- 1Department of Neurosurgery and Brain and Nerve Research Laboratory, The First Affiliated Hospital of Soochow University, Suzhou, China
- 2Suzhou Medical College of Soochow University, Suzhou, China
- 3Department of Neurology, The First Affiliated Hospital of Soochow University, Suzhou, China
Background: The novel coronavirus disease 2019 (COVID-19) has rapidly spread worldwide and created a tremendous threat to global health. Growing evidence suggests that patients with COVID-19 have more severe acute ischemic stroke (AIS). However, the overall efficacy and safety of recanalization therapy for AIS patients infected by the SARS-CoV-2 virus is unknown.
Methods: The PRISMA guideline 2020 was followed. Two independent investigators systematically searched databases and ClinicalTrials.gov to identify relevant studies published up to 31 March 2022. AIS patients who received any recanalization treatments were categorized into those with COVID-19 and those without COVID-19. The main efficacy outcomes were patients' functional independence on discharge and successful recanalization, and the safety outcomes were in-hospital mortality and symptomatic intracranial hemorrhage. Subgroup analyses were implemented to assess the influence of admission National Institutes of Health Stroke Scale and different recanalization treatments on the outcomes. STATA software 12.0 was used for the statistical analysis.
Results: This systematic review and meta-analysis identified 10 studies with 7,042 patients, including 596 COVID-19 positive patients and 6,446 COVID-19 negative patients. Of the total patients, 2,414 received intravenous thrombolysis while 4,628 underwent endovascular thrombectomy. COVID-19 positive patients had significantly lower rates of functional independence at discharge [odds ratio (OR) 0.30, 95% confidence interval (CI) 0.15 to 0.59, P = 0.001], lower rates of successful recanalization (OR 0.40, 95% CI 0.24 to 0.68, P = 0.001), longer length of hospital stay (weighted mean difference 5.09, 95% CI 1.25 to 8.94, P = 0.009) and higher mortality rates (OR 3.38, 95% CI 2.43 to 4.70, P < 0.0001). Patients with COVID-19 had a higher risk of symptomatic intracranial hemorrhage than the control group, although the difference did not reach statistical significance (OR 2.34, 95% CI 0.99 to 5.54, P = 0.053).
Conclusions: Compared with COVID-19 negative AIS patients who received recanalization treatments, COVID-19 positive patients turned out to have poorer outcomes. Particular attention needs to be paid to the treatments for these COVID-19 patients to decrease mortality and morbidity. Long-term follow-up is necessary to evaluate the recanalization treatments for AIS patients with COVID-19.
Systematic review registration: https://inplasy.com/inplasy-2022-4-0022/, identifier: INPLASY202240022.
Introduction
Since the first case of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was reported in late December 2019, the world has witnessed an overwhelming global coronavirus infectious disease 2019 (COVID-19) pandemic. The COVID-19 pandemic has led to millions of confirmed cases and deaths now (1). Acute cerebrovascular diseases are frequently reported in patients with COVID-19 infection, and the most common manifestation is acute ischemic stroke (AIS) (2). An observational study reported an AIS prevalence of 4.6% in patients with COVID-19 infection (3), which is higher than that reported in patients without COVID-19 (4), or patients with influenza (5) or SARS (6). What's worse, AIS has been reported to be one of the most severe complications of COVID-19 infection. There are accumulating reports suggesting that COVID-19 patients with AIS present with worse functional outcomes and higher mortality than COVID-19 patients without AIS (7–10). Additional studies revealed that the potential stroke mechanisms in COVID-19 are hypercoagulopathy, angiotensin-converting enzyme 2 inhibition, and cardioembolism (11). A multicenter retrospective study of South India suggested that hypertension and atrial fibrillation were more common in the COVID-19 related stroke group than in historical controls (9). In addition, during the COVID-19 infection, hypercoagulability arises as a “sepsis-induced like coagulopathy” and may predispose patients to AIS (12). Nannoni et al. (2) suggested that hypercoagulation could lead to an increased risk of cerebral thrombosis and/or thromboembolism, which may be the reason why AIS is common in patients with COVID-19.
