
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol., 12 January 2023
Sec. Experimental Therapeutics
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.985288
This article is part of the Research TopicApplication and Evaluation of Acupuncture in the Treatment of Neurological DiseasesView all 28 articles
Background: Acupuncture (AT) successfully regulates overactive bladder (OAB) symptoms. However, previous systematic reviews and meta-analyses have not provided sufficient evidence. This review presents the current evidence of the efficacy of AT in the management of OAB symptoms.
Methods and analyses: A total of 12 databases were searched from their inception: PubMed, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), and AMED databases; five Korean medical databases; and three Chinese medical databases. Study selection, data extraction, and assessment were independently performed by two researchers. The risk of bias was assessed using the Cochrane risk of bias assessment tool. RevMan 5.4.1 software was used for data aggregation, and the Grades of Recommendations, Assessment, Development and Evaluation (GRADE) assessment was used to evaluate the quality of the study outcomes.
Results: A total of 30 studies were included in this review. Compared with the sham AT group, the AT group exhibited significant effects in reducing overactive bladder symptom scores (OABSS) [mean difference (MD): −1.13, 95% confidence interval (CI): −2.01 to −0.26, p = 0.01 I2 = 67%] and urinary frequency [standardized mean difference (SMD): −0.35, 95% CI: −0.62 to −0.08, I2 = 0%]. The AT group showed an equivalent effect as drug therapy in reducing OABSS (MD: −0.39, 95% CI: – 1.92 to 1.13, p = 0.61, I2 = 94%) and urinary frequency (MD: 0.74, 95% CI: −0.00 to 1.48, p = 0.05, I2 = 71%) with fewer adverse events [risk ratio (RR): 0.38, 95% CI: 0.16–0.92, p = 0.03, I2 = 58%]. The AT plus drug therapy group had a more favorable effect than drug therapy alone for reducing OABSS (MD: −2.28, 95% CI: −3.25 to −1.31, p < 0.00001, I2 = 84%) and urinary frequency (MD: −2.34, 95% CI: −3.29 to −1.38, p < 0.00001, I2 = 88%). The GRADE assessment demonstrated that the level of evidence was mostly low or very low given the high risk of bias and small sample sizes.
Conclusion: AT had more favorable effects than sham AT in reducing OAB symptoms. AT improved OAB symptoms as effectively as conventional drug therapy, and the combination of AT and drug therapy had more favorable effects than drug therapy alone. However, more rigorous studies are needed to enhance the level of evidence.
Systematic review registration: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42014010377, identifier: PROSPERO [CRD42014010377].
Overactive bladder (OAB) refers to urinary urgency that is accompanied by increased frequency and nocturia with or without urgency urinary incontinence in the absence of urinary tract infection (UTI) or other obvious pathology (1). The symptoms of OAB are due to involuntary contractions of the detrusor muscle during the filling phase of the micturition cycle, so-called detrusor overactivity (1). However, only 64% of patients with OAB have uro-dynamically proven detrusor overactivity. Therefore, OAB is a syndrome characterized as a “symptom-based diagnosis” (2).
Once a diagnosis of OAB has been made, most patients progress through a stepwise treatment path from conservative options to medical treatments and finally surgical treatments. As a first step, conservative management includes modifying behaviors and interventions, such as pelvic muscle exercises (3). The second step, pharmacotherapy, employs antimuscarinic agents, which have an antagonistic action on muscarinic receptors throughout the body, therefore affecting both involuntary detrusor contraction and increased sensory afferent signaling. However, despite these effects, antimuscarinic agents have several uncomfortable side effects resulting in an overall poor adherence profile with 17–35% of patients still taking their prescribed drug after 1 year (4). Dry mouth is the most common side effect, and other symptoms, such as blurred vision, constipation, erythema, fatigue, increased sweating, nausea, and vomiting, have also been reported (5). The third step, which includes surgical treatments, such as neuromodulation or botulinum toxin injection, is more invasive; therefore, patients with those conditions may seek other treatment options (6).
The mechanisms underlying acupuncture (AT) for neuromodulation of the bladder are not precisely understood. However, urodynamic evidence of detrusor overactivity suggests that AT suppresses uninhibited bladder contractions (7). Furthermore, AT stimulation seems to pass information via sensory ganglia to the spinal cord and via interneurons to modulate the activity of motor neurons in the brainstem that controls autonomic function, including urogenital activity, such as detrusor and sphincter muscle activity (8). These findings suggest that AT may help to improve OAB symptoms.
This review aims to systematically evaluate the evidence for the safety and effectiveness of AT for patients with OAB from randomized controlled trials (RCTs).
This protocol was registered with The International Prospective Register of Systematic Reviews (PROSPERO) (CRD42014010377). The reporting of this review adheres to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (9).
Electronic databases, including MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), AMED, five Korean databases [KoreaMed, the Korean Traditional Knowledge Portal (KTKP), DBpia, the Research Information Service System (RISS), and the Korean Studies Information Service System (KISS)], and three Chinese databases [China National Knowledge Infrastructure (CNKI), the Chongqing VIP Chinese Science and Technology Periodical Database (VIP), and the Wanfang Database], were searched from their inception to February 2022. Our search strategy included keywords, such as “acupuncture,” “overactive bladder,” “detrusor instability,” and “urinary urgency,” in English, Chinese, and Korean. The search terms for each database are listed in Supplementary material 1.
Only RCTs were included in this systematic review. We excluded trials, case studies, case series, qualitative studies, and uncontrolled trials. Trials that failed to provide detailed results were also excluded. RCTs published in the form of abstracts were included. No language restrictions were imposed.
We included studies that involved patients with OAB regardless of age, sex, and race. We excluded OAB studies that involved patients with neurological disease, for example, OAB in Parkinson's disease or stroke.
Studies evaluating all types of AT with and without electrical stimulation were included. Studies were included if AT was used as the only intervention. We also included trials in which the control group received general conventional care, such as a behavioral approach, conventional drug treatments, and sham AT (interventions mimicking “true” AT/true treatment). The acceptability of sham AT as a valid control is highly controversial (10, 11), and we planned to analyze the results using subgroup analysis. Pragmatic trials that compared AT with any other treatments (e.g., conventional drugs/exercise and education) were included. Studies were excluded if the AT was a part of a complex intervention. We excluded warm needle AT and fire AT. Studies investigating other methods of AT point stimulation without needle insertion [e.g., acupressure, transcutaneous electrical nerve stimulation (TENS), pressed studs, and laser stimulation] were excluded. Trials were excluded if the study design did not allow for the evaluation of the effectiveness of AT (e.g., use of a treatment with unproven efficacy in the control group or a comparison between two different forms of AT) or if the study adopted comparisons between treatments or groups that were expected to have similar effects to AT (e.g., moxibustion).
- OABSS: overactive bladder symptom scores.
- Frequency: number of daily urinary events.
- Incontinence: number of daily incontinence events.
- Response rate: number of patients whose OAB symptoms improved/total participants.
- Adverse events (AEs).
Two reviewers (J-JL and J-WH) independently screened the titles and abstracts of the studies identified in the search, assessed the criteria for study selection, and recorded their decisions based on predefined criteria. Another reviewer (J-IK) resolved any disagreements in the study selection. The study selection process was documented and summarized in a PRISMA flow diagram. The process is presented in Figure 1.
