- 1Clinical Medical College, Jining Medical University, Jining, China
- 2Department of Neurosurgery, Affiliated Hospital of Jining Medical University & Shandong Provincial Key Laboratory of Stem Cells and Neuro-Oncology, Jining, China
- 3Medical Research Center, Affiliated Hospital of Jining Medical University, Jining Medical University, Jining, China
Hemoglobin (Hb) and lipid metabolism are critical in the pathophysiology of moyamoya disease (MMD), and Hb and triglycerides (TGs) both play roles in the development of cerebrovascular illness. However, there is little evidence of a link between Hb and TGs in patients with MMD. This study aimed to determine the association between Hb and TGs in patients who had recently been diagnosed with MMD. From March 2013 to December 2018, 337 patients clinically diagnosed with MMD were admitted to our hospital. Among these, 235 were selected for analysis in this retrospective, cross-sectional study. Each patient's clinical features were documented. For analysis, we used univariate analysis, smoothed-curve fitting, and multivariable, piecewise linear regression. Overall, the mean±standard deviation patient age was 48.14 ± 11.24 years, 44.68% were men, and the mean Hb concentration was 135.72 ± 18.99 g/L. After controlling for relevant confounders, smoothed-curve fitting revealed a nonlinear association between the Hb and TG concentrations (P = 0.0448). When the Hb concentration was below 141 g/L, multivariate piecewise linear regression analysis revealed a significant association between the Hb and TG concentrations [β: 0.01, 95% confidence interval (CI): 0.00, 0.01; P = 0.0182], although the association disappeared above this threshold (β:−0.00, 95% CI:−0.01, 0.01; P = 0.4429). In individuals newly diagnosed with MMD, there is a significant correlation between Hb and TGs, which may be connected to MMD pathogenesis.
Introduction
Moyamoya disease (MMD) is a chronically occlusive cerebrovascular illness marked by stenosis of the terminal portions of both internal carotid arteries (ICAs), which leads to the creation of an aberrant network of collateral vessels. Its progressive phase can often lead to ischemic and hemorrhagic strokes (1–3). Thus, given the high risk of stroke in patients with MMD, it is particularly important to prevent the occurrence and development of this disease. However, despite more than 60 years of continuous research, its mechanisms remain unclear. Recent research suggests that TGs is a risk factor for cerebrovascular diseases, including carotid artery stenosis and intracranial artery stenosis (4, 5). Therefore, exploring the factors associated with abnormal lipid metabolism could facilitate our knowledge of the its fundamental mechanics. In a previous study, we discovered that uric acid and triglycerides (TGs) had a substantial beneficial relationship, and the early prevention of hyperuricemia and lipid abnormalities was associated with a decrease in the incidence of MMD (6).
Hb is a cellular protein that binds to oxygen (7, 8). There is a positive association between the Hb concentration and inflammation in previously and currently infected populations (7, 9, 10). Furthermore, the Hb concentration is a well-known independent risk factor for stroke and a poor prognosis (11, 12). In general, both Hb and TGs play crucial roles in the pathology and physiology of cerebrovascular disease, although the relationship between the two has not been elucidated.
Taken together, MMD is a cerebrovascular disease that frequently leads to a stroke, while Hb and TGs are both linked to the development of a stroke. Therefore, this study aimed to investigate whether Hb and TGs are independently associated with each other among patients newly diagnosed with MMD in China. Clarifying the relationship between the two may help to predict the type of stroke induced by MMD and to better understand their role the development of MMD.
Methods
Study design
The link between Hb and TGs was studied using a retrospective, cross-sectional design. The Hb concentration was defined as the independent variable and the TG concentration as the predictor variable.
Study population
All data used in this study were acquired from the computerized medical record system of the Affiliated Hospital of Jining Medical University. The information we gathered did not contain any personal information, to protect patient privacy. Informed consent was not required because this cohort study was retrospective. The hospital's institutional review board approved this study.