Intravenous thrombolysis (IVT) was the main systemic reperfusion therapy for AIS and was usually performed within 4.5 h of symptom onset (13, 14), while effective endovascular therapy showed improved outcomes and expanded the window period of therapy in AIS (15). However, the pandemic of COVID-19 has notable impacts on the treatments and management of AIS patients, and recanalization therapy during the pandemic could be challenging. A study from China reported that both prehospital (onset-to-door time) and posthospital (door-to-needle time) delay were prolonged remarkably, and the proportion of patients with AIS who received IVT treatment decreased significantly (16). Pooled analysis of July et al. (17) showed a significant reduction in mechanical thrombectomy performed during the pandemic than during the pre-pandemic period. Untimely recognition of stroke due to quarantine, delayed patient arrivals, COVID-19 screening before admission, and preparation of protective equipment for stroke team members, may lead to the missing of the therapeutic window (18). While in some researches, the recanalization therapies were not delayed during the COVID-19 pandemic (9, 19, 20).
Because of the rapidly increasing number of AIS complications combined with COVID-19 infections, it is of vital importance to have an in-depth understanding of the impact of COVID-19 on recanalization therapy for these patients. However, the published literature was limited to case reports, case series, and observational studies. The overall effect of COVID-19 on the outcomes of recanalization therapy for AIS patients has not been adequately assessed. Thus, we performed a meta-analysis to evaluate the efficacy and safety of recanalization therapy for COVID-19 patients who suffered from AIS.
Methods
Data availability
The authors declare that all supporting data are available within the article and in the Supplemental material.
Study protocol
We registered our study on INPLASY website (Register number INPLASY202240022) and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement (21).
Eligibility criteria
We set the inclusion criteria as follows: (1) study type: retrospective, prospective cohort study, or randomized controlled trial (RCT) study design; (2) language: published in English; (3) participants: AIS patients (≥18 years) received any recanalization treatments, with or without COVID-19 infection; COVID-19 infection was laboratory-confirmed by PCR or antigen test. (4) interventions: patients were categorized into those with COVID-19 versus those without COVID-19, and treated with IVT, intraarterial thrombolysis (IAT), endovascular thrombectomy (EVT), or a combination of these recanalization interventions; (5) outcomes: including efficacy and safety outcomes. The primary efficacy outcome was functional independence on discharge (modified Rankin Scale, mRS 0–2). The second efficacy outcome was successful recanalization indicated by Thrombolysis in Cerebral Infarction (TICI) or modified TICI (mTICI) scores ≥ 2 b/3. Other efficacy outcomes include the length of hospital stay (days), time (min) from stroke onset to treatment (onset-to-needle in those who received IVT or combined therapy; onset-to-groin puncture in those who received EVT or combined therapy), and time (min) from door to treatment (door-to-needle in those who received IVT or combined therapy; door-to-groin puncture in those who received EVT or combined therapy). The safety outcomes were in-hospital mortality and symptomatic intracranial hemorrhage (sICH), defined as worsening of 4 or more points in NIHSS attributed to hemorrhagic transformation. Included studies were not requested to supply all the outcomes mentioned above.
We set the exclusion criteria as follows: (1) review, commentary, letter, case reports, case-series, or observational studies without a control group. (2) Not all the AIS patients included in the study received recanalization interventions. (3) Suspected/ probable cases of SARS-CoV-2 infection.
Search strategy
Two independent investigators (ZiW and HT) systematically searched MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov to identify relevant studies published up to 31 March 2022. We searched Medical Subject Headings (MeSH) terms and keywords (in the title/abstract) in multiple combinations, including COVID-19, SARS-CoV-2, 2019-nCoV, stroke, cerebrovascular disease, cerebral infarction, thrombolysis, thrombectomy, thrombolytic, revascularization, recanalization. The detailed search strategy was described in Supplementary Table S1. Additionally, relevant systematic reviews and meta-analyses were also screened independently and manually to ensure a more comprehensive search.