All articles were read by two independent reviewers (J-JL and J-WH), who extracted data from the articles according to predefined criteria. The extracted data included the author's name(s), year of publication, sample size, age, sex, OAB duration, AT intervention, control intervention, main outcomes, adverse effects, and authors' conclusion. For the extraction of intervention-related information, the revised Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA) items were used to describe the details of AT treatments used in each study (12). When the reported data were insufficient or unclear, the author contacted the first author or corresponding authors by e-mail or telephone to request missing data or clarify data.
Two authors (J-JL and J-WH) independently extracted the data from the included trials. The Cochrane risk of bias tool (13) was used to assess the internal validity of each study. The following characteristics were assessed: (1) random sequence generation, (2) allocation concealment, (3) the blinding of participants and personnel, (4) the blinding of outcome assessment, (5) incomplete outcome data, (6) selective outcome reporting, and (7) other sources of bias (we evaluated baseline imbalance). This review uses “L, U, and H” as keys for these evaluations, where “L” (low) indicates a low risk of bias, “U” (unclear) indicates that the risk of bias is unclear, and “H” (high) indicates a high risk of bias. Disagreements were resolved by discussion among all authors. Information regarding the risk of bias assessment for the included studies is presented in a table, and the results and implications are critically discussed.
We used the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) system to evaluate the level of evidence (14). We initially gave four points to each outcome in each RCT and then lowered the total scores for defects in bias risks, inconsistency, indirectness, inaccuracy, and publication bias. Bias risks included erroneous randomization methods, absence of allocation concealment, insufficient blinding, and excessive data loss to follow-up. The inconsistencies mainly involved different interventions or evaluation techniques. Indirectness principally included two categories: (1) lack of direct comparison between the two groups and (2) outcome measure sensitivity to direct evaluation of efficacy. Inaccuracy was mainly evaluated based on the width of the confidence interval (CI). Publication bias was related to an unpublished study (usually containing negative results) by the investigator. The quality of evidence was categorized as high, moderate, low, or very low quality.
All statistical analyses were conducted by the Cochrane Collaboration's software Review Manager (RevMan) v.5.4.1 for Windows (The Nordic Cochrane Center, Copenhagen, Denmark). Differences between the intervention and control groups were assessed. In the analysis of clinical efficacy, dichotomous data were assessed in terms of risk ratios (RRs), and continuous data were assessed in terms of mean differences (MDs). Dichotomous and continuous variables were expressed as efficacy values with 95% confidence intervals (CIs). In cases of outcome variables assessed using different scales, the standardized MD (SMD) was used instead of the weighted MD (WMD). If heterogeneity was detected (defined by heterogeneity tests with a chi-square test of p < 0.1 and Higgins of I2 ≥ 50%), subgroup analyses were performed to determine the cause of clinical heterogeneity. A random-effects model was used to assess combined effect sizes from efficacy variables, and substantial clinical heterogeneity was expected across the included studies based on diversity among the interventions, study designs, and other conditions. An albatross plot showing the effects of direction and size range by p-value and the given sample size was generated for each included study. Publication bias was assessed using funnel plots if more than 10 studies were available (15).
A total of 1,110 studies were identified in the search of 12 databases. A total of four studies were added based on other identified sources. A total of 344 articles were eliminated due to duplication. A total of 770 studies were screened by reading the titles and abstracts, and 63 articles remained. Notably, 33 articles were excluded due to the reasons described in Figure 1 after the full texts of the 63 articles were read. Finally, 30 studies (16–45) met the inclusion criteria, and a meta-analysis was conducted. The PRISMA flowchart of the search process is shown in Figure 1.
A total of 25 studies were conducted in China (19–28, 30–42, 44, 45), two in the United Kingdom (29, 43), one in the United States (16), one in Turkey (18), and one in Hong Kong (17). The main characteristics of the 30 included studies are presented in Table 1. Seven studies compared AT with sham AT (16–22), and 13 studies compared AT with drug therapy (18, 20, 23–33). Two of the studies had two control groups: sham AT and drug therapy (18, 20). One study had two intervention groups: AT and AT plus drug therapy (25). Ten studies used AT plus drug therapy as an intervention and drug therapy alone as a control (25, 34–42). Three studies compared AT plus standard care with standard care alone (43–45).
Rationales for using AT were reported in 97% of studies. These studies were mainly based on traditional Chinese medicine (TCM) theory (16–29, 31, 33–36, 38–45).
All studies reported acupoints, and the more frequently used acupoints included SP6 (17 studies) (16–19, 24–29, 31, 34, 35, 39, 41, 43, 44), BL23 (17 studies) (17, 19, 23–25, 27–29, 31, 35, 38–42, 44, 45), BL28 (15 studies) (16, 17, 23–25, 27–29, 31, 35, 38, 39, 42, 44, 45), CV4 (15 studies) (16–18, 23, 25–27, 29, 31, 34, 35, 39, 40, 43, 44), BL32 (15 studies) (17, 19, 21, 23–25, 27, 31–33, 35, 39, 41, 44, 45), and CV3 (11 studies) (19, 23, 25, 27, 29, 31, 34, 35, 39, 43, 44).
In total, 19 studies selected the “de qi” response (16, 18, 21, 22, 26, 31, 33–36, 38–40, 42, 43). All studies reported needle stimulation methods. The most frequently used retention time was 30 min (17 studies) (17, 21–24, 31, 33, 35, 37, 38, 40–44). The treatment period varied from 1 to 12 weeks, and the largest number of studies was conducted over 4 weeks (16, 18, 22–24, 26, 27, 30, 38, 42). The details of the STRICTA domains are shown in Supplementary material 2.
The risk of bias in these studies is presented in Figure 2. A total of 14 studies had a low risk of bias regarding random sequence generation (16–21, 26, 29, 30, 34, 37, 39, 40, 43), whereas 16 studies did not provide detailed information about their random generation method (22–25, 27, 28, 31–33, 35, 36, 38, 41, 42, 44, 45). Notably, five studies reported the allocation concealment method (16, 17, 20, 26, 43), whereas the other 25 studies did not mention the allocation concealment method (18, 19, 21–25, 27–42, 44, 45). A total of seven studies had a low risk of performance bias given that these studies selected AT as the intervention and sham AT as the control (16–22). The remaining 23 studies had a high risk of performance bias. Given the distinct differences between the intervention and control groups, patients or clinicians could not be blinded (18, 23–45). In five studies, blinding was performed by the investigators (16, 17, 21, 26, 29). In one study, blinding was not performed, but it was judged to not affect the evaluation of results given that every participant wrote their symptom scores by themselves (34). In one study, the practitioner and outcome investigators were the same people given the lack of resources (43). The other 23 studies did not report the blinding of the outcome assessor (18–20, 22–25, 27, 28, 30–33, 35–42, 44, 45). Twenty-six studies had a low risk of attrition bias given that 19 of these studies had no dropout rate (19, 22–25, 27, 28, 31, 33, 34, 36–42, 44, 45); six had a low dropout rate, which is small enough to not affect the results (17, 20, 21, 30, 32, 35); and one had moderate dropout, but the reasons were similar in both the intervention and control groups (16). Four studies had high dropout rates (18, 26, 29, 43). Two studies were conducted in accordance with their protocols (17, 26), whereas the other 28 studies did not report the protocol or disclose all prespecified and expected results (16, 18–25, 27–45). Three studies reported adequate details to eliminate various biases (16, 17, 26), whereas the other 27 studies did not report sufficient information (18–25, 27–45).