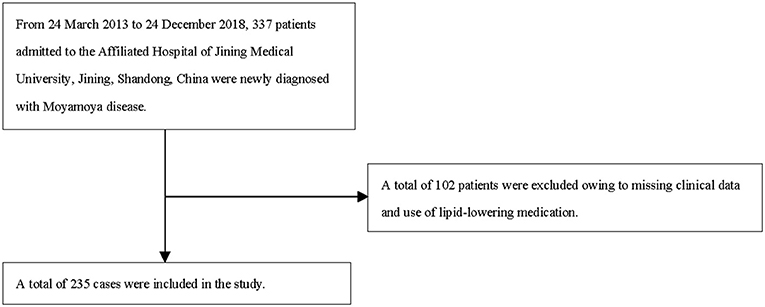
Data of 235 patients were initially collected. The start and end dates for inclusion were March 2013 and December 2018, respectively. The MMD (Spontaneous Occlusion of the Circle of Willis) Guidelines for Diagnosis and Treatment (2012 Edition) were used to guide the clinical strategy for each participant (13). The following cerebral angiography results were required for diagnosis: (1) in the arterial phase, stenosis or blockage of the intracranial ICA, parietal part of the anterior cerebral artery, and/or middle cerebral artery; (2) anomalies in vascularization next to an endothelial orstenotic lesion in the first cycle; and (3) observations in (1) and (3) must be consistent with those in (2). The following were the exclusion criteria: (1) short-term use of anemia-correcting drugs, (2) atherosclerosis, (3) autoimmune disease, (4) meningitis, (5) brain tumors, (6) Down syndrome, (7) cerebrovascular lesions detected via head irradiation, (8) head injury, (9) von Recklinghausen's disease, (10) age <18 years and (11) other conditions. We only included patients newly diagnosed with MMD who were admitted to our hospital. Further exclusion criteria were patients with myeloproliferative illnesses receiving toxic treatments, pregnant women, breastfeeding women, patients currently using diuretics or lipid-regulating medicines, patients with renal or liver illness, and patients on antinociceptive drugs.
Variables
Hb and TG concentrations were measured at the start of the study and used as continuous variables. Briefly, the department nurse collected the patients' peripheral venous blood while they were fasting, instantly submitting it to the laboratory. All measurements were performed at our hospital laboratory by laboratory technicians and physicians.
The covariates in this investigation were divided into three categories: (1) demographic data; (2) variables previously reported to affect the Hb and/or TG concentration; and (3) variables identified based on our clinical experience. As a result, multivariable models were constructed, adjusting for the following: (1) quantitative variables: sex, smoking status, and alcohol intake and (2) continuous variables: age and body mass index (BMI). All these variables were obtained at baseline.
Equations
Continuous variables are displayed as means ± standard deviations, and categorical variables are displayed as frequencies and percentages. All analyses were performed using the R statistical package (http://www.R-project.org, R Foundation for Statistical Computing, Vienna, Austria) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). Statistical significance was defined as a P < 0.05 (two-tailed). For details of the statistical methods, please see the Supplementary material.
Results
Clinical features
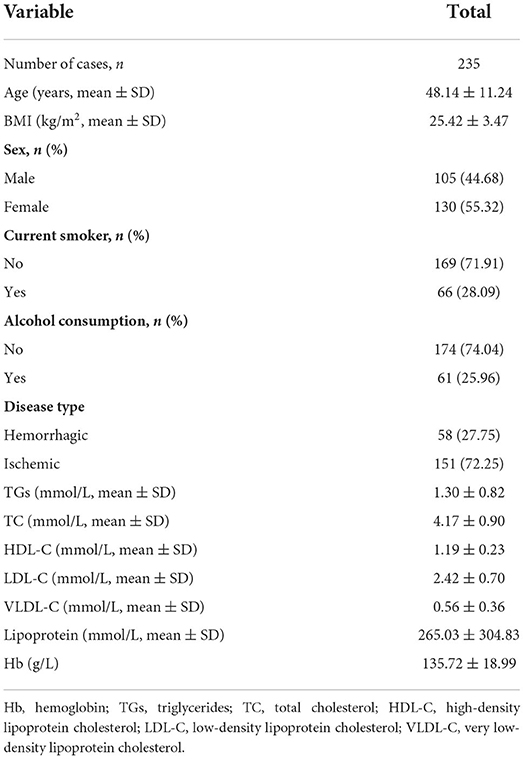
We included 235 patients were identified for the final data analysis (Figure 1). They had an average age of 48.14 ± 11.24 years; 44.68% were men, and 27.75% had hemorrhagic MMD (Table 1). The TG and Hb concentrations were 1.30 ± 0.82 mmol/L and 135.72 ± 18.99 g/L, respectively.