Study selection and data collection
According to the eligibility criteria mentioned above, two investigators (XW and XY) independently evaluated all records retrieved from the databases and relevant systematic reviews or meta-analyses. Disagreements were resolved by discussion or by another independent investigator (ZC). After selection and evaluation, data from the included studies were extracted, including study authors and year, publications, study design, basic information of the patients, inclusion and exclusion criteria, and outcome events.
Risk of bias
The risk of bias for each included study was assessed using the Methodological Index for Non-randomized Studies (MINORS) for all included studies (22). MINORS contains 12 items relating to potential areas of bias. Each item receives a score from 0 to 2, resulting in overall scores ranging from 0 to 24. Two investigators performed the assessment independently (ZiW and HT). Disagreements were solved between the two investigators by consensus or by another independent investigator (ZC).
Statistical analysis
STATA software 12.0 (STATA Corp., College Station, Texas, USA) was used for the statistical analysis. The Meta-Analyses were based on a random-effects model. Weighted mean difference (WMD) and 95% confidence interval (CI) were calculated for the continuous outcomes. Odds ratio (OR) and 95% CI values were calculated for the dichotomous outcomes. A funnel plot was used to investigate possible publication bias, true heterogeneity and other methodological irregularities. Cochrane's Q test and I2 were used for calculating outcome heterogeneity. Sensitivity analysis was also performed to explore the stability of the consolidated results. For all the analyses, two-tailed tests were performed, and P < 0.05 was considered to be statistically significant.
Results
Search results and study characteristics
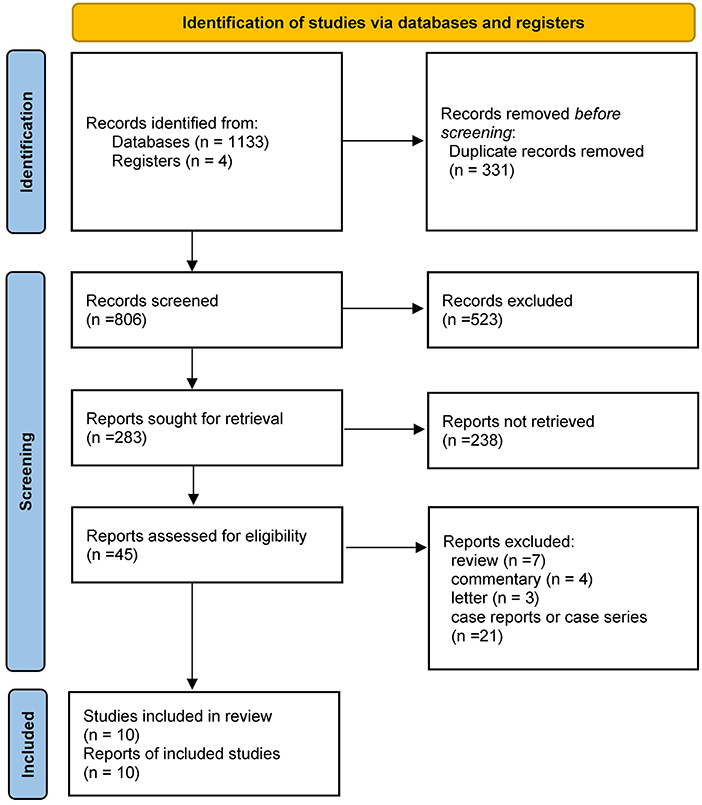
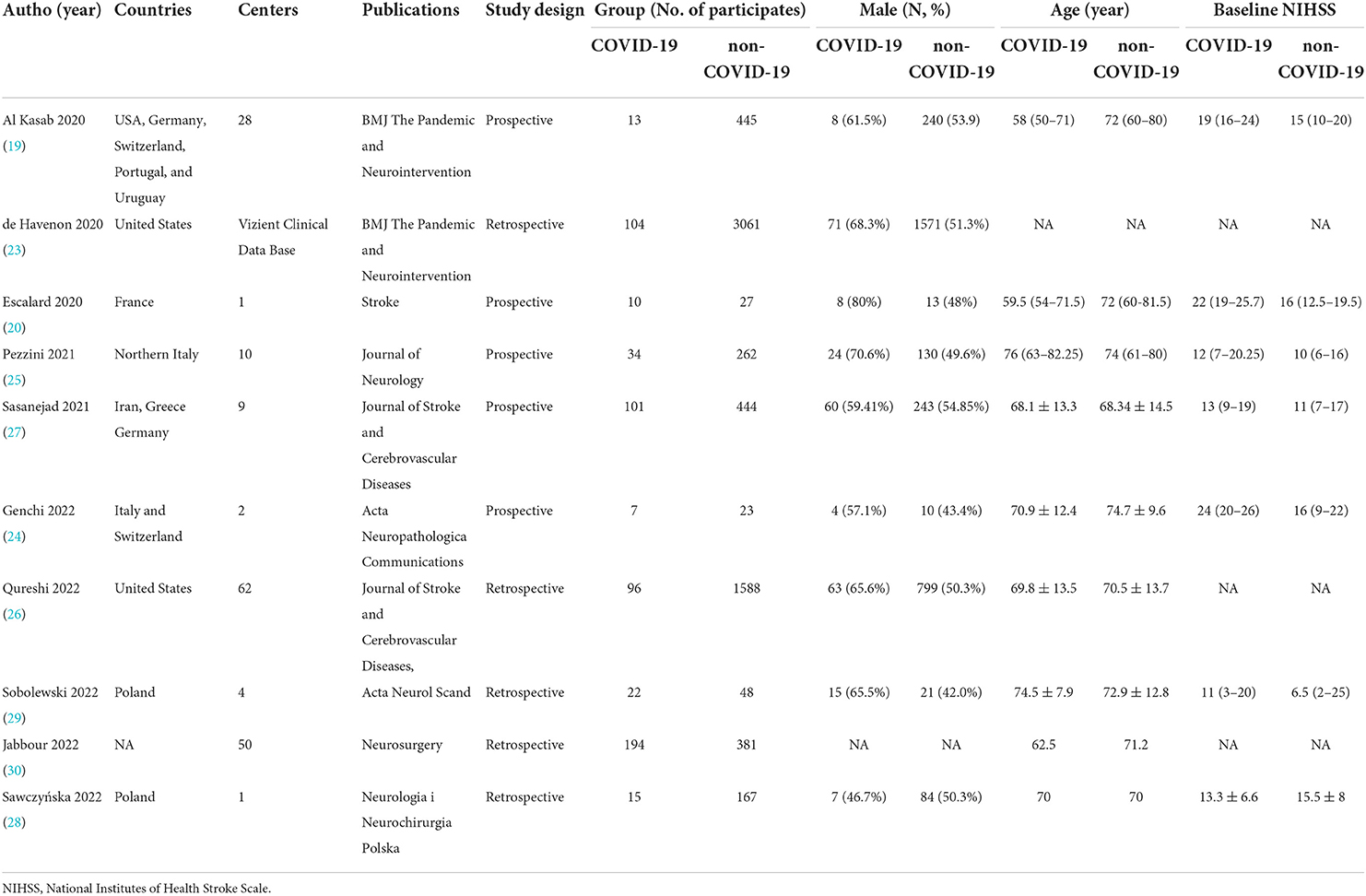
MEDLINE, EMBASE, CENTRAL, and ClinicalTrials.gov provided 1,137 titles and abstracts for review. Of these, a total of 331 articles were excluded due to duplication. After screening, 45 full articles were further assessed for eligibility. Eventually, ten studies (19, 20, 23–30) containing 7,042 patients (596 in the COVID-19 positive group and 6,446 in the COVID-19 negative group) were selected for qualitative synthesis (Figure 1). Five were retrospectively designed and five were prospectively designed. Six studies mainly focused on EVT treatment, two on IVT treatment, and two have data on both EVT and IVT treatments. The main characteristics of the included studies were listed in Table 1. Other details of the studies were shown in the supplementary materials (Supplementary Table S2).