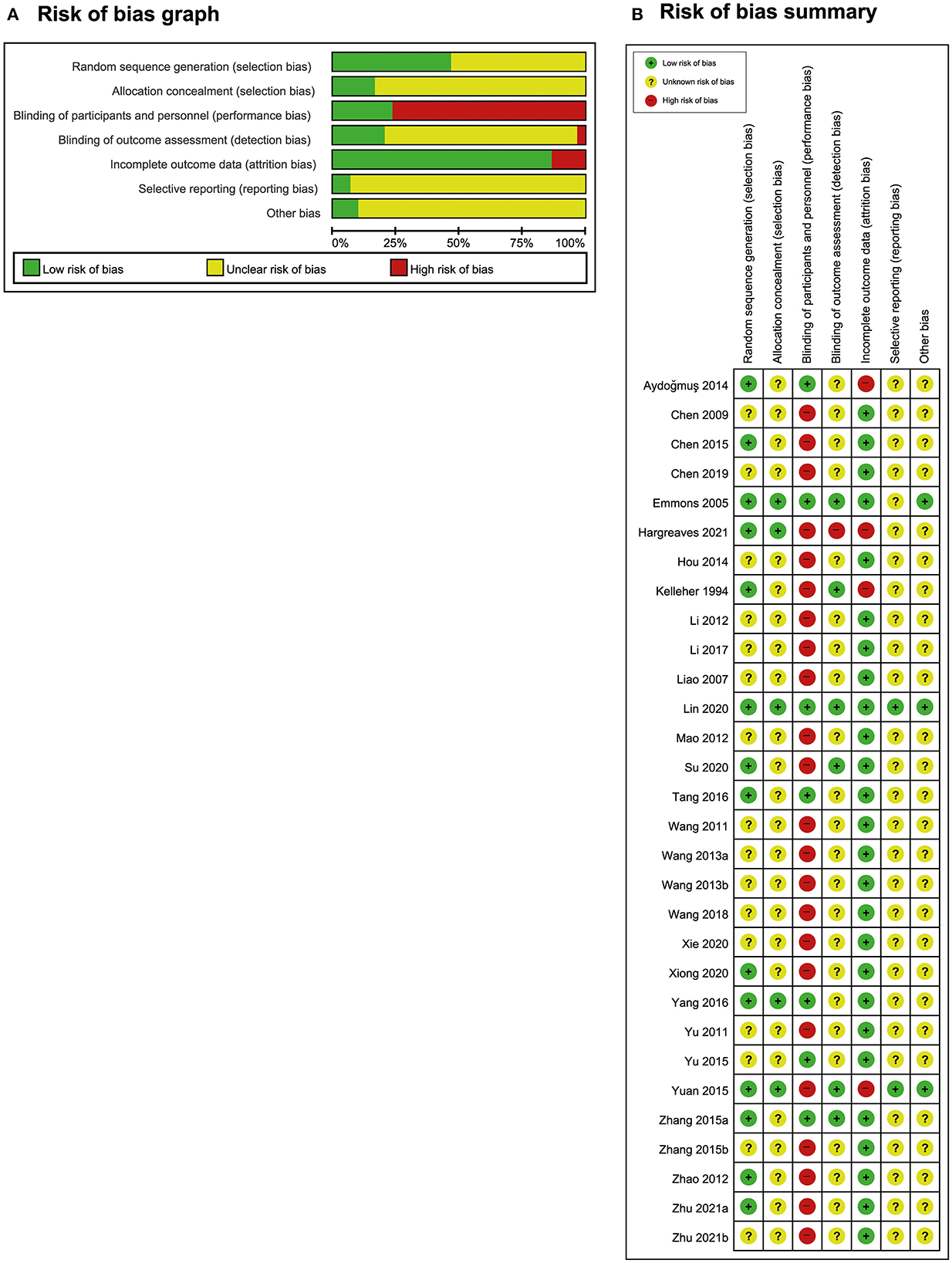
Figure 2. Risk of bias. (A) Risk-of-bias graph and (B) risk-of-bias summary: The present authors' judgments regarding the risk of each form of bias in all included studies.
Seven RCTs compared the effects of AT with sham AT (16–22). Three RCTs reported OABSS. In one study, AT exhibited an effect equivalent to sham AT (17), whereas the other two studies showed favorable effects of AT on reducing OABSS compared with sham AT (19, 21). Meta-analysis revealed that AT showed a more favorable effect than sham AT, and heterogeneity was high (MD: −1.13, 95% CI: −2.01 to −0.26, p = 0.01, I2 = 67, Figure 3A).
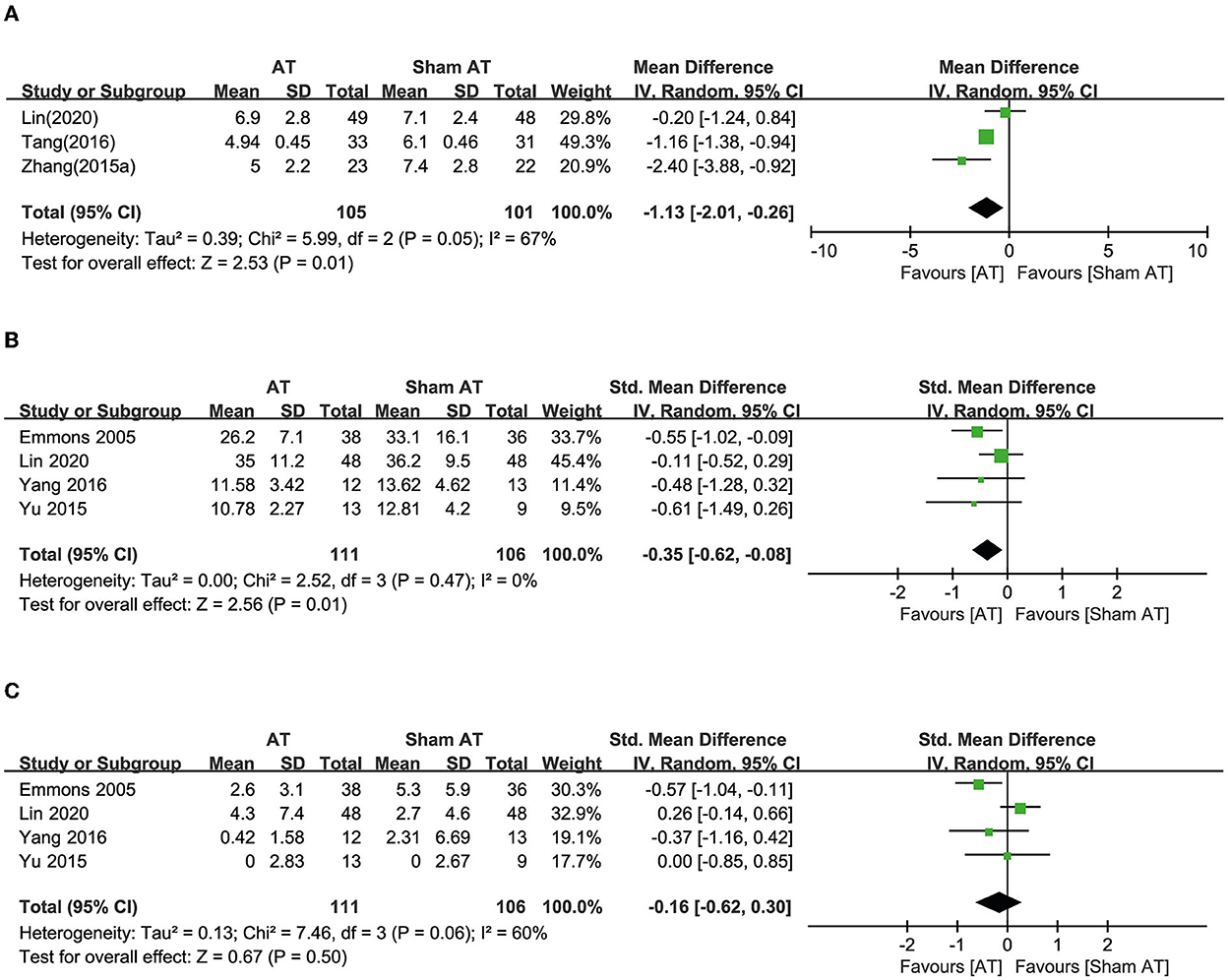
Figure 3. Forest plot of (A) OABSS, (B) frequency, and (C) incontinence according to the comparison of AT vs. sham AT.