Univariate analysis for TGs
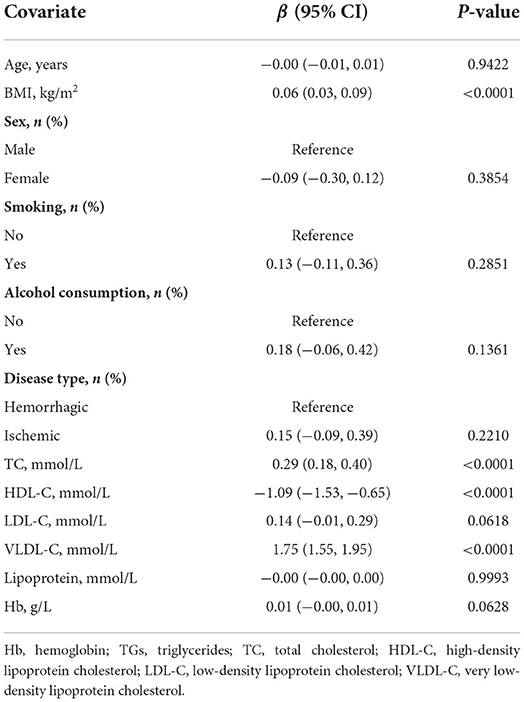
Table 2 displays the results of the univariate analyses. Therein, age, sex, smoking status, drinking habits, ischemic disease type, low-density lipoprotein cholesterol concentration, lipoprotein concentration, and Hb concentration were not linked with TG concentration. Furthermore, high-density lipoprotein cholesterol was negatively associated with TG concentration (β: −1.09, 95% confidence interval [CI]: −1.53, −0.65), whereas BMI (β: 0.06, 95% CI: 0.03, 0.09), total cholesterol concentration (β: 0.29, 95% CI: 0.18, 0.40), and very low-density lipoprotein cholesterol (β: 1.75, 95% CI: 1.55, 1.95) were positively associated with TG concentration.
Unadjusted and adjusted linear regression results
Models were created to explore the indirect effects of Hb on the TG concentration after confounders were removed (multivariable linear regression). The effect sizes (β) and 95% CIs are shown in Table 3. The model-based effect size in TG concentration in the unadjusted model (Model I) is estimated for a 1 g/L increase in the Hb concentration. For sensitivity analysis, Hb was transformed from a continuous to a categorical variable (quartiles). The P-value for the trend in the Hb concentration in the fully adjusted model was similar to the results when Hb concentration was treated as a continuous variable.