Efficacy outcome
Four studies reported functional independence on discharge (mRS 0–2). According to the meta-analysis, the COVID-19 positive group showed significantly lower rates of functional independence than the COVID-19 negative group at discharge (OR 0.30, 95% CI 0.15 to 0.59, P = 0.001; Figure 2A). However, statistically significant evidence of heterogeneity across the five studies was found (I2 = 62.7%, P = 0.045). A sensitivity analysis was performed to detect the source of this statistical heterogeneity, demonstrating that the statistics were robust (Supplementary Figure S1).
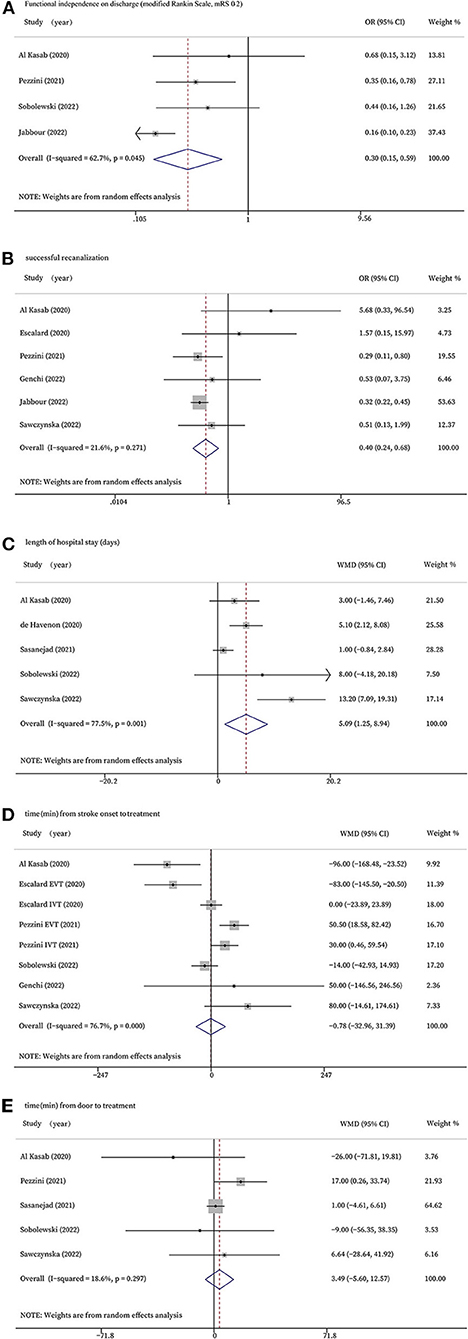
Figure 2. Forest plots for efficacy outcomes. (A): Functional independence on discharge (modified Rankin Scale, 0-2); (B): successful recanalization; (C): length of hospital stay (days); (D): time (min) from stroke onset to treatment; (E): time (min) from door to treatment.
Six studies that treated patients with EVT evaluated the rates of successful recanalization. The forest plot showed COVID-19 positive patients had significantly lower rates of successful recanalization (OR 0.40, 95% CI 0.24 to 0.68, P = 0.001, I2 = 21.6%; Figure 2B).
Five of the studies investigated the length of hospital stay and found stroke patients with COVID-19 infection stayed in the hospital significantly longer than those without COVID-19 infection (WMD 5.09, 95% CI 1.25 to 8.94, P = 0.009, I2 = 77.5%; Figure 2C). The sensitivity analysis validated the robustness of the results (Supplementary Figure S2).
Six studies reported time from stroke onset to treatment, and two of the studies had data of onset-to-needle in those who received IVT and onset-to-groin puncture in those who received EVT, respectively. There was no significant difference between the two groups in terms of time from stroke onset to treatment (WMD −0.78, 95% CI −32.96 to 31.39, P = 0.962, I2 = 76.7%; Figure 2D). The sensitivity analysis validated the robustness of the results (Supplementary Figure S3).
Five studies reported time from door to treatment, but no significant difference was found (WMD 3.49, 95% CI −5.60 to 12.57, P = 0.452, I2 = 18.6%; Figure 2E).
