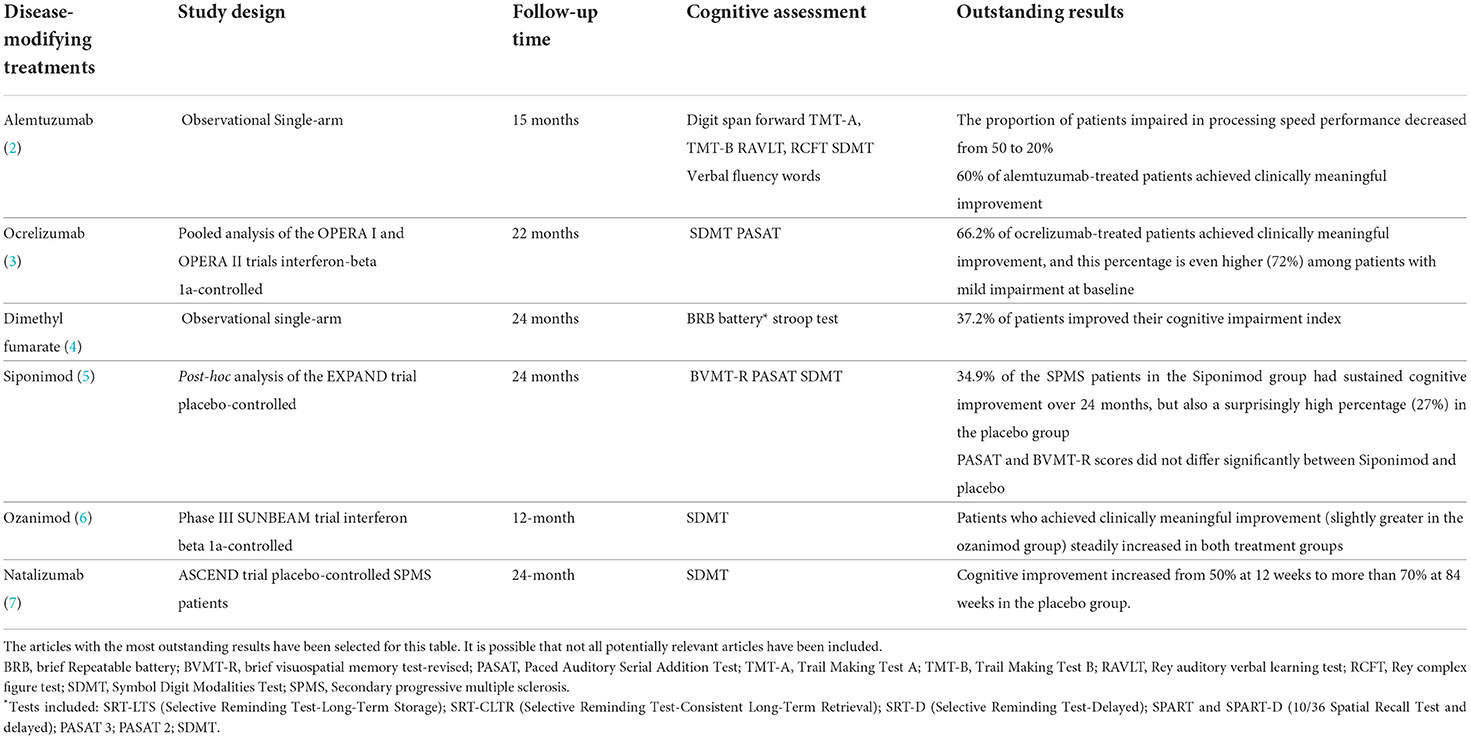
Table 1. Unreasonable results of studies analyzing the effects of disease-modifying treatments on cognitive outcomes.
This is not a comprehensive review of the cognitive assessment literature's methodological weaknesses, which can be found elsewhere (1), but instead a wake-up call that provides researchers and trialists with controversial findings that merit much further reflection before moving the research forward.
The mean change from baseline in processing speed scores has consistently been higher at month 6, and, after that, it steadily improves in successive evolutions (2–12), The best example is the observation reported by Koch et al. (7), who found that SDMT scores steadily increased overall 28 testing sessions throughout the 2 years of follow-up in the ASCEND trial in secondary progressive multiple sclerosis (SPMS), Similarly, Morrow et al. (10) reported average SDMT baseline scores of 46.8 and an average final score of 62.2 at week 48 (an average improvement of 32.9% over baseline) in 660 natalizumab-treated patients with MS; and Woelfle et al. (11) observed an average improvement of 25.4% correct responses over baseline with electronic SMDT. The effect is not mitigated by using Z-scores, as evidenced by our results in which the percentage of patients who underwent cognitive improvement at 12 months (defined by an increase of 0.5 standard deviations) was twice as frequent as those who experienced cognitive worsening (26.6 vs. 11.4%) (9).