Association between Hb and TG concentrations
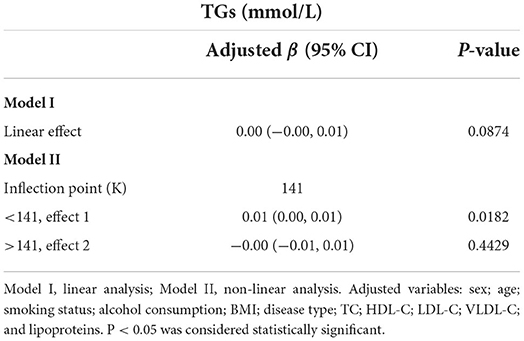
Figure 2 illustrates the smooth curve fitting after controlling for possible confounders. The TG concentration had a non-linear relationship with the Hb concentration. The threshold effect was further investigated using curve fitting, as summarized in Table 4. A significant positive correlation between Hb and TG concentrations was identified when the Hb concentration was below 141 g/L (β: 0.01, 95% CI: 0.00, 0.01; P = 0.0182). When the Hb concentration was more than 141 g/L, there was no clinically significant link between the two parameters (β: −0.00, 95% CI −0.01, 0.01; P = 0.4429).

Figure 2. Association between Hb (g/L) and TGs (mmol/L). (A) smooth fitted curve of Hb and TGs, (B) scatter plot for the distribution of Hb and TGs. The solid red line represents the smooth curve fit between the variables. The blue bands represent the 95% CI of the fit. The model is adjusted for sex; age; smoking status; alcohol consumption; BMI; disease type; TC; HDL-C; LDL-C; VLDL-C; lipoproteins.
Subgroup analysis
Smooth fitted curves were also plotted separately for patients with hemorrhagic MMD and those with ischemic MMD (Figures 3, 4, respectively). Both subgroups exhibited a positive relationship between the TG and Hb concentrations.
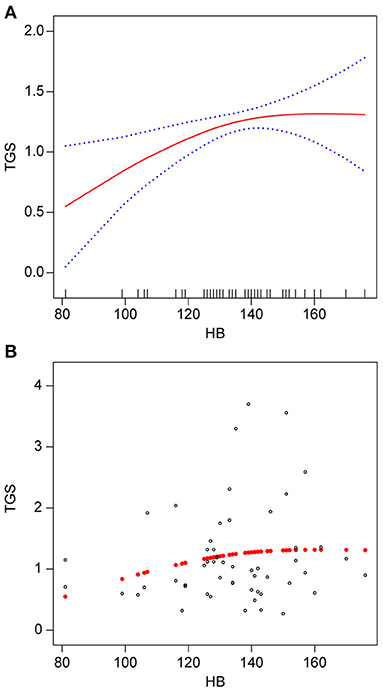
Figure 3. Association between Hb (g/L) and TGs (mmol/L) in patients with hemorrhagic MMD. (A) smooth fitted curve of Hb and TGs, (B) scatter plot for the distribution of Hb and TGs. The solid red line represents the smooth curve fit between the variables. The blue bands represent the 95% CI of the fit. The model was adjusted for age; smoking status; alcohol consumption; BMI; disease type; TC; HDL-C; LDL-C; VLDL-C; and lipoproteins.
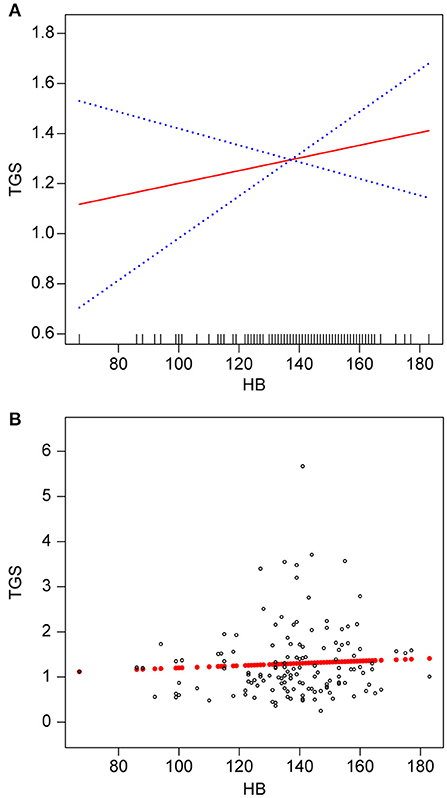
Figure 4. Association between Hb (g/L) and TGs (mmol/L) in patients with ischemic MMD. (A) smooth fitted curve for Hb and TGs, (B) scatter plot for the distribution of Hb and TGs. The solid red line represents the smooth curve fit. The blue bands represent the 95% CI of the fit. The model was adjusted for age; smoking status; alcohol consumption; BMI; disease type; TC; HDL-C; LDL-C; VLDL-C; and lipoproteins.
