- 1Department of Radiology and Nuclear Medicine, Xuanwu Hospital, Capital Medical University, Beijing, China
- 2Beijing Key Laboratory of Magnetic Resonance Imaging and Brain Informatics, Beijing, China
- 3Department of Neurosurgery, Xuanwu Hospital, Capital Medical University, Beijing, China
- 4China International Neuroscience Institute (China-INI), Beijing, China
- 5Department of Library, Beijing Luhe Hospital, Capital Medical University, Beijing, China
- 6Beijing Key Laboratory of Clinical Epidemiology, School of Public Health, Capital Medical University, Beijing, China
- 7Neuroendovascular Program, Massachusetts General Hospital and Harvard Medical School, Boston, MA, United States
- 8Department of Neurology, Loma Linda University Health, Loma Linda, CA, United States
- 9Department of Library, Xuanwu Hospital, Capital Medical University, Beijing, China
Background: Active cancer (AC) is a known risk factor for stroke and a common comorbidity among patients being considered for treatment with endovascular thrombectomy (EVT). This systematic review and meta-analysis aimed to evaluate the current evidence for the feasibility, efficacy, and safety of EVT for patients with AC.
Methods: MEDLINE, EMBASE, and the Cochrane Library were searched for relevant randomized controlled trials (RCTs) and observational studies which met the inclusion criteria for EVT in patients with AC. Studies were excluded due to the mismatch of data format, article type, and group design. The risk of bias was assessed through different scales according to the study design. I2 statistics were used to evaluate the heterogeneity. Funnel plots were used to evaluate publication bias.
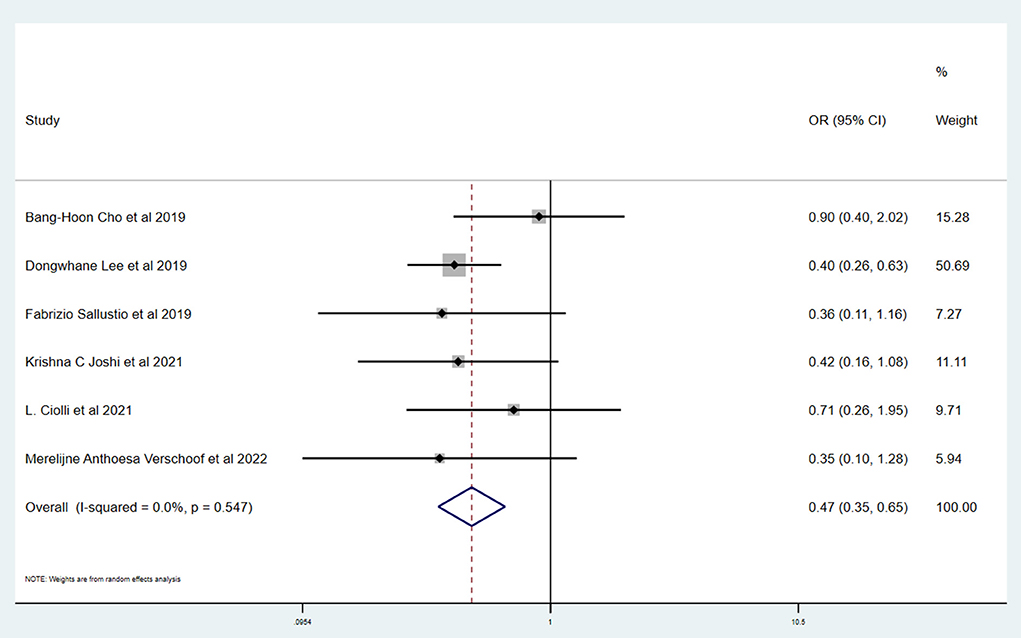
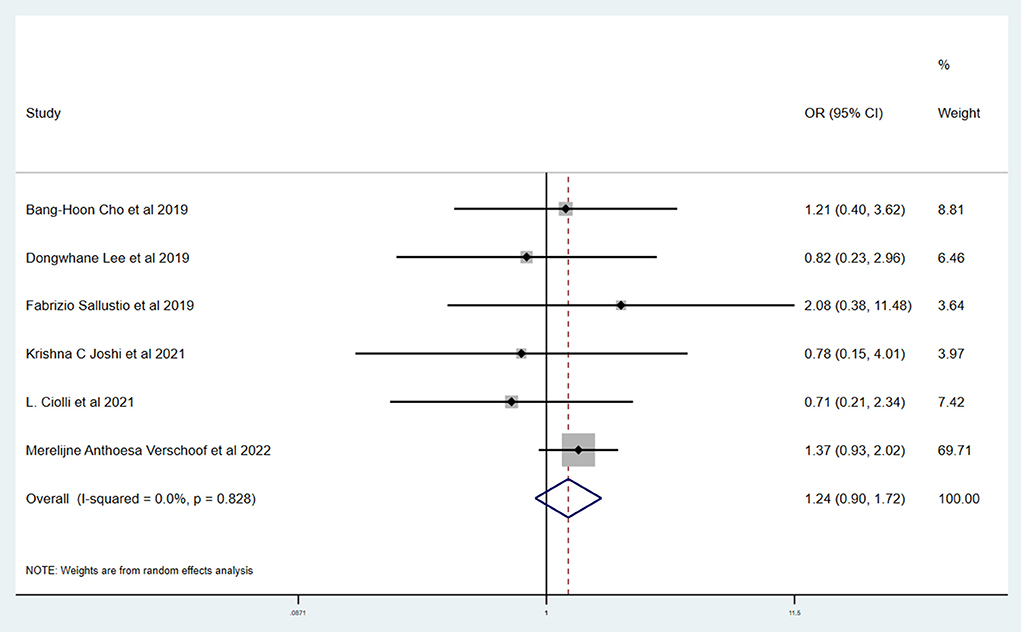
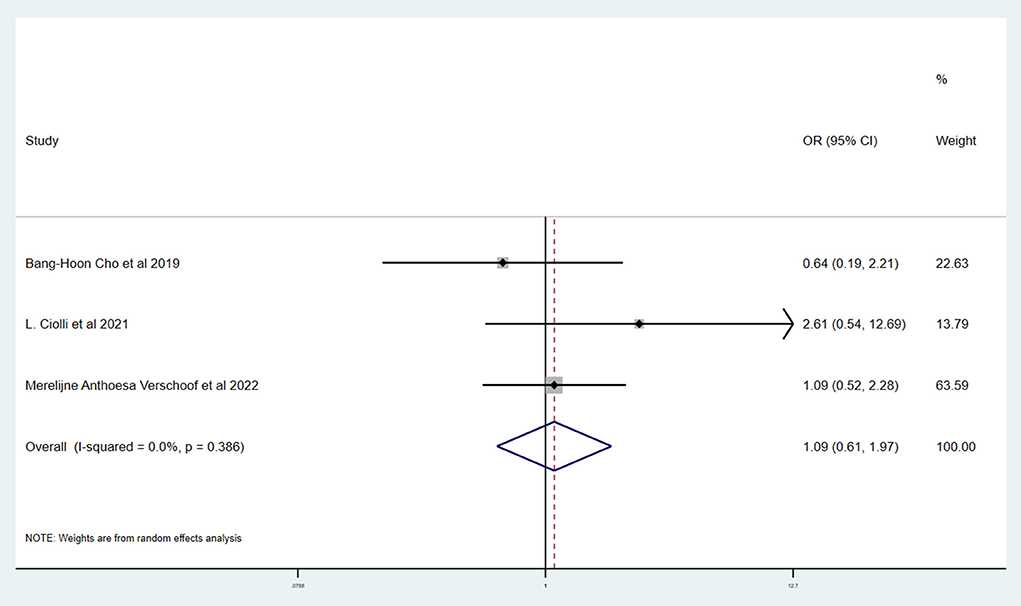
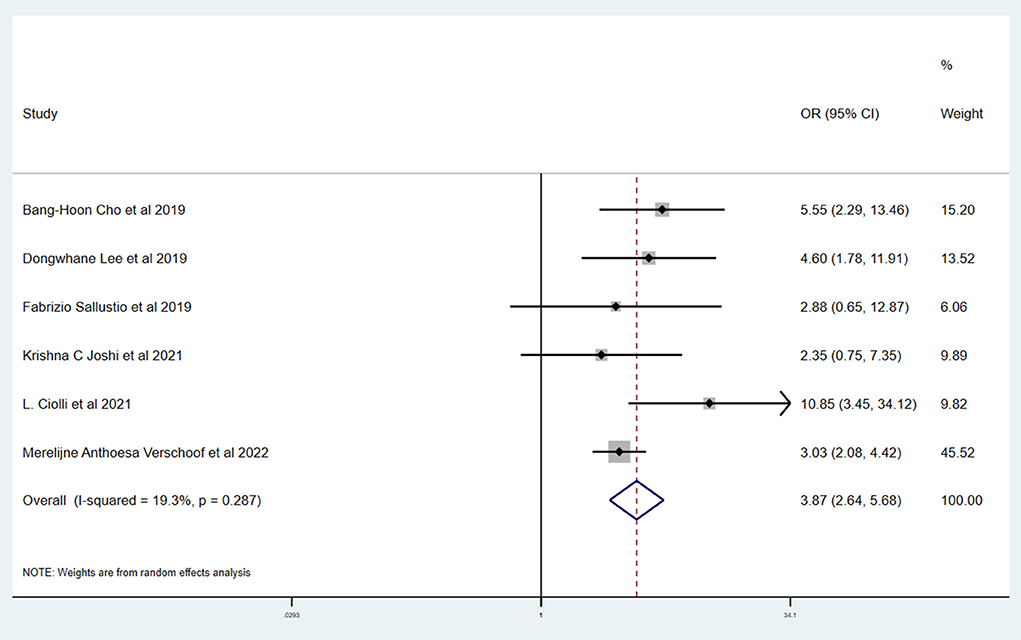
Results: A total of six studies and 3,657 patients were included. Compared to without active cancer (WC) patients, patients with AC had a significantly higher proportion of in-hospital mortality (OR 3.24; 95% CI, 1.03–10.15). The estimated rate of favorable outcome of six studies was lower in patients with AC than in patients with WC (OR 0.47; 95% CI, 0.35–0.65). For 90-day mortality of four studies, the AC group had a higher proportion when compared with the WC group (OR 3.87; 95% CI, 2.64–5.68). There was no difference between rate of six studies of successful recanalization (OR 1.24; 95% CI, 0.90–1.72) and four studies of symptomatic ICH (OR 1.09; 95% CI, 0.61–1.97) comparing AC and WC.