Safety outcome
The safety outcomes include in-hospital mortality and sICH. As shown in Figure 3A, eight studies had the data of mortality. The forest plot indicated that stroke patients with COVID-19 infection who received recanalization treatments were associated with significantly higher mortality than those without COVID-19 infection (OR 3.38, 95% CI 2.43 to 4.70, P < 0.0001, I2 = 27.9%; Figure 3A). According to our meta-analyses, patients in COVID-19 positive group experienced more sICH than the control group, while the difference did not reach statistical significance (OR 2.34, 95% CI 0.99 to 5.54, P = 0.053, I2 = 0.0%; Figure 3B).
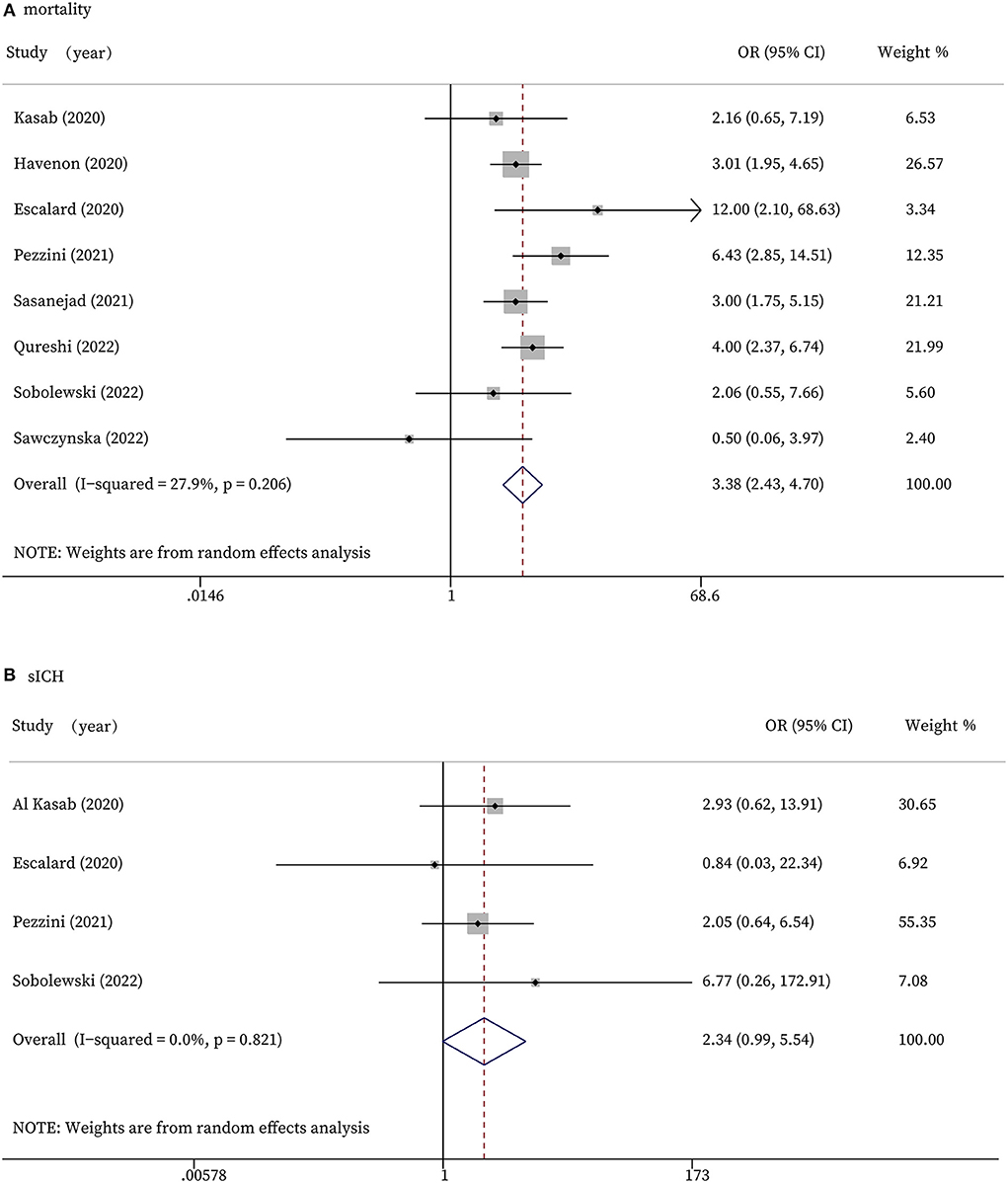
Figure 3. Forest plots for safety outcomes. (A): In-hospital mortality; (B): Symptomatic intracranial hemorrhage.