Discussion
We observed a non-linear positive correlation between Hb and TG levels in patients with MMD. Further sensitivity analysis suggested a critical positive relationship between Hb and TG levels. Up to an Hb concentration of 141 g/L, we discovered that the TG concentration increased with the Hb concentration. However, above that threshold, there was no association between these two parameters. Upon stratified analysis of patients with hemorrhagic MMD and those with ischemic MMD, the Hb concentration was positively associated with the TG concentration in both groups.
MMD is an uncommon cerebrovascular illness marked by the creation of an aberrant network of collateral vessels, often leading to ischemic and hemorrhagic strokes (1, 2). The Hb level is an easily accessible and sensitive clinical indicator that reflects the physiological status of the body (7, 13). A recent study (12) showed a U-shaped association between hemoglobin concentration and stroke sequelae and recurrence, with either too high or too low hemoglobin concentrations being associated with stroke disability, death and recurrence. Meanwhile, a multicentre study (14) noted that elevated hemoglobin concentrations within 3 months of onset were associated with poor prognosis in men but not significantly in women with cerebral hemorrhage. Several previous publications (15–17) suggest that dyslipidaemia is a known risk factor for cerebrovascular disease. A high concentration of TGs is an independent risk factor for ICA stenosis, which is strongly linked to the development of MMD (5). A potential link between the pathophysiology of MMD and aberrant lipid metabolism has recently been demonstrated (18). Our team found a positive association between SUA and TG in a previous study (6) and concluded that early prevention of dyslipidemia could help reduce the incidence of cerebrovascular disease. Our data demonstrated that the Hb and TG concentrations of Chinese patients diagnosed with MMD were positively related after adjusting for other factors. These findings suggest a potential overlapping mechanism between Hb concentration and abnormal lipid metabolism.
Different derivatives of Hb are formed through oxidation, each exerting different pro-oxidative and pro-inflammatory effects, which can increase the sensitivity of vascular endothelial cells to oxidant-mediated injury and cause lipid peroxidation through the release of heme and redox-active iron, thereby leading to inflammation of the vascular wall (19, 20). Several researchers (21, 22) consider oxidative derivatives formed by Hb important causes of cerebrovascular disease. Those conclusions are largely in line with what we discovered.
To the best of our knowledge, there is limited information on the relationship between lipid metabolism and Hb in patients with MMD. We are also not aware of previous studies on the relationship between Hb and TGs in individuals newly diagnosed with MMD. Future MMD predictive models may benefit from our results, possibly leading to the development of a clinically accessible indicator for MMD diagnosis.
Our study had several strengths. First, we explored the non-linearity of the relationship between the two primary parameters. Second, as this was an observational study, we employed rigorous statistical adjustments to minimize the effects of influencing factors.
This study also had certain limitations. First, our study population comprised patients newly diagnosed with MMD in Southwest China, and we excluded certain categories of patients, which may limit the generalizability of our findings. Second, family history is a significant feature of patients with MMD, although only a few family members with MMD were discovered in the data we collected.
In summary, we revealed in the current study a link between Hb and TGs in patients recently diagnosed with MMD; this link may be related to the development of MMD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by the Affiliated Hospital of Jining Medical University Institutional Review Board (approval number: 2021C107). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
GL contributed to conception and formal analysis. YS contributed to data curation, resources, and writing the original draft. FJ contributed to funding acquisition, project administration, validation, and reviewing and editing the original draft. HZ contributed to investigation. SF contributed to methodology. JL contributed to software. YL contributed to supervision. CC contributed to visualization. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Research Fund for Lin He's Academician Workstation of New Medicine and Clinical Translation in Jining Medical University, JYHL2018FMS16, and the Key Research and Development Program of Jining Science and Technology (2018SMNS005), a Project of Shandong Province Medical Health and Technology Development Program (2014WS0518). The funders had no role in the study design; in the collection, analysis or interpretation of the data; in the writing of the report; or in the decision to submit the paper for publication.