Conclusion: Patients with AC are less likely to have a favorable outcome and have a higher risk of mortality after EVT. Further studies are warranted for this unique patient population.
Introduction
Cancer is a widely known risk factor of acute ischemic stroke (AIS), especially among patients with active cancer (AC) which was diagnosed within 6 months or during the admission period, requires chemotherapy or surgical treatment within 6 months, or was recurrent, metastatic, or inoperable (1). Several mechanisms related to malignancy theoretically increase the risk of AIS, such as hypercoagulation state, migratory thrombosis, and tumor embolus (2–4). Also, AC-related stroke is associated with a higher morbidity in several studies (5–8). Indeed, about 10% of hospitalized patients with AIS had AC (9–11). Unfortunately, patients with AC are often ineligible for intravenous thrombolysis (IVT) due to various reasons, such as bleeding tendency and recent prior surgery (12, 13).
Endovascular thrombectomy (EVT) has revolutionized acute stroke care and is recommended as the first-line treatment for AIS due to large vessel occlusion (LVO) (14, 15). Whether EVT benefits AC-related stroke patients to a similar degree remains uncertain. In a previous meta-analysis including a relatively limited number of studies, the AC group had a comparable rate of successful recanalization and symptomatic intracerebral hemorrhage (sICH) compared to the control group, but a lower rate of favorable outcome (modified Rankin Scale ≤ 2) and a higher rate of mortality (16). However, some recent clinical studies indicated that rate of favorable outcome may be similar between the two groups, which differs from the aforementioned meta-analysis (4, 5). Other studies suggested that patients with active cancer are more likely to have any cerebral hemorrhage (4, 6). Thus, this article aims to investigate the safety and effectiveness of EVT in AC-related stroked patients, so as to provide clinicians with the most comprehensive and updated evidence for decision-making in clinical practice.
Methods
This study was conducted according to the statement of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (17).
Search strategy
Studies for inclusion were identified by two independent reviewers (CS and ZS) from the three databases: MEDLINE, EMBASE, and the Cochrane Library. Eligible studies were restricted from database inception until 24 January 2022 in the English language. The terms “ischemic stroke”, “brain ischemia”, “cancer”, “neoplasm”, “embolectomy”, “mechanical thrombectomy”, and “endovascular thrombectomy” were applied in our search strategy for potentially relevant studies. The detailed search strategy is presented in the online Supplementary material (Supplement File 1).
Study eligibility
The criteria for study design were specified according to the Population, Intervention, Comparison, Outcome (PICO) model.
Patient selection criteria
Inclusion criteria included adult patients (age ≥ 18 years) with AIS due to LVO, including anterior or posterior circulation occlusions, undergoing EVT. These were divided into active cancer (AC) group and without active cancer (WC) group according to the presence of AC. Active cancer was defined as cancer that was diagnosed within 6 months or during the admission period, requires chemotherapy or surgical treatment within 6 months, or was recurrent, metastatic, or inoperable (1). Arterial occlusion is confirmed by either computed tomographic angiography (CTA), magnetic resonance angiography (MRA), or digital subtraction angiography (DSA). We did not collect any primary data from patients, so ethics approval was deemed unnecessary by our IRB given there was a minimal patient risk.
Intervention
Mechanical thrombectomy with modern devices, such as stent retrievers or aspiration catheters, for patients is available to additional intravenous thrombolysis.
Outcomes
At least one of the following items was reported:
Primary outcomes:
1. Favorable outcome defined as modified Rankin Score (mRS) of 0–2 or equal to pre-stroke score at 90 days.
2. Symptomatic intracranial hemorrhage (SICH) was diagnosed if a new intracranial hemorrhage was associated with any of the following conditions: (1) NIHSS score increased >4 points than that immediately before worsening; (2) NIHSS score increased >2 points in one category; (3) deterioration of neurological status led to intubation, hemicraniectomy, external ventricular drain placement, or other major medical or surgical intervention, according to the second European Australasian Acute Stroke Study classification (ECASS II) (18).
Secondary outcomes:
1. Successful recanalization (MTICI 2b-3) determined by post-interventional DSA.
2. Mortality at 90-day follow-up.
3. In-hospital mortality.
Comparison
The comparator was patients of the WC group, who also received EVT without limitation of additional intravenous thrombolysis.
Studies
We included RCTs and observational studies, including cohort studies and case-controlled studies. Other types of articles such as abstracts, conference reports, and case reports were excluded. Studies which did not report the above outcomes or extractable complications were also excluded.
Selection of studies and data extraction
Two reviewers (ZF and ZS) independently searched the databases to include eligible studies. In the initial stage of screening, titles, keywords, and abstracts were reviewed, and irrelevant studies were excluded. Subsequently, full articles of all the remaining studies were obtained and carefully reviewed to assess eligibility, and reasons for inclusion or exclusion of studies were documented in detail. Conflicts in study selection between two reviewers were resolved by a third reviewer (HZ).
The extraction of data from included studies was conducted by two independent reviewers (LD and HZ) using a standardized data extraction form. The extracted information of included studies was as follows: (1) characteristics of the study, such as publication time, country, and the number of patients; (2) demographic characteristics, such as age, gender, cancer type, medical history, laboratory results, site of occlusion by angiography, and intervention characteristics; (3) aforementioned outcomes such as sICH and favorable outcome. The resolution of disagreement regarding data extraction was achieved through the assistance of another reviewer (XB). For missing or ambiguous data in included studies, clarification of data through direct contact with the corresponding authors by e-mail was attempted.