Four RCTs reported urinary frequency, and one of these studies showed a favorable effect of AT compared with sham AT (16). The other three RCTs showed equivalent effects (17, 20, 22). Meta-analysis revealed that AT had more favorable effects than sham AT on reducing urinary frequency (SMD: −0.35, 95% CI: −0.62 to −0.08, p = 0.01, I2 = 0%, Figure 3B).
Four RCTs reported urinary incontinence. One showed a favorable effect of AT compared with sham AT (16), but three showed equivalent effects (17, 20, 22). Meta-analysis revealed that AT exhibited effects equivalent to sham AT on reducing urinary incontinence (SMD: −0.16, 95% CI: −0.62 to 0.30, p = 0.50, I2 = 60%, Figure 3C).
One RCT reported the response rate and showed a favorable effect of AT compared with sham AT (RR: 3.76, 95% CI: 1.78 to 7.94, p = 0.0005) (19).
Thirteen RCTs used anticholinergic conventional drug therapy as a control (18, 20, 23–33). Four RCTs reported OABSS. Two showed equivalent effects of AT (23, 25), and one showed a favorable effect of AT (32). In contrast, the other study reported a favorable effect of drug therapy (30). A meta-analysis demonstrated equivalent effects of AT with drug therapy on reducing OABSS (MD: −0.39, 95% CI: −1.92 to 1.13, p = 0.61, I2 = 94%, Figure 4A).
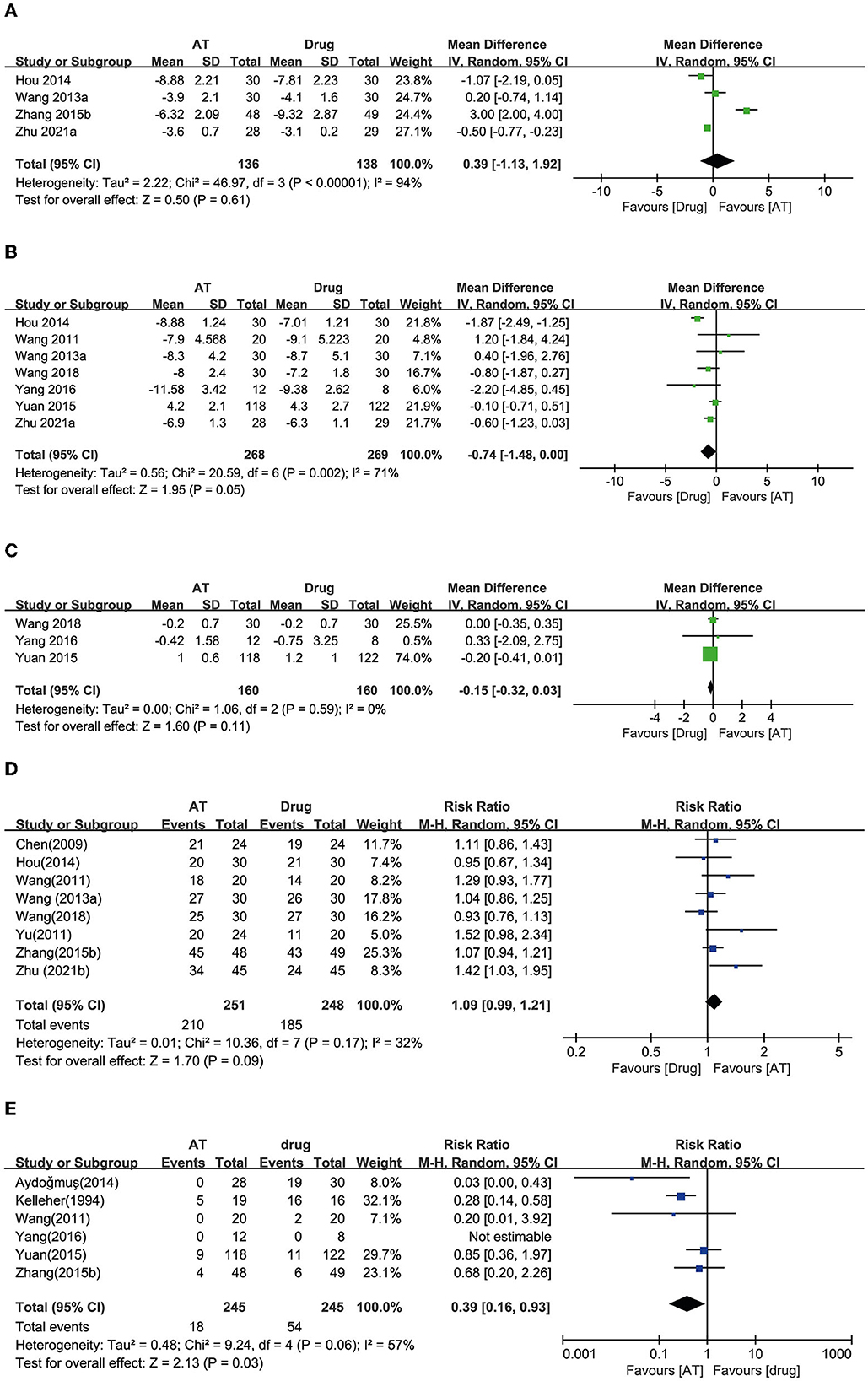
Figure 4. Forest plot of (A) OABSS, (B) frequency, (C) incontinence, (D) response rate, and (E) adverse effects according to the comparison of AT vs. drug therapy.
Seven RCTs reported urinary frequency, and six of these studies showed equivalent effects of AT with drug therapy on reducing urinary frequency (20, 23, 24, 26, 27, 30). The other reported a favorable effect of drug therapy (25). Meta-analysis revealed that AT exhibited effects equivalent to drug therapy on reducing urinary frequency (MD: 0.74, 95% CI: −0.00 to 1.48, p = 0.05, I2 = 71%, Figure 4B).
Three RCTs reported the number of daily incontinence episodes, and all of them showed that AT had equivalent effects to drug therapy (20, 24, 26). Through the meta-analysis, AT had equivalent effects with drug therapy on reducing urinary incontinence (MD: 0.15, 95% CI: −0.03 to 0.32, p = 0.11, I2 = 0%, Figure 4C).