Acknowledgments
The authors thank all the healthcare technicians in the Department of Neurosurgery, Affiliated Hospital of Jining Medical University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.994341/full#supplementary-material
References
1. Fuentes AM, Chiu RG, Mehta AI. Disparities in the symptomatic presentation of Moyamoya disease in the United States: a nationwide all-payer analysis. J Clin Neurosci. (2021) 87:92–6. doi: 10.1016/j.jocn.2021.02.019
2. Akiyama Y, Mikami T, Mikuni N. Deep learning-based approach for the diagnosis of Moyamoya disease. J Stroke Cerebrovasc Dis. (2020) 29:105322. doi: 10.1016/j.jstrokecerebrovasdis.2020.105322
3. Ge P, Ye X, Zhang Q, Liu X, Deng X, Zhao M, et al. Clinical features, surgical treatment, and outcome of intracranial aneurysms associated with Moyamoya disease. J Clin Neurosci. (2020) 80:274–9. doi: 10.1016/j.jocn.2020.09.006
4. Dei Cas M, Carrozzini T, Pollaci G, Potenza A, Nava S, Canavero I, et al. Plasma lipid profiling contributes to untangle the complexity of moyamoya arteriopathy. Int J Mol Sci. (2021) 22:13410. doi: 10.3390/ijms222413410
5. Kitagami M, Yasuda R, Toma N, Shiba M, Nampei M, Yamamoto Y, et al. Impact of hypertriglyceridemia on carotid stenosis progression under normal low-density lipoprotein cholesterol levels. J Stroke Cerebrovasc Dis. (2017) 26:1793–800. doi: 10.1016/j.jstrokecerebrovasdis.2017.04.010
6. Ma W, Cui C, Feng S, Li G, Han G, Hu Y, et al. Serum uric acid and triglycerides in Chinese patients with newly diagnosed moyamoya disease: a cross-sectional study. BioMed Res Int. (2019) 2019:9792412. doi: 10.1155/2019/9792412
7. Ahankari AS, Kabra P, Tata LJ, Hayter M, Fogarty AW. Two measures of systemic inflammation are positively associated with haemoglobin levels in adolescent girls living in rural India: a cross-sectional study. Trop Med Int Health. (2021) 26:327–34. doi: 10.1111/tmi.13524
8. Agyemang AA, Kvist SV, Brinkman N, Gentinetta T, Illa M, Ortenlöf N, et al. Cell-free oxidized hemoglobin drives reactive oxygen species production and pro-inflammation in an immature primary rat mixed glial cell culture. J Neuroinflammation. (2021) 18:42. doi: 10.1186/s12974-020-02052-4
9. Karling P, Lundgren D, Eklöf V, Palmqvist R, Hultdin J. Improved monitoring of inflammatory activity in patients with ulcerative colitis by combination of faecal tests for haemoglobin and calprotectin. Scand J Clin Lab Invest. (2019) 79:341–6. doi: 10.1080/00365513.2019.1622148
10. Greffeuille V, Fortin S, Gibson R, Rohner F, Williams A, Young MF, et al. Associations between zinc and hemoglobin concentrations in preschool children and women of reproductive age: an analysis of representative survey data from the biomarkers reflecting inflammation and nutritional determinants of anemia (BRINDA) project. J Nutr. (2021) 151:1277–85. doi: 10.1093/jn/nxaa444
11. Chang JY, Lee JS, Kim BJ, Kim JT, Lee J, Cha JK, et al. Influence of hemoglobin concentration on stroke recurrence and composite vascular events. Stroke. (2020) 51:1309–12. doi: 10.1161/STROKEAHA.119.028058