Assessment of risk bias and heterogeneity
Two reviewers (QT and XW) independently assessed the risk of bias for each included study. The Cochrane Collaboration criteria were applied in the process of selection of RCTs (Supplementary File 2). The Newcastle–Ottawa scale was used for observational studies, such as cohort studies and case-control studies (Supplementary File 3) (19). The heterogeneity of pooled outcomes was evaluated by I2 statistic. If I2 is <20%, the heterogeneity was considered acceptable. The Mantel–Haenszel method for fixed-effects estimation was applied if heterogeneity was mild or moderate. For substantial heterogeneity of outcomes, we conducted meta-regression and sensitivity analyses to explore the potential source of heterogeneity.
Measures of treatment effect
A meta-analysis on a specific result was performed only when there were at least two suitable studies for analysis. If there were insufficient suitable studies for meta-analysis, the results were described in the narrative. We adapted OR with 95% CIs for dichotomous data and the mean differences (MD) with 95% CIs for continuous data. The standard of p-value < 0.05 was considered statistically significant. The Stata statistical software (version 15.0, Stata Corp, College Station, Texas, USA) was used for data analysis and heterogeneity assessment.
Results
Study selection and study characteristics
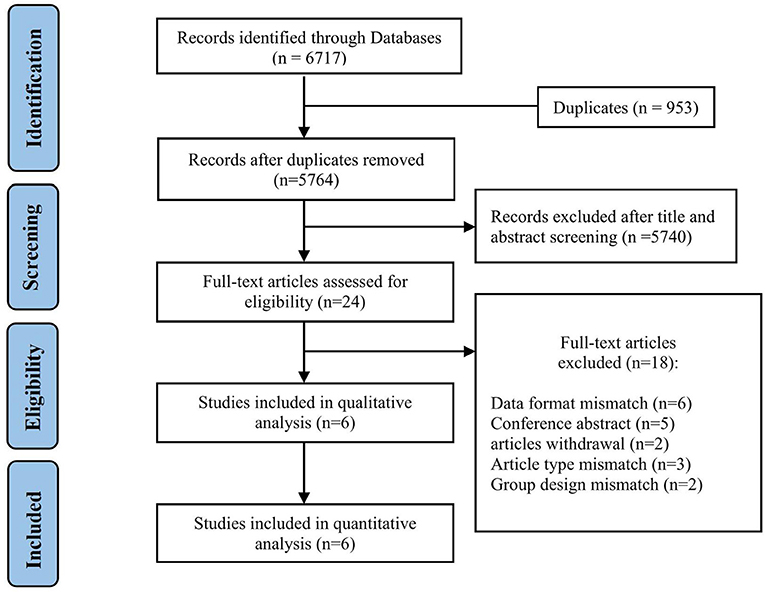
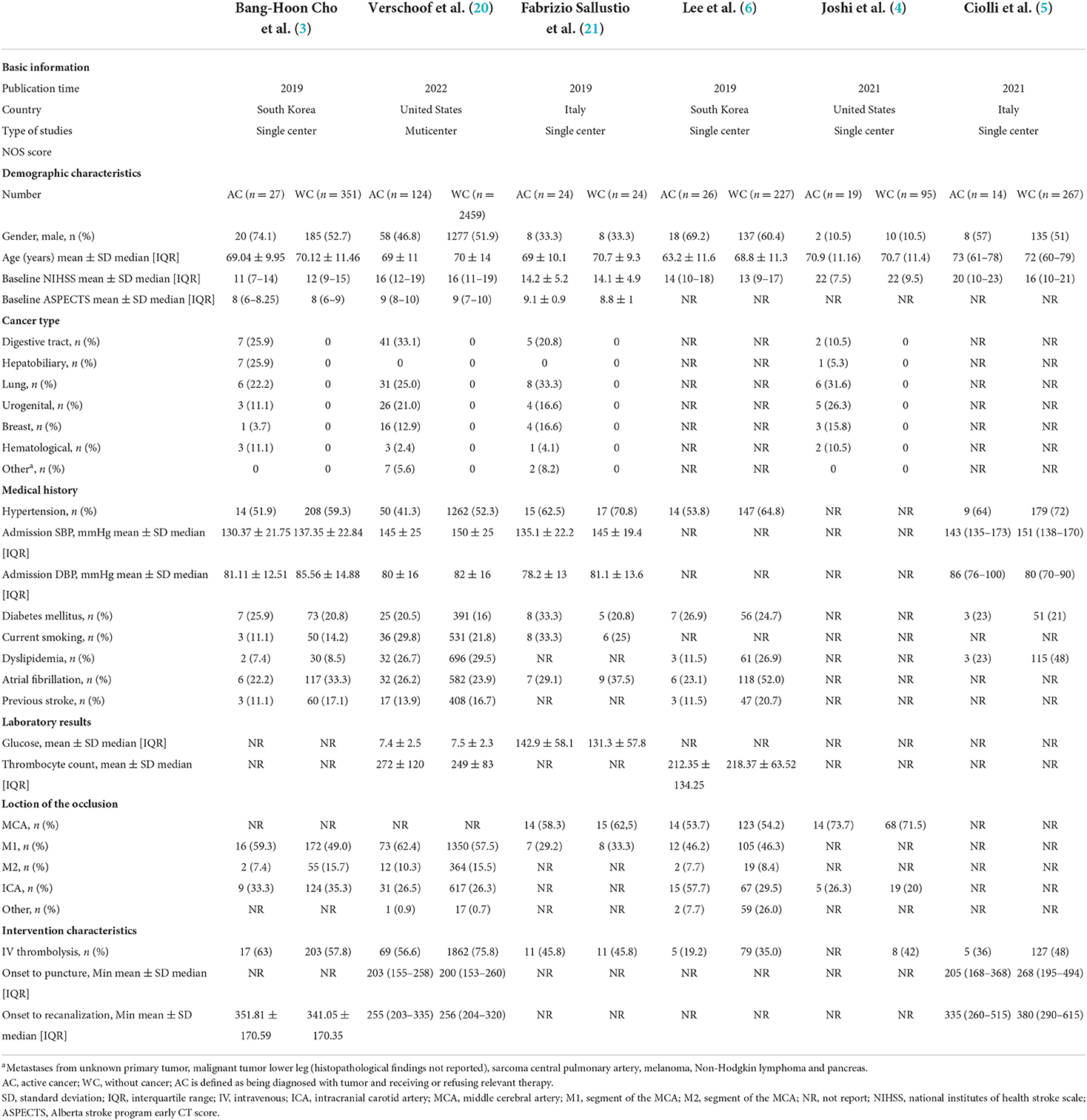