Eight RCTs reported the response rate, and seven of them showed equivalent effects of AT and drug therapy (23–25, 27, 28, 32, 33). One RCT showed a favorable effect of AT compared with drug therapy (31). Through the meta-analysis, AT showed equivalent effects as drug therapy on the response rate (RR: 1.09, 95% CI: 0.99 to 1.21, p = 0.09, I2 = 32%, Figure 4D).
Six studies reported the AEs of participants, and three of the studies showed an incidence of AT equivalent to that of drug therapy (26, 27, 32). Two RCTs reported a significantly lower incidence of side effects with AT compared with drug therapy (18, 29). AEs could not be estimated in one RCT because it reported that both groups experienced no AEs (20). Meta-analysis revealed that AT showed a significantly lower incidence of AEs compared with drug therapy (RR: 0.39, 95% CI: 0.16 to 0.93, p = 0.03, I2 = 57%, Figure 4E).
A total of 10 RCTs used the combination of AT and drug therapy as an intervention, and the same anticholinergic conventional drug therapies served as a control (25, 34–42). Five RCTs reported OABSS, and all of them showed favorable effects of AT plus drug therapy for reducing OABSS compared with drug therapy alone (25, 34, 35, 37, 38). The meta-analysis revealed that the combination of AT and drug therapy showed favorable effects compared with drug therapy alone (MD: −2.28, 95% CI: −3.25 to −1.31, p < 0.00001, I2 = 84%, Figure 5A).
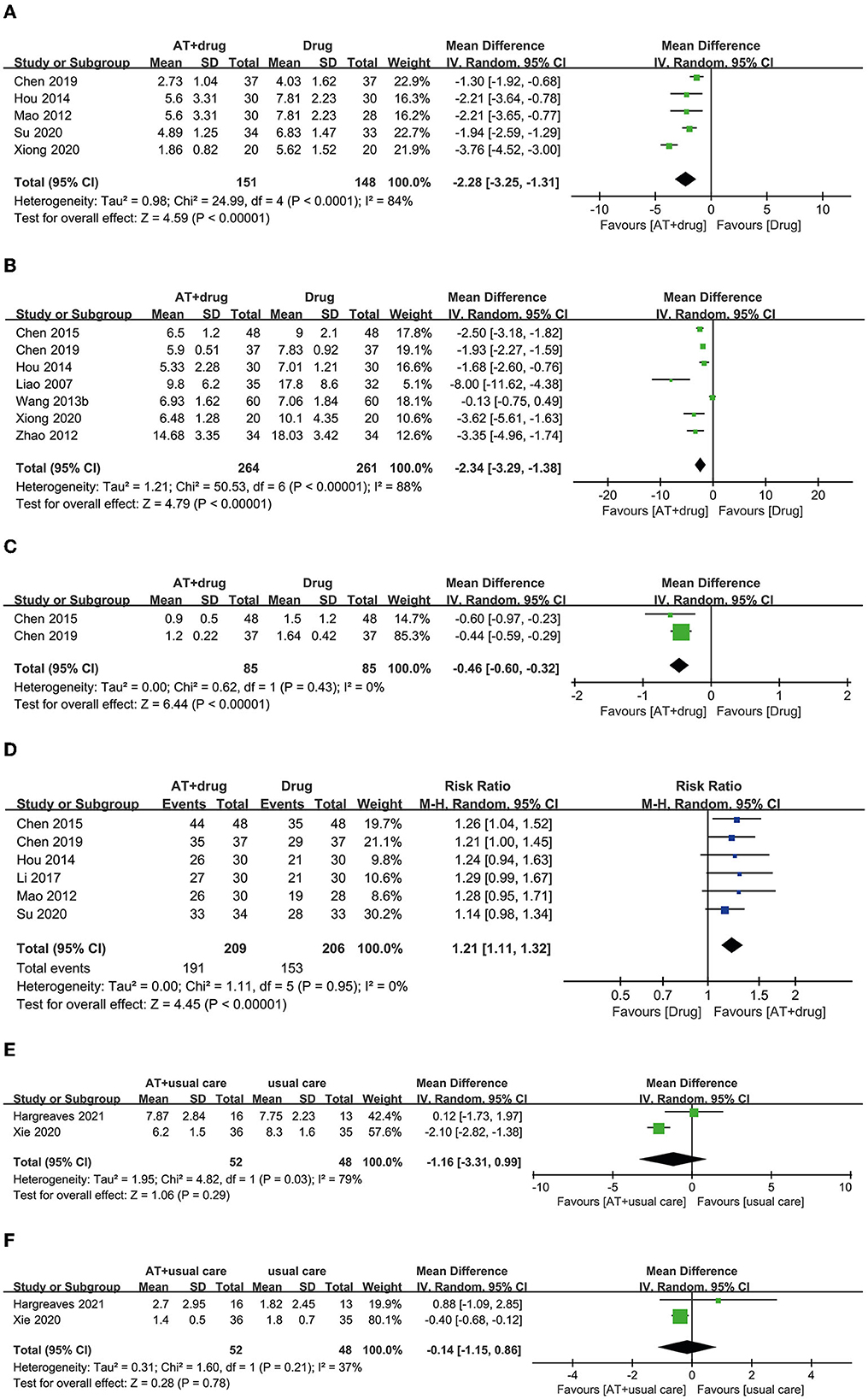
Figure 5. Forest plot of (A) OABSS, (B) frequency, (C) incontinence, and (D) response rate according to the comparison of AT plus drug therapy vs. drug therapy. (E) Frequency, (F) incontinence according to the comparison of AT plus usual care vs. usual care.
Seven RCTs reported urinary frequency, and six of them showed favorable effects of AT plus drug therapy for reducing urinary frequency compared with drug therapy alone (25, 37–40, 42). In one RCT, the combination of AT and drug therapy had an equivalent effect on drug therapy alone (41). Meta-analysis revealed that AT plus drug therapy had favorable effects on reducing urinary frequency compared with drug therapy alone (MD: −2.34, 95% CI: −3.29 to −1.38, p < 0.00001, I2 = 88%, Figure 5B).
Two RCTs reported the number of patients experiencing urinary incontinence, and both RCTs showed more favorable effects of AT plus drug therapy for reducing urinary incontinence than drug therapy alone (38, 40). A meta-analysis also revealed favorable effects of the combination of AT and drug therapy over drug therapy alone (MD: −0.46, 95% CI: −0.60 to −0.32, p < 0.00001, I2 = 0%, Figure 5C).
Six RCTs reported the response rate, and four RCTs showed equivalent effects for the combination of AT plus drug therapy and drug therapy alone (25, 34–36). However, two RCTs showed that AT combined with drug therapy had more favorable effects on the response rate than drug therapy alone (38, 40). Meta-analysis revealed that the combination of AT and drug therapy had a more favorable effect on the response rate than drug therapy alone (RR: 1.21, 95% CI: 1.11 to 1.33, p < 0.00001, I2 = 0%, Figure 5D).
Three studies selected AT plus usual care as an intervention and usual care as a control (43–45). Two RCTs reported urinary frequency. One of these RCTs showed equivalent effects (43). In contrast, the other showed a more favorable effect of AT plus usual care compared with usual care alone (44). Meta-analysis revealed equivalent effects of AT plus usual care and usual care alone for reducing urinary frequency (MD: −1.16, 95% CI: −3.31 to 0.99, p = 0.29, I2 = 79%, Figure 5E).