12. Liu Q, Wang X, Wang Y, Wang C, Zhao X, Liu L, et al. Both ends of values in the hemoglobin spectrum are associated with adverse stroke outcomes. Cerebrovasc Dis. (2022) 51:36–44. doi: 10.1159/000517868
13. Kumar Y, Dogra A, Kaushik A, Kumar S. Progressive evaluation in spectroscopic sensors for non-invasive blood haemoglobin analysis-a review. Physiol Meas. (2022) 43:2. doi: 10.1088/1361-6579/ac41b7
14. Zhang S, Shu Y, Li W, Wei C, Deng A, Cheng Y, et al. High haemoglobin levels and mortality in males with intracerebral haemorrhage: a retrospective cohort study. BMJ. (2022) 12:e048108. doi: 10.1136/bmjopen-2020-048108
15. Tahir A, Martinez PJ, Ahmad F, Fisher-Hoch SP, McCormick J, Gay JL, et al. An evaluation of lipid profile and pro-inflammatory cytokines as determinants of cardiovascular disease in those with diabetes: a study on a Mexican American cohort. Sci Rep. (2021) 11:14197. doi: 10.1038/s41598-021-93445-9
16. Kopčeková J, Lenártová P, Mrázová J, GaŽarová M, Habánová M, Jančichová K. The relationship between seeds consumption, lipid profile and body mass index among patients with cardiovascular diseases. Rocz Panstw Zakl Hig. (2021) 72:145–53. doi: 10.32394/rpzh.2021.0159
17. Hsu HY, Lin CJ, Lee YS, Wu TH, Chien KL. Efficacy of more intensive lipid-lowering therapy on cardiovascular diseases: a systematic review and meta-analysis. BMC Cardiovasc Disord. (2020) 20:334. doi: 10.1186/s12872-020-01567-1
18. Mineharu Y, Miyamoto S. RNF213 and GUCY1A3 in Moyamoya disease: key regulators of metabolism, inflammation, and vascular stability. Front Neurol. (2021) 12:687088. doi: 10.3389/fneur.2021.687088
19. Saucedo CL, Courtois EC, Wade ZS, Kelley MN, Kheradbin N, Barrett DW, et al. Transcranial laser stimulation: mitochondrial and cerebrovascular effects in younger and older healthy adults. Brain Stimul. (2021) 14:440–9. doi: 10.1016/j.brs.2021.02.011
20. Bale G, Mitra S, de Roever I, Sokolska M, Price D, Bainbridge A, et al. Oxygen dependency of mitochondrial metabolism indicates outcome of newborn brain injury. J Cereb Blood Flow Metab. (2019) 39:2035–47. doi: 10.1177/0271678X18777928
21. Bachi ALL, Barros MP, Vieira RP, Rocha GA, de Andrade PBM, Victorino AB, et al. Combined exercise training performed by elderly women reduces redox indexes and proinflammatory cytokines related to atherogenesis. Oxid Med Cell Longev. (2019) 2019:6469213. doi: 10.1155/2019/6469213
Keywords: moyamoya disease, internal carotid arteries, cerebrovascular disease, triglycerides, hemoglobin
Citation: Su Y, Li G, Zhao H, Feng S, Lu Y, Liu J, Chen C and Jin F (2022) The relationship between hemoglobin and triglycerides in moyamoya disease: A cross-sectional study. Front. Neurol. 13:994341. doi: 10.3389/fneur.2022.994341
Received: 14 July 2022; Accepted: 19 August 2022;
Published: 08 September 2022.
Edited by:
Yasushi Takagi, Tokushima University, JapanCopyright © 2022 Su, Li, Zhao, Feng, Lu, Liu, Chen and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Jin, amluZmVuZ3Nkam5AMTYzLmNvbQ==
 Yu Su
Yu Su Genhua Li2
Genhua Li2 Jilan Liu
Jilan Liu