The impact of the practice effect on the improved SDMT performance is so large that a simple change in the order of the key symbols has been shown to completely reverse the increase in SDMT scores and returned it to baseline values (12).
Although this improvement has been chiefly considered short-term learning- effect as most of it occurred up to the third repetition, there was still a significant improvement from the third repetition onward (11, 12). For instance, Roar et al. (12) reported that the SDMT performance improved by 1.2 points/test during the first 6 months and by 0.4 points/test thereafter.
Even more concerning is that this practice effect seems more significant than the reported between-group mean difference in longitudinal cognitive outcomes, which raises questions about whether the differences observed in favor of some treatments are meaningful. A secondary analysis of the EXPAND trial of siponimod in SPMS reported a between-group mean difference in SDMT scores of 2.3 points between baseline and 24 months in favor of siponimod compared to placebo (5), and a pooled analysis of the OPERA, I, and OPERA II studies reported a between-group difference in SDMT scores of 1.3 points in favor of ocrelizumab compared to interferon beta1a (3). Although these differences are statistically significant, they seem futile compared to the observed magnitude of SDMT score change related to practice effects.
When using the proposed four-point definition of clinically meaningful change, patients are much more likely to experience cognitive improvement than worsening throughout their longitudinal cognitive assessment.
The most unexpected result has been observed in SPMS patients, in whom cognitive improvement increased from around 50% of participants at 12 weeks to more than 70% at 84 weeks, including those in the placebo group (7). In the post hoc EXPAND trial, 27% of the SPMS patients in the placebo group had sustained cognitive improvement over 24 months (5), which does not fit with the expected relentless decline of cognitive function that many people with SPMS are supposed to experience. As a whole, more than half of the patients regardless of the DMT received, have been reported to show a clinically meaningful change in their cognitive performance in just a few months, which we consider to be utterly unreasonable. Table 1 summarizes some of the main astonishing results.
While the field researchers are well aware of the practice effects challenges, it is concerning that the studies aimed to assess the effects of DMTs on cognitive evolution have systematically overlooked the practice effects in interpreting their results. For example, the SDMT practice effect is wholly disregarded in the OPERA and the SUNBEAM studies (3, 6). Furthermore, these studies expedite and amplify the learning effects by using short and frequent retesting intervals (at 12-week intervals in the OPERA study (3) and 6-month intervals in the EXPAND (5) and SUNBEAM (6) studies), which contribute to exacerbating the improved SDMT performance.
First, given that without a comparator group is impossible to disentangle whether longitudinal cognitive performance improvement represents practice or treatment effects, the meaningfulness of the uncontrolled observational studies, which represent the majority of the published literature (1), should be questioned.
Regarding randomized controlled trials, it is mandatory to precisely define which approaches have been used to control for practice effect. The following methodological proposal could partially mitigate the practice effect and help strengthen the effect of a given DMT on the cognitive evolution: (i) Assess longitudinal performances also in randomized clinical control groups; (ii) Use adequate retesting intervals by performing longitudinal cognitive tests less frequently (i.e., at 12-month intervals); (iii) Discard the results from the first cognitive performances. If the frequency of SDMT testing is conducted monthly or at 6-month intervals, as in clinical trials, the results from the first 2–3 cognitive performances should be discarded. This approach would not be necessary if annual cognitive examinations were applied. Regardless of the frequency of the SDMT administration, we firmly believe that the first ever cognitive evaluation should be removed, and therefore the second cognitive measurement (providing the cognitive assessment is not conducted at monthly intervals) could be accepted as the baseline cognitive evaluation on which to assess the DMT effects on longitudinal cognitive performance; (iv) Apply parallel or alternate forms of SDMT which have shown excellent reliability (13). Regarding this last point, it should be stressed that although it appears that practice effects are modestly mitigated by using alternating forms (13), the reality is that to date, no study has been specifically designed to determine to what extent the use of alternate forms decreases the learning effect compared to the continued use of the same SDMT key. Since electronic tests [e.g., the iPad®-based Processing Speed Test (PST)] randomly generate a new key for each new administration (14), we believe it would be particularly interesting to assess the extent to which the learning effect is mitigated by comparing the rearranged key-based electronic tests with the paper-and-pencil SDMT in both MS patients and controls. Until these differences are adequately defined through specifically designed studies, we believe we should be cautious in asserting that using SDMT alternate forms can per se prevent the learning effects-relate cognitive improvement. Indeed, one-third of untreated SPMS patients showed cognitive improvement despite using alternate SDMT forms in the EXPAND study (5).