Two RCTs reported urinary incontinence. One RCT showed an equivalent effect (43), whereas the other showed a more favorable effect of AT plus usual care than usual care alone (44). The meta-analysis revealed equivalent effects of AT plus usual care and usual care alone for reducing urinary incontinence (MD: −0.14, 95% CI: −1.15 to 0.86, p = 0.78, I2 = 37%, Figure 5F).
Only one RCT reported the response rate, and a more favorable effect of AT plus usual care was noted compared with usual care alone (44).
A total of 15 studies did not report AEs (19, 23–25, 28, 30, 31, 33, 36–38, 41, 42, 44, 45). In seven studies, AEs of AT did not occur (18, 20, 27, 34, 35, 39, 40). Eight studies reported minor AEs, such as needling pain, bruising, and bleeding. However, no severe AEs were reported (16, 17, 21, 22, 26, 29, 32, 43).
For the continuous outcomes, including pain, function, and QoL, most points were scattered and accumulated on the right side of the plot with many points clustered around the null line, failing to show specific effects of AT on these outcomes (Figures 6A, B). For the total effective rate, the points were scattered across the contour lines (Figure 6C). All the points were clustered on the positive association side of the plot, indicating that ginseng is favorable for the management of OAB by AT.
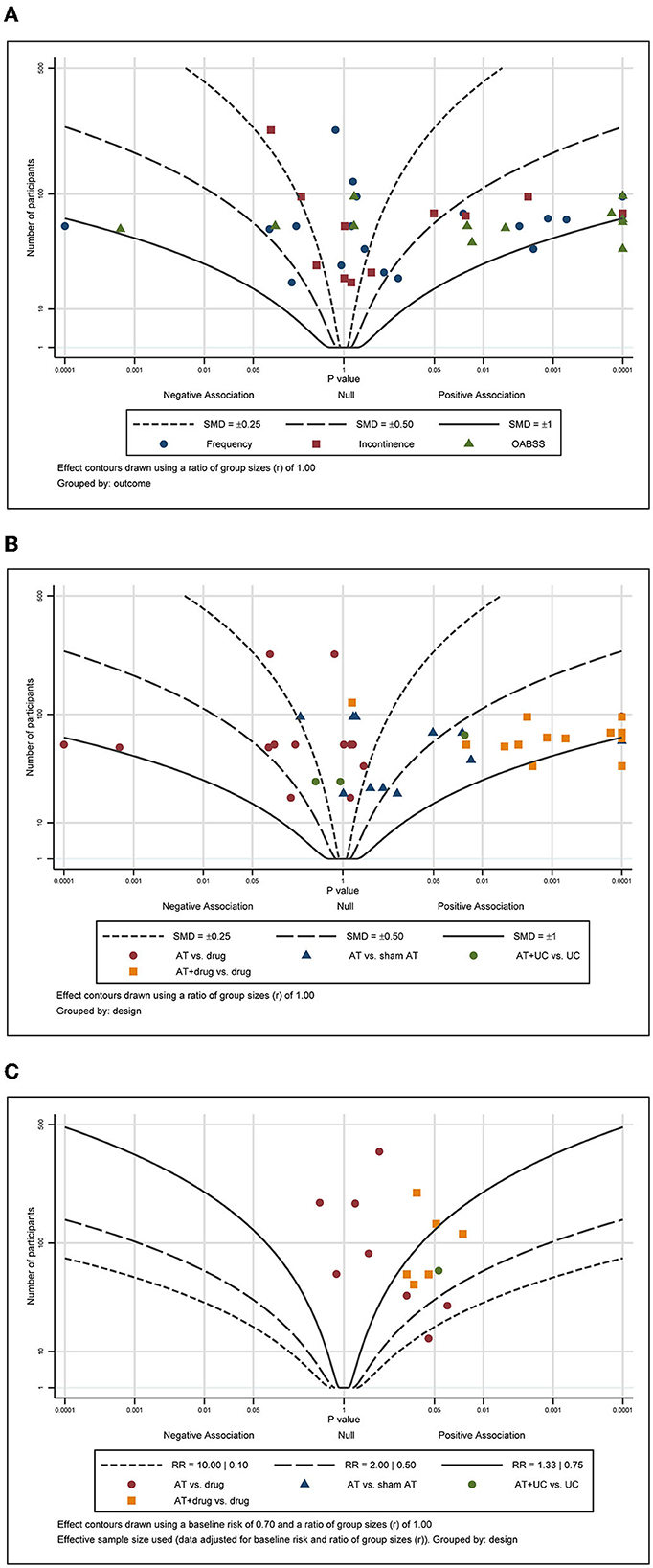
Figure 6. Albatross plot of (A) frequency, (B) incontinence, and (C) response rate according to the comparison of MA plus standard care vs. standard care.
The certainty of evidence (CoE) was assessed using the GRADEpro program, and a summary of the findings, including studies with low or very low CoE, is shown in Table 2.
In this review, the advantages and possibilities of the use of AT for the treatment of OAB were identified. In the AT vs. sham AT comparison, significant effects in reducing OABSS and improving the response rate were noted for AT with very low certainty of evidence (CoE). Moreover, AT had a more favorable effect on reducing urinary frequency than sham AT with a low CoE. No significant differences in reducing urinary incontinence with a very low CoE were noted. However, AT exhibited effects equivalent to anticholinergic conventional drug therapy for reducing OABSS, urinary frequency, and urinary incontinence with a very low CoE. AT also had an equivalent effect as drug therapy for enhancing the response rate with a low CoE. Furthermore, the incidence of adverse effects was significantly lower with ATs compared with drug therapy with a low CoE. The combination of AT with drug therapy had a more favorable effect on reducing OABSS than drug therapy alone with a very low CoE. For reducing urinary frequency and incontinence, the combination of AT with drug therapy had a more favorable effect than drug therapy alone with a low CoE. Moreover, the combination of AT with drug therapy had a more favorable effect on enhancing the response rate than drug therapy alone with a low CoE. In the comparison of AT plus usual care with usual care, the AT plus usual care exhibited equivalent effects on reducing urinary frequency and incontinence with a very low CoE. However, regarding the response rate, the combination of AT and usual care had a more favorable effect with a very low CoE.
Our review aimed to evaluate and complete the evidence from recent RCTs of AT for the treatment of patients with OAB. Compared with two previous systematic reviews (46, 47), we identified 18 new RCTs (17, 18, 24, 27–31, 33–39, 43–45) and successfully assessed the evidence for therapy. The results of our review are different from those of the two previously published reviews. One previous review (47) showed that AT may be beneficial for reducing micturition, incontinence, and nocturia episodes, whereas the other review (46) failed to report favorable effects of AT for reducing symptoms of OAB compared with several types of controls. When we examined the results of AT for OAB symptoms, our results showed beneficial effects of AT compared with sham AT. However, one review did not show significant effects of AT compared with sham AT (47). Only two RCTs were included in the meta-analysis of electroacupuncture (EA) vs. sham EA, and EA had no favorable effect on reducing urinary frequency, urgency, or incontinence compared with sham EA. EA showed a favorable effect on decreasing nocturia. Another study (46) performed a meta-analysis of AT vs. sham AT on reducing OABSS based on two RCTs and did not show a significant effect. Instituting an appropriate sham AT condition is always a difficult factor in designing a study to determine the effects of AT. OAB affects the psychology of an individual, and sham AT for OAB was previously reported to produce a placebo effect in ~33–56% of participants (48). Emmons and Otto postulated a 40% placebo effect and a 59% treatment effect and explained that larger RCTs are needed to statistically show the effects of AT (16). Thus, it seems that the effect of sham AT, which has not been identified in previous reviews, appeared in this review, which includes more studies. However, since the size of the effect is not large, more studies are needed to collect the data. More consideration is needed to establish an appropriate sham AT to demonstrate the effect of AT appropriately.