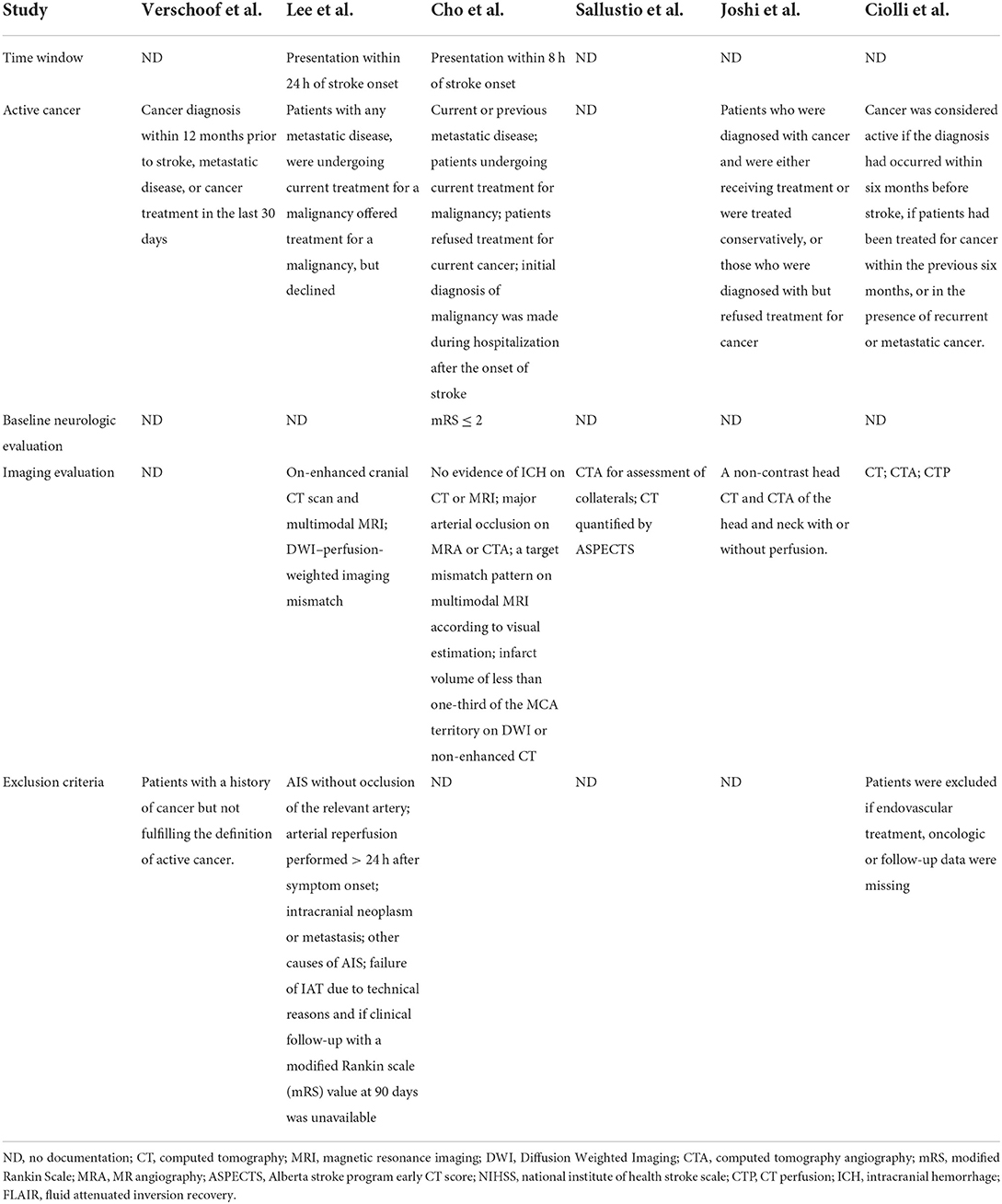
We found 5,764 references, abstracts, and related clinical trials from the three electronic databases and clinical trial registries. Among the results, 24 full-text articles were retrieved after initial checks, and six studies were finally eligible for inclusion in the qualitative and quantitative analysis. The process of study selection and reasons for exclusion are summarized in Figure 1. Table 1 shows the characteristics of included studies and patients. A total of six studies and 3,657 patients met inclusion criteria (3–6, 20). All studies were published after 2019, two were conducted in Italy, two were conducted in the USA, and two were performed in South Korea. There was one multicenter study, and the remaining were single-center studies. The inclusion and exclusion criteria for patients included in the individual studies are summarized in Table 2. The inclusion criteria consisted of the time window, cancer diagnosis, and baseline neurological and imaging evaluations. The number of patients in each included study ranged from 48 to 2,583, and the ratio of men to women was equal. The site of occlusion as determined by angiography was mostly located at anterior circulation, especially the internal carotid artery and M1 segment of the middle cerebral artery. The estimated times of onset to groin puncture and onset to revascularization ranged from 153–494 to 203–615 min, respectively.