Importantly, although processing speed is the core domain of cognitive impairment in MS, the effects of DMTs on other cognitive domains less susceptible to the learning effects remain uninvestigated. Thus, although integrating neuropsychological batteries into MS daily clinical practice remains challenging due to the need for at least 20 minutes of one-on-one testing for every patient, the inclusion in randomized controlled trials of other cognitive domains such as episodic memory seems reasonable. Noteworthy, Siponimod had an impressive benefit on SDMT scores compared to the placebo group. Still, there were no differences regarding the memory outcome measured as the BVMT-R (brief visuospatial memory test-revised) (5).
Although establishing a 4-point change in the SMDT as a benchmark for clinically meaningful change was objectively based on deterioration in vocational status predictions (15), the reality is that its systematic application in the literature has yielded irrational results, as described in point 2.2.
The strict application of such a narrow difference might imply that high baseline performers could reach their boundary sooner and, therefore, are much more vulnerable to a 4-point decrease. On the other hand, low baseline performers could have more room for improvement due to a floor effect. Indeed, if we take a closer look at the SUNBEAM results, we can see that the 12-month SDMT-worsened group was a much higher baseline performer than the 12-month SDMT-improved group (raw score: 52.3 vs. 45.1; standardized z-score: +0.38 vs. −0.15) (6), which means that the lower the baseline SDMT score is, the greater the opportunity to show a 4-point improvement. This analysis is of great importance, as the greater percentage of clinically meaningful improvements reported in favor of some DMTs could be due to an unadjusted imbalance in the group's proportion of baseline SDMT performance levels rather than a specific treatment effect on cognitive evolution.
These results involve high stakes, as it raises questions about the convenience of the SDMT as a longitudinal outcome measure of disease progression, at least over the 2-year follow-up of most studies. If the community research does not want longitudinal cognitive assessment to mask disease progression, a reframing of how we define cognitive improvement/ worsening is urgently needed. For instance, a decline of 8 or more raw score points, double the current threshold, has recently been described as the most helpful threshold for capturing a statistically reliable individual change on the SDMT (16).
We still prefer to use relative (e.g., a 10% loss of SDMT score) or adjusted [reduction of 0.5 standard deviations (Z-score)] score losses rather than raw score losses.
Moreover, based on these relative or adjusted definitions, we propose a new approach in which the information inherent in practice effects could be exploited as a new helpful outcome measure. In this approach, three different groups would be established based on the patient's SDMT learning curves: (1) the group with the expected physiological continuous SDMT improvement related to gaining practice. In a practice effect context, we consider this group represents a much more accurate definition than the term clinically meaningful improvement. (2) The group who remain cognitive unchanged, which should be interpreted with caution as these are patients who are not able to turn the benefit of practice effect into improvement, and (3) the group with cognitive worsening that would represent those patients with neurodegenerative damage that no longer have the brain reserve availability to maintain their SDMT scores despite the benefit of the practice effects.
Another approach that might offer more insight into the potential beneficial effects of DMTs on cognitive function would include stratifying by some baseline cognitive performance subgroups (e.g., cognitive impairment at baseline, patients with the highest baseline cognitive score, etc.), thus providing information on the rate of change in cognitive functioning across different groups.
Despite all these caveats, we are convinced that longitudinal cognitive assessment is a useful measure of disease progression and that DMTs, particularly high effective DMTs (9), have a protective effect on disease-related cognition. However, identifying and critiquing cognitive-related methodological limitations are paramount before moving the research forward. We strongly believe that using some easy-to-adopt measures, such as more adequate retesting intervals (at 12-month intervals) and replacing the 4-point threshold with more reasonable measures, might overcome cognitive-related methodological challenges.
AL-F initiated the project. AL-F, EM, and JB-L made intellectual contributions, read, revised, and approved the final manuscript.
JB-L is supported by the National Institutes of Health, Bethesda, MD, USA (NINDS #R01 NS39422), the European Commission (grant ICT-2011-287739, NeuroTREMOR), the Ministry of Economy and Competitiveness (grant RTC-2015-3967-1, NetMD—platform for the tracking of movement disorder), and the Spanish Health Research Agency (grants FIS PI12/01602 and FIS PI16/00451). The Spanish Health Research Agency and the Spanish Office of Science and Technology supported NEDICES. Information about collaborators and detailed funding of the NEDICES study can be found on the following webpage (https://ciberned.es/proyectos/nedices-1.html%20).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Landmeyer NC, Bürkner PC, Wiendl H, Ruck T, Hartung HP, Holling H, et al. Disease-modifying treatments and cognition in relapsing-remitting multiple sclerosis: a meta-analysis. Neurology. (2020) 94:e2373–83. doi: 10.1212/WNL.0000000000009522
2. Riepl E, Pfeuffer S, Ruck T, Lohmann H, Wiendl H, Meuth SG, et al. Alemtuzumab improves cognitive processing speed in active multiple sclerosis-A longitudinal observational study. Front Neurol. (2018) 8:730. doi: 10.3389/fneur.2017.00730
3. Benedict RHB, de Seze J, Hauser SL, Kappos L, Wolinsky JS, Zheng H, et al. Impact of ocrelizumab on cognition in patients at increased risk of Progressive disease. In: Poster presented at the 70th American Academy of Neurology (AAN) Annual Meeting; April 21–27, Los Angeles, CA (2018).