Based on our assessment, the risk of bias is high in each of the included studies, potentially leading to false positives. Regarding performance bias, 23 studies had a high risk of bias based on the difference between intervention and control as well as AT treatment or drug administration. Although AT has to penetrate the skin and requires time for retention, medication is taken orally as prescribed. Therefore, blinding is difficult because patients can easily distinguish whether they are receiving AT or taking medications. Of the RCTs included in this study, there were no studies using AT and sham AT or drug and placebo drugs interchangeably. Moreover, AT could not be blinded to the performer (18, 23–45). Additional independent studies in different countries are required to determine the generalizability of these results given that 26 studies were conducted in China (17, 19–28, 30–42, 44, 45).
This review has some limitations. First, many included RCTs had an unclear risk of bias given that these studies did not report particular details. Second, despite the large number of RCTs, the outcomes were very diverse, so a large-scale meta-analysis could not be performed. Therefore, the level of evidence is mostly low or very low. Third, the frequency of AT intervention ranged from 2 per day to < 1 per week. Fourth, the suitable design of a sham AT condition remains a difficult problem.
Future studies on OAB treatment with AT should report their study design in more detail to obtain a high level of evidence. Moreover, if OABSS, response rate, adverse effects, and quality of life scores are commonly reported in these studies, we can obtain results with a larger population, definitively demonstrate the advantages of AT, and achieve a higher level of consensus. Studies should also be performed that could inform AT guidelines regarding acupoints, retention times, frequency, and treatment period for the application of AT for OAB in the clinical field.
In conclusion, AT had more favorable effects than sham AT in reducing OAB symptoms. AT showed equivalent effects as anticholinergic drugs with fewer AEs. The combination of AT and drug therapy had more favorable effects on OAB than drug therapy alone. However, the level of evidence is low due to the high risk of bias and the small sample size. Future well-designed research is needed to obtain a higher level of evidence to apply AT for the treatment of OAB.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
J-JL and J-IK: conceptualization. J-JL and J-WH: data curation and resources. J-JL, J-WH, MSL, and J-IK: formal analysis and writing—original draft. J-JL, J-WH, JHJ, and T-YC: investigation. J-JL, J-WH, and MSL: methodology. MSL and J-IK: project administration and supervision. J-WH and J-IK: software. JHJ and T-YC: writing—review and editing. All authors have read and approved the final manuscript.
T-YC, JHJ, and MSL were supported by the Korea Institute of Oriental Medicine, Korea (KSN2022210 and KSN2022240). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.985288/full#supplementary-material
1. Haylen BT, Ridder DD, Freeman RM, Swift SE, Berghmans B, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. (2010) 29:4–20. doi: 10.1002/nau.20798
2. Hashim H, Abrams P. Is the bladder a reliable witness for predicting detrusor overactivity? J Urol. (2006) 175:191–4. doi: 10.1016/S0022-5347(05)00067-4
3. Hutchinson A, Nesbitt A, Joshi A, Clubb A, Perera M. Overactive bladder syndrome: management and treatment options. Aust J Gen Pract. (2020) 49:593–8. doi: 10.31128/AJGP-11-19-5142
4. Corcos J, Przydacz M, Campeau LJW, Hickling D, Honeine C, Radomski SB, et al. CUA guideline on adult overactive bladder. Can Urol Assoc J. (2017) 11:E142–73. doi: 10.5489/cuaj.4586
5. Chapple CR, Khullar V, Gabriel Z, Muston D, Bitoun CE, Weinstein D. The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis. Eur Urol. (2008) 54:543–62. doi: 10.1016/j.eururo.2008.06.047
6. Wibisono E, Rahardjo HE. Effectiveness of short term percutaneous tibial nerve stimulation for non-neurogenic overactive bladder syndrome in adults: a meta-analysis. Acta Med Indones. (2015) 47:188–200.
7. Minni B, Capozza N, Creti G, De Gennaro M, Caione P, Bischko J. Bladder instability and enuresis treated by acupuncture and electro-therapeutics: early urodynamic observations. Acupunct Electrother Res. (1990) 15:19–25. doi: 10.3727/036012990816358333
8. Burnstock G. Purinergic signalling in the lower urinary tract. Acta Physiol. (2013) 207:40–52. doi: 10.1111/apha.12012
9. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007
10. Birch S, Lee MS, Kim T-H, Alraek T. On defining acupuncture and its techniques: a commentary on the problem of sham. Integr Med Res. (2022) 11:100834. doi: 10.1016/j.imr.2022.100834
11. Birch S, Lee MS, Kim T-H, Alraek T. Historical perspectives on using sham acupuncture in acupuncture clinical trials. Integr Med Res. (2022) 11:100725. doi: 10.1016/j.imr.2021.100725
12. MacPherson H, Altman DG, Hammerschlag R, Youping L, Taixiang W, White A, et al. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT statement. PLoS Med. (2010) 7:e1000261. doi: 10.1371/journal.pmed.1000261
13. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
14. Liu J-P. GRADE methods in traditional medicine. Integr Med Res. (2022) 11:100836. doi: 10.1016/j.imr.2022.100836
15. Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
16. Emmons SL, Otto L. Acupuncture for overactive bladder: a randomized controlled trial. Obstetr Gynecol. (2005) 106:138–43. doi: 10.1097/01.AOG.0000163258.57895.ec
17. Lin ZX, Chan NHT, Kwan YK, Chan YT, Zhang H, Tam KYS, et al. A randomized controlled trial to assess the effectiveness and safety of acupuncture for overactive bladder: a study in Hong Kong population. Chin Med. (2020) 15:108. doi: 10.1186/s13020-020-00388-w
18. Aydogmus Y, Sunay M, Arslan H, Aydn A, Adiloglu AK, Sahin H. Acupuncture versus solifenacin for treatment of overactive bladder and its correlation with urine nerve growth factor levels: a randomized, placebo-controlled clinical trial. Urol Int. (2014) 93:437–43. doi: 10.1159/000358202
19. Tang KM, Ming SR, Feng QV, Jiang F, Chen YL. A clinical research of the overactive bladder treatment using acupuncture combined with a radiation of the needling sensation. J Shanghai Univ Trad Chin Med. (2016) 30:35–7,51. doi: 10.16306/j.1008-861x.2016.02.009
20. Yang WY. Electro-Acupuncture Treatment of Overactive Bladder With Baliao and Huiyang (Master dissertation). Beijing: Beijing University of Chinese Medicine (2016).
21. Zhang J, Cheng W, Cai M. Effects of electroacupuncture on overactive bladder refractory to anticholinergics: a single-blind randomised controlled trial. Acupunct Med. (2015) 33:368–74. doi: 10.1136/acupmed-2015-010770
22. Yu HZ. Clinical Exploration of Electroacupuncture in the Treatment of Hypermobility of the Upper Wrist (Master dissertation). Beijing University of Chinese Medicine, Cnki (2015).