Subgroup analyses
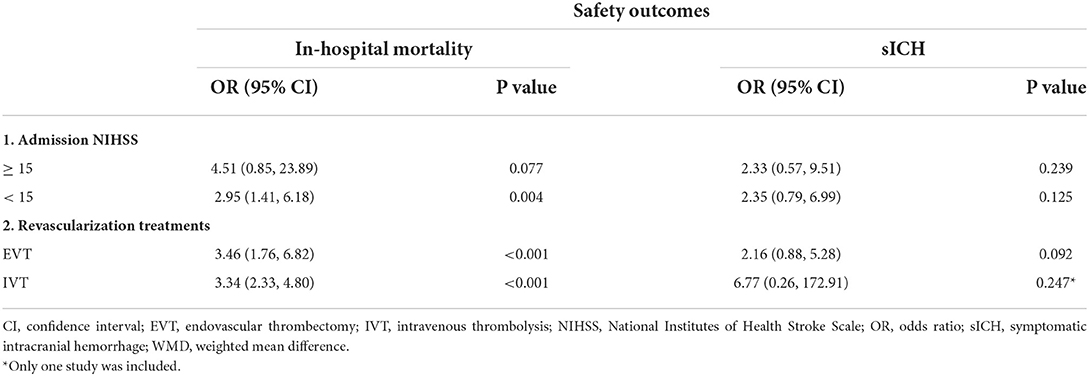
Subgroup analyses were implemented to assess the influence of median admission National Institutes of Health Stroke Scale (NIHSS) and different recanalization treatments on the outcomes. In studies that included patients with admission NIHSS < 15, patients without COVID-19 infection were associated with higher rates of functional independence on discharge (OR 0.38, 95% CI 0.20 to 0.72, P = 0.003; Table 2 and Supplementary Figure S4A) and successful recanalization (OR 0.36, 95% CI 0.16 to 0.80, P = 0.013; Table 2 and Supplementary Figure S4B) than patients with COVID-19 infection. In subgroup studies of different recanalization treatments on the outcomes, we found that in studies with patients with EVT treatment, patients with COVID-19 infection were associated with lower rates of functional independence on discharge (OR 0.27, 95% CI 0.12 to 0.60, P = 0.001; Table 2 and Supplementary Figure S6A) and longer length of hospital stay (WMD 6.57, 95% CI 1.71 to 11.43, P = 0.008; Table 2 and Supplementary Figure S6B). No significant difference was found between the time from stroke onset/door to treatment according to the subgroup analyses of different recanalization treatments or admission NIHSS (Table 2).
In terms of mortality, significant difference was found in studies that included patients with admission NIHSS < 15 (OR 2.95, 95% CI 1.41 to 6.18, P = 0.004; Table 3 and Supplementary Figure S5A). In studies that included patients with different recanalization treatments, both EVT and IVT treatment subgroup indicated that patients with COVID-19 infection leaded to higher mortality (EVT: OR 3.46, 95% CI 1.76 to 6.82, P < 0.001; IVT: OR 3.34, 95% CI 2.33 to 4.80, P < 0.001; Table 3 and Supplementary Figure S7A). No significant difference of sICH was found according to the subgroup analyses of different recanalization treatments or admission NIHSS (Table 3). All the forest plots for subgroup analyses are available within the Supplemental material.
Risk of bias in included studies
The risk of bias in the ten studies based on the MINORS quality assessment was considered low, and none was excluded (Supplementary Table S3). The symmetrical funnel plot indicated no risk of publication bias (Supplementary Figure S8).