4. Amato MP, Goretti B, Morra VB, Gallo P, Zaffaroni M, Onofrj M, et al. Effects of 2-year treatment with dimethyl fumarate on cognition and functional impairment in patients with relapsing-remitting multiple sclerosis. Neurol Sci. (2020) 41:3185–93. doi: 10.1007/s10072-020-04320-w
5. Benedict RHB, Tomic D, Cree BA, Fox R, Giovannoni G, Bar-Or A, et al. Siponimod and cognition in secondary progressive multiple sclerosis: expand secondary analyses. Neurology. (2021) 96:e376–86. doi: 10.1212/WNL.0000000000011275
6. DeLuca J, Schippling S, Montalban X, Kappos L, Cree BAC, Comi G, et al. Effect of ozanimod on symbol digit modalities test performance in relapsing MS. Mult Scler Relat Disord. (2021) 48:102673. doi: 10.1016/j.msard.2020.102673
7. Koch MW, Mostert J, Repovic P, Bowen JD, Uitdehaag B, Cutter G. Is the Symbol Digit Modalities Test a useful outcome in secondary progressive multiple sclerosis? Eur J Neurol. (2021) 28:2115–20. doi: 10.1111/ene.14732
8. Rorsman I, Petersen C, Nilsson PC. Cognitive functioning following one-year natalizumab treatment: a non-randomized clinical trial. Acta Neurol Scand. (2018) 137:117–24. doi: 10.1111/ane.12833
9. Labiano-Fontcuberta A, Costa-Frossard L, Sainz de la Maza S, Rodríguez-Jorge F, Chico-García JL, Monreal E. The effect of timing of high-efficacy therapy on processing speed performance in multiple sclerosis. Mult Scler Relat Disord. (2022) 64:103959. doi: 10.1016/j.msard.2022.103959
10. Morrow S, O'Connor P, Polman C, Goodman A, Kappos L, Lublin F, et al. Evaluation of the Symbol Digit Modalities test (SDMT) and MS neuropsychological screening questionnaire (MSNQ) in natalizumab-treated MS patients over 48 weeks. Mult Scler. (2010) 16:1385–92. doi: 10.1177/1352458510378021
11. Woelfle T, Pless S, Wiencierz A, Kappos L, Naegelin Y, Lorscheider J. Practice effects of mobile tests of cognition, dexterity, and mobility on patients with multiple sclerosis: data analysis of a smartphone-based observational study. J Med Internet Res. (2021) 23:e30394 doi: 10.2196/30394
12. Roar M, Illes Z, Sejbaek T. Practice effect in Symbol Digit Modalities Test in multiple sclerosis patients treated with natalizumab. Mult Scler Relat Disord. (2016) 10:116–22. doi: 10.1016/j.msard.2016.09.009
13. Benedict RH, Smerbeck A, Parikh R, Rodgers J, Cadavid D, Erlanger D. Reliability and equivalence of alternate forms for the Symbol Digit Modalities Test: implications for multiple sclerosis clinical trials. Mult Scler. (2012) 18:1320–5. doi: 10.1177/1352458511435717
14. Rao SM, Losinski G, Mourany L, Schindler D, Mamone B, Reece C, et al. Processing speed test: validation of a self-administered, iPad®-based tool for screening cognitive dysfunction in a clinic setting. Mult Scler. (2017) 23:1929–37 doi: 10.1177/1352458516688955
15. Morrow SA, Drake A, Zivadinov R, Munschauer F, Weinstock-Guttman B, Benedict RH. Predicting loss of employment over three years in multiple sclerosis: clinically meaningful cognitive decline. Clin Neuropsychol. (2010) 24:1131–45. doi: 10.1080/13854046.2010.511272
Keywords: information processing, cognitive dysfunction, randomized controlled (clinical) trial, multiple sclerosis, practice effect
Citation: Labiano-Fontcuberta A, Monreal E and Benito-León J (2022) Time to rethink the reported disease-modifying treatment effects on cognitive outcomes: Methods and interpretative caveats. Front. Neurol. 13:995690. doi: 10.3389/fneur.2022.995690
Received: 16 July 2022; Accepted: 08 August 2022;
Published: 01 September 2022.
Edited by:
Francesco Patti, University of Catania, ItalyReviewed by:
Amgad Droby, Tel Aviv Sourasky Medical Center, IsraelCopyright © 2022 Labiano-Fontcuberta, Monreal and Benito-León. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrés Labiano-Fontcuberta, Z2FuZGhpbGFiaWFub0Bob3RtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.