23. Wang B, Xiao YP, Fan K, Huang CJ. Treatment of female overactive bladder with acupuncture. J Beijing Univ Trad Chin Med. (2013) 36:713–6. doi: 10.3969/j.issn.1006-2157.2013.10.014
24. Wang QK, Lin ML. Comparison of the effectiveness of acupuncture and solifenacin alone in the treatment of overactive bladder. Chin Health Care Nutr. (2018) 28:142. doi: 10.3969/j.issn.1004-7484.2018.25.205
25. Hou CH, Gao WX, Hu SW. Acupuncture combined M receptor antagonist optimization overactive bladder clinical research. Hubei J Trad Chin Med. (2014) 36:40–1.
26. Yuan Z, He C, Yan S, Huang D, Wang H, Tang W. Acupuncture for overactive bladder in female adult: a randomized controlled trial. World J Urol. (2015) 33:1303–8. doi: 10.1007/s00345-014-1440-0
27. Wang XL, Shi YS. Clinical study on acupuncture treatment of overactive bladder (kidney-qi deficiency type) in elderly women. Guide China Med. (2011) 9:176–7. doi: 10.3969/j.issn.1671-8194.2011.32.137
28. Yu XZ, Wang JQ. 24 cases of overactive bladder treated by acupuncture. Shandong J Trad Chin Med. (2011) 30:245–6. doi: 10.16295/j.cnki.0257-358x.2011.04.039
29. Kelleher CJ, Filshie J, Burton G, Khullar V, Cardozo LD. Acupuncture and the treatment of irritative bladder symptoms. Acupunct Med. (1994) 12:9–12. doi: 10.1136/aim.12.1.9
30. Zhu WJ, Zhang Q, Sheng DY, Peng Y. Clinical study on awn needle combined with low frequency electrical stimulation of the pudendal nerve in the treatment of overactive bladder. J Pract Trad Chin Med. (2021) 37:1273–5.
31. Zhu ZB, Bi QL. Analysis of the value of acupuncture in the treatment of frequent urination. Clin Res. (2021) 29:113–4.
32. Zhang YJ, Qin YQ, Xue GH, Zhang RJ, Zhuo XW. A clinical research of the overactive bladder treatment using acupuncture at baliao point combined with interference electron input. Chin Health Care Nutr. (2015) 25:46–7.
33. Chen ZL, Han C, Wang GW. 24 cases of overactive bladder treated by electroacupuncture. J Clin Acupunct Moxibust. (2009) 25:22–22. doi: 10.3969/j.issn.1005-0779.2009.08.016
34. Su YY, Zhao D, Lin MQ. Clinical observation of urine three-needling combined with tolterodine in treating overactive bladder. J Guangzhou Univ Tradi Chin Med. (2020) 37:1495–500. doi: 10.13359/j.cnki.gzxbtcm.2020.08.016
35. Mao HH. Acupuncture Combined M Receptor Antagonist Optimization Overactive Bladder Clinical Research (Master dissertation). Hubei: Hubei University of Chinese Medicine (2012).
36. Li XL, Liu XH, Zhao GJ Li Y, Cao DN Li A, Xu YJ, Liang J, et al. Influence of Yuanluo Tongjing acupuncture on urinary frequency in middle-aged and elderly men. Chin J Gerontol. (2017) 37:6167–9. doi: 10.3969/j.issn.1005-9202.2017.24.070
37. Xiong C, Tang Y, Shi RT, Zhou YH, Sun SD, Xiang YL, et al. Therapeutic effect of myofascial trigger point electroacupuncture technology on the treatment of overactive bladder syndrome in female. J Central South Univ. (2020) 45:155–9. doi: 10.11817/j.issn.1672-7347.2020.180790
38. Chen SL, Zhao SL, Chen QX. Effect of combined acupuncture and medicine on postmenopausal overactive bladder and urine NGF levels. Shanghai J Acu-mox. (2019) 38:1150–3. doi: 10.13460/j.issn.1005-0957.2019.10.1150
39. Zhao L. Acusector Overactive Bladder in 68 Cases of Disease Clinical Observation (Master dissertation). Henan: Henan University of Traditional Chinese Medicine (2012).
40. Chen X, Chen J, Tang SL. Efficacy observation of electroacupuncture combined with tolterodine in the treatment of 48 cases of female overactive bladder. Hainan Med J. (2015) 127–8. doi: 10.3969/j.issn.1003-6350.2015.01.0043
41. Wang YF, Pan ZL, Chen JJ, Zhang W, Meng L. Clinical study of overactive bladder treated by electric needle therapy. Pract Clin J Integrat Trad Chin West Med. (2013) 13:1–3. doi: 10.3969/j.issn.1671-4040.2013.03.001
42. Liao XQ, Tang H, Xiao P. Electroacupuncture at Zhongjiao point for the treatment of 35 cases of bladder hyperactivity syndrome. J Clin Acupunct Moxibust. (2007) 23:35–6. doi: 10.3969/j.issn.1005-0779.2007.01.023
43. Hargreaves E, Harding C, Clarkson C. Acupuncture in addition to standard conservative treatment for overactive bladder; a feasibility trial for a randomized controlled study. Neurourol Urodyn. (2021) 40:1770–9. doi: 10.1002/nau.24741
44. Xie N, Yang Y. Clinical observation of applying acupuncture and nursing intervention in the treatment of overactive bladder. J Sichuan Trad Chin Med. (2020) 38:202–5.
45. Li ZH, Ren MY, Wang J. Clinical research of acupuncture therapy and nursing intervention for the treatment of female overactive bladder syndrome. J Nurses Train. (2012) 27:609–10. doi: 10.3969/j.issn.1002-6975.2012.07.016
46. Mak TC, Chen HY, Cho WC. Acupuncture for overactive bladder in adults: a systematic review and meta-analysis. Acupunct Med. (2019) 37:321–31. doi: 10.1136/acupmed-2017-011528
47. Zhao Y, Zhou J, Mo Q, Wang Y, Yu J, Liu Z. Acupuncture for adults with overactive bladder: a systematic review and meta-analysis of randomized controlled trials. Medicine. (2018) 97:e13437. doi: 10.1097/MD.0000000000013437
Keywords: acupuncture, overactive bladder, systematic review, meta-analysis, grade
Citation: Lee J-J, Heo J-W, Choi T-Y, Jun JH, Lee MS and Kim J-I (2023) Acupuncture for the treatment of overactive bladder: A systematic review and meta-analysis. Front. Neurol. 13:985288. doi: 10.3389/fneur.2022.985288
Received: 03 July 2022; Accepted: 16 December 2022;
Published: 12 January 2023.
Edited by:
Zhaoxiang Bian, Hong Kong Baptist University, Hong Kong SAR, ChinaReviewed by:
Hai-Yan Yin, Chengdu University of Traditional Chinese Medicine, ChinaCopyright © 2023 Lee, Heo, Choi, Jun, Lee and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Myeong Soo Lee, 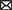 ZHJtc2xlZUBnbWFpbC5jb20=; Jong-In Kim,
ZHJtc2xlZUBnbWFpbC5jb20=; Jong-In Kim, 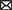 aGFubjg0MDBAaGFubWFpbC5uZXQ=
aGFubjg0MDBAaGFubWFpbC5uZXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.