Meta-analysis of primary and secondary outcomes
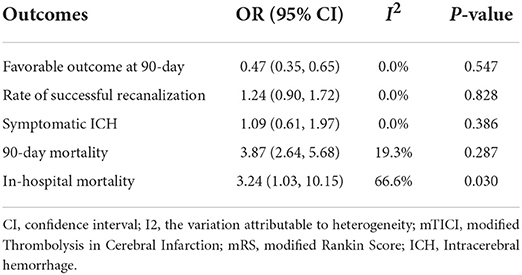
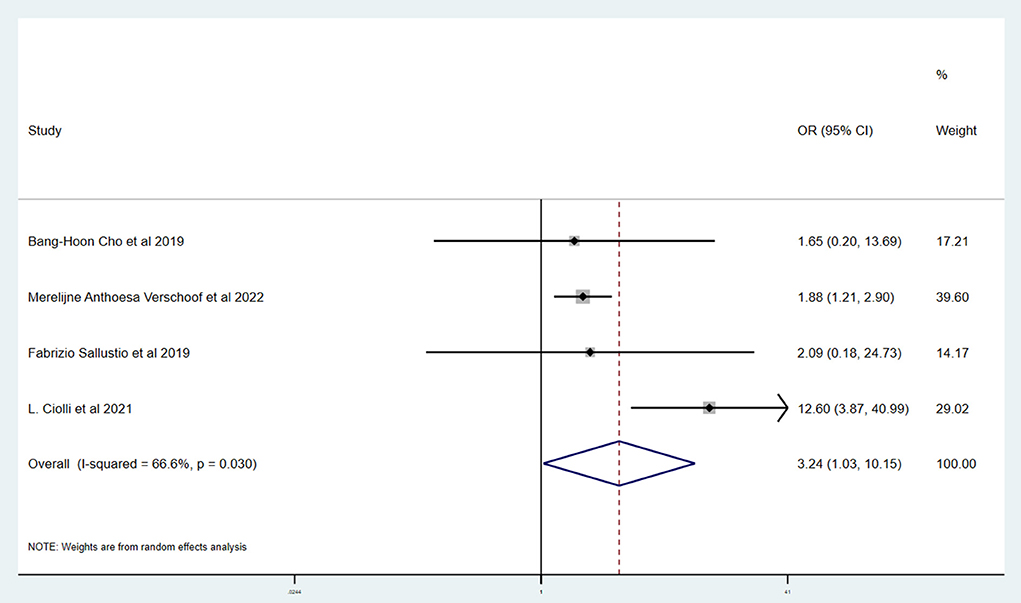
Table 3 shows the primary and secondary outcomes for patients with AC and WC. In four studies and 3,140 patients, compared with patients with WC, patients with AC had a significantly higher proportion of in-hospital mortality (OR, 3.24; 95% CI, 1.03–10.15, p = 0.030; I2 = 66.6%). The estimated rate of favorable outcome of six studies and 3,657 patients was lower in patients with AC than in patients with WC (OR, 0.47; 95% CI, 0.35–0.65, p = 0.547; I2 = 0.0%). The outcome of four studies and 3,345 patients of 90-day mortality showed that the AC group had a higher proportion when compared with the WC group (OR, 3.87; 95% CI, 2.64–5.68; p = 0.287; I2 = 19.3%). There was no difference between rate of successful recanalization (OR, 1.24; 95% CI, 0.90–1.72; p = 0.828; I2 = 0.0%) in six studies and 3,657 patients and symptomatic ICH (OR, 1.09; 95% CI, 0.61–1.97; p = 0.386; I2 = 0.0%) in four studies and 3,140 patients between groups AC and WC.
Risk of bias
The Newcastle–Ottawa scale was used to assess the bias risk of observational studies, such as case-control studies, with the majority of included studies being low-risk. Funnel plots were used to explore the publication bias, with the results demonstrating no evident reporting bias. The outcomes of the above analyses are presented in Figures 2–6.
Discussion
This systematic review and meta-analysis summarized the safety and efficacy of EVT for AIS patients with AC. The successful recanalization proportion (OR, 1.24; 95% CI, 0.90–1.72; p = 0.828; I2 = 0.0%) and rate of symptomatic ICH (OR, 1.09; 95% CI, 0.61–1.97; p = 0.386; I2 = 0.0%) in patients with AC were comparable with patients with WC. However, patients with AC had a significantly lower rate of favorable outcome (OR, 0.47; 95% CI, 0.35–0.65, p = 0.547; I2 = 0.0%). We also showed a tendency for a higher proportion of in-hospital mortality (OR, 3.24; 95% CI, 1.03–10.15, p = 0.030; I2 = 66.6%) and 90-day mortality (OR, 3.87; 95% CI, 2.64–5.68; p = 0.287; I2 = 19.3%) in patients with AC.
The similar rate of successful recanalization and symptomatic ICH in patients with AC and WC supports the safety of EVT in patients with AC. However, our study found that there was a significant difference in 90-day favorable outcome and mortality after EVT in patients with and without active cancer. The presence of analytical abnormalities or hemostatic abnormalities is common in cancer patients, and it may be a major cause for the poorer prognosis of these patients. The level of platelet in blood of cancer patients often decreases, which may result in the tendency of bleeding and a higher rate of complications. What is more, the growth of cancer consumes a large amount of body's energy and may bring to malnutrition, thus giving rise to the poor prognosis. In addition, EVT will inevitably lead to the injury of vessels and plaque and trigger the inner repairing mechanism. Accumulation of platelet is a reaction of inflammation, which is a stimulation to malignant cancer and is likely to worsen the post-surgery prognosis. It should be noticed that, according to recent articles, under certain circumstances, thrombosis is a physiological process that constitutes an intrinsic effector mechanism of innate immunity, whereas the rapid update of cancer cells will result in blood internal environment disorder and disable the defense system by thrombosis (22).
Intravenous thrombolysis (IVT), given to many patients in our meta-analysis, may be associated with higher risks in patients with AC. However, we did not observe a difference in hemorrhage rates and other studies have reported that IVT using alteplase is safe and effective for patients with AC (23, 24). The condition of cancer itself may also contribute to a higher rate of mortality. Stroke symptoms and related disability may influence decisions regarding cancer treatment, such as tumor resection and chemotherapy (5). Therefore, the death of patients in the AC group may be related to cancer and tumor progression. It is necessary to carry out more research on the stage, grade, and type of tumor to further analyze the mortality due to cancer.
Our study has several limitations. First, we could not evaluate the effects of cancer-related factors, including the cancer staging, brain metastasis, treatment status, and life expectancy due to insufficient data. Second, selection bias is inevitable in our study selection process. Furthermore, several included studies had small sample sizes, and there were inconsistent outcome measures. All of the included studies are retrospective, and only one was multicenter. More data are needed to confirm our conclusion.
Conclusion
AC is likely to influence patient outcomes after EVT and may hold a higher risk of mortality. While there were similar rates of reperfusion and hemorrhage, further high-quality studies are warranted to better understand long-term outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LD contributed to the initial idea for this study. CS, QT, and XW developed and revised the search strategy. LD and HZ finished the study design. BY was consulted about the clinical issues. LD, ZF, and HZ contributed to the original draft. LD, ZF, HZ, CS, QT, AD, RR, ZS, XG, XW, and BY were responsible for the revision of the draft. All authors approved the final work before submission.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.992825/full#supplementary-material
References
1. Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, et al. Low-molecular-weight heparin vs. a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. (2003) 349:146–53. doi: 10.1056/NEJMoa025313
2. Schwarzbach CJ, Schaefer A, Ebert A, Held V, Bolognese M, Kablau M, et al. Stroke and cancer: the importance of cancer-associated hypercoagulation as a possible stroke etiology. Stroke. (2012) 43:3029–34. doi: 10.1161/STROKEAHA.112.658625
3. Cho BH, Yoon W, Kim JT, Choi KH, Kang KW, Lee JH, et al. Outcomes of endovascular treatment in acute ischemic stroke patients with current malignancy. Neurol Sci. (2020) 41:379–85. doi: 10.1007/s10072-019-04103-y
4. Joshi KC, Grewal P, Beer-Furlan A, Vargas A, Osteraas N, Dafer R, et al. Endovascular thrombectomy for acute ischemic stroke in patients with cancer: a propensity-matched analysis. J Neurointerv Surg. (2021). doi: 10.1136/neurintsurg-2021-018211. [Epub ahead of print].
5. Ciolli L, Bigliardi G, Ferraro D, Maffei S, Vandelli L, Dell'Acqua ML, et al. Efficacy of mechanical thrombectomy in patients with ischemic stroke and cancer. J Clin Neurosci. (2021) 91:20–2. doi: 10.1016/j.jocn.2021.06.029
6. Lee D, Lee DH, Suh DC, Kwon HS, Jeong DE, Kim JG, et al. Intra-arterial thrombectomy for acute ischaemic stroke patients with active cancer. J Neurol. (2019) 266:2286–93. doi: 10.1007/s00415-019-09416-8
7. Rinaldo L, Cloft HJ, Rangel Castilla L, Rabinstein AA, Brinjikji W. Utilization rates of tissue plasminogen activator and mechanical thrombectomy in patients with acute stroke and underlying malignancy. J Neurointerv Surg. (2019) 11:768–71. doi: 10.1136/neurintsurg-2018-014480
8. Yoo J, Kim YD, Park H, Kim BM, Bang OY, Kim HC, et al. Immediate and long-term outcomes of reperfusion therapy in patients with cancer. Stroke. (2021) 52:2026–34. doi: 10.1161/STROKEAHA.120.032380
9. Navi BB, Iadecola C. Ischemic stroke in cancer patients: a review of an underappreciated pathology. Ann Neurol. (2018) 83:873–83. doi: 10.1002/ana.25227
10. Sanossian N, Djabiras C, Mack WJ, Ovbiagele B. Trends in cancer diagnoses among inpatients hospitalized with stroke. J Stroke Cerebrovasc Dis. (2013) 22:1146–50. doi: 10.1016/j.jstrokecerebrovasdis.2012.11.016
11. Lindvig K, Moller H, Mosbech J, Jensen OM. The pattern of cancer in a large cohort of stroke patients. Int J Epidemiol. (1990) 19:498–504. doi: 10.1093/ije/19.3.498
12. Merkler AE, Marcus JR, Gupta A, Kishore SA, Leifer D, Patsalides A, et al. Endovascular therapy for acute stroke in patients with cancer. Neurohospitalist. (2014) 4:133–5. doi: 10.1177/1941874413520509
13. Masrur S, Abdullah AR, Smith EE, Hidalgo R, El-Ghandour A, Rordorf G, et al. Risk of thrombolytic therapy for acute ischemic stroke in patients with current malignancy. J Stroke Cerebrovasc Dis. (2011) 20:124–30. doi: 10.1016/j.jstrokecerebrovasdis.2009.10.010
14. Campbell BCV, Donnan GA, Lees KR, Hacke W, Khatri P, Hill MD, et al. Endovascular stent thrombectomy: the new standard of care for large vessel ischaemic stroke. Lancet Neurol. (2015) 14:846–54. doi: 10.1016/S1474-4422(15)00140-4
15. Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al. 2015 American heart association/American stroke association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2015) 46:3020–35. doi: 10.1161/STR.0000000000000074
16. Eun MY, Jeon ET, Seo KD, Lee D, Jung JM. Reperfusion therapy in acute ischemic stroke with active cancer: a meta-analysis aided by machine learning. J Stroke Cerebrovasc Dis. (2021) 30:105742. doi: 10.1016/j.jstrokecerebrovasdis.2021.105742
17. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
18. Krishnan R, Mays W, Elijovich L. Complications of mechanical thrombectomy in acute ischemic stroke. Neurology. (2021) 97(20 Suppl 2):S115–25. doi: 10.1212/WNL.0000000000012803
19. Lo CK, Mertz D, Loeb M. Newcastle–Ottawa scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. (2014) 14:45. doi: 10.1186/1471-2288-14-45
20. Verschoof MA, Groot AE, de Bruijn S, Roozenbeek B., Bart van der Worp H, Dippel DWJ, et al. Clinical outcome after endovascular treatment in patients with active cancer and ischemic stroke: a MR CLEAN registry substudy. Neurology. (2022) 98:E993–E1001. doi: 10.1212/WNL.0000000000013316
21. Sallustio F, Mascolo AP, Marrama F, Koch G, Alemseged F, Davoli A, et al. Safety and efficacy of reperfusion therapies for acute ischemic stroke patients with active malignancy. J Stroke Cerebrovasc Dis. (2019) 28:2287–91. doi: 10.1016/j.jstrokecerebrovasdis.2019.05.018
22. Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. (2013) 13:34–45. doi: 10.1038/nri3345
23. Caruso P, Ajcevic M, Furlanis G, Ridolfi M, Lugnan C, Cillotto T, et al. Thrombolysis safety and effectiveness in acute ischemic stroke patients with pre-morbid disability. J Clin Neurosci. (2020) 72:180–84. doi: 10.1016/j.jocn.2019.11.047
Keywords: acute ischemic stroke, endovascular thrombectomy, active cancer, meta-analysis, systematic review
Citation: Duan L, Fu Z, Zhao H, Song C, Tian Q, Dmytriw AA, Regenhardt RW, Sun Z, Guo X, Wang X and Yang B (2022) Outcomes after endovascular thrombectomy for acute ischemic stroke patients with active cancer: A systematic review and meta-analysis. Front. Neurol. 13:992825. doi: 10.3389/fneur.2022.992825
Received: 13 July 2022; Accepted: 23 September 2022;
Published: 20 October 2022.
Edited by:
Yuping Tang, Fudan University, ChinaReviewed by:
Antonio Cruz Culebras, Ramón y Cajal University Hospital, SpainMyzoon Ali, University of Glasgow, United Kingdom
Copyright © 2022 Duan, Fu, Zhao, Song, Tian, Dmytriw, Regenhardt, Sun, Guo, Wang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Yang, eWFuZ2Jpbl84MUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Linyan Duan
Linyan Duan Zhaolin Fu
Zhaolin Fu Hengxiao Zhao
Hengxiao Zhao Chengyu Song
Chengyu Song Qiuyue Tian
Qiuyue Tian Adam A. Dmytriw
Adam A. Dmytriw Robert W. Regenhardt
Robert W. Regenhardt Ziyi Sun3,4
Ziyi Sun3,4 Xiaofan Guo
Xiaofan Guo Xue Wang
Xue Wang