Discussion
This study evaluated the efficacy and safety of recanalization therapy for AIS patients with COVID-19. We found that COVID-19 positive patients had significantly lower rates of functional independence at discharge, lower rates of successful recanalization, longer lengths of hospital stay, and higher mortality rates.
According to our study, the COVID-19 positive group who received recanalization therapy showed significantly lower rates of functional independence at discharge than COVID-19 negative group. Additionally, AIS patients with COVID-19 infection had a considerably longer length of hospital stay than those without COVID-19 infection. The previous report showed that ischemic stroke is more severe in patients with COVID-19, with a higher rate of patients who needed treatment in an ICU (31). In addition, patients with COVID-19 infection were more likely to have pneumonia, respiratory failure, acute kidney injury, septic shock, cardiac arrest, and require intubation or mechanical ventilation (26). These findings indicated that COVID-19 probably affected stroke patients through additional mechanisms. Therefore, these patients generally stayed in hospital for a longer period of time and have lower rates of functional independence at discharge. AIS patients with COVID-19 who were treated with EVT also had significantly lower rates of successful recanalization, as revealed by our study. COVID-19 may predispose patients to thrombosis, which is initially derived from the interaction of SARS-CoV-2 with ACE2; this will result in dysregulation of angiotensin signaling, subsequent inflammation, and tissue injury (32). This mechanism may also have a negative effect on the successful recanalization.
No significant difference between the two groups in the time from stroke onset to treatment was found. And our further subgroup analyses for the onset-to-needle time in AIS patients who received IVT and onset-to-groin puncture in those who received EVT still showed no significant difference between the two groups. Moreover, the time from door to treatment showed no significant difference. According to some studies, the time to treatment was significantly prolonged in COVID-19 positive patients with AIS (25, 28). Such a delay may be due to: the shortage of stroke team members, deceleration of evaluations, adherence to traffic restriction, and practice of preventive measures (33). However, in some research, the door-to-needle time was shortened through multidisciplinary collaboration and continuous process optimization despite the challenges posed by the COVID-19 pandemic (34). The study from Mathew et al. (9) also showed that the time from stroke onset to presentation to the hospital was reduced in stroke patients with COVID-19. This was an interesting observation that could be explained by less traffic on the roads due to the quarantine and the presence of other systemic symptoms that brought these patients to the hospital sooner. In addition, the time of enrollment of the patients may also be one of the important characteristics. As in the beginning of the pandemic, the in-hospital pathways were heavily affected, and preparation for the protective and specific treatment processes were inadequate, so time-to-treatment was frequently increased. As the stroke teams had time to adapt their processes, the previously identified delays were reduced. Studies that focused on comparisons between different COVID-19 periods with AIS patients infected by the SARS-CoV-2 virus in the same medical institution will further explain the phenomenon. Several studies including patients who were admitted with a principal diagnosis of AIS and received recanalization therapy reported that the time to treatment was similar in the COVID-19 positive group and COVID-19 negative group (19, 29). These different conditions in different areas or countries might explain that no significant difference was found between the time from stroke onset to treatment or the time from door to treatment. This might indicate that, as COVID-19 infection did not delay patients' recanalization treatment, the poorer outcomes of these patients might be due to the COVID-19 disease itself.
Regarding the safety outcomes of our meta-analyses, the mortality rate was significantly higher among patients who received recanalization therapy in the COVID-19 positive group than among those in the COVID-19 negative group, which was consistent with prior reports. Richer et al. found that the in-hospital mortality rate was significantly higher in patients with AIS and concurrent COVID-19 than in COVID-19 negative patients (35). Also, elevated d-dimer levels supported the increased risk of thromboembolism in COVID-19 patients and thus might increase their mortality rate (36). In addition, de Havenon et al. observed that more COVID-19 positive AIS patients were intubated, and had acute coronary syndrome, acute renal failure, and pulmonary emboli, which might also increase the mortality rate (37). The sICH is caused by an abnormally permeable blood-brain barrier resulting from ischemia of the capillary endothelium that allows for the leakage of blood cells (38). It is one of the severe complications of recanalization therapy in patients with AIS. According to our analysis, sICH occurred more frequently in COVID-19 positive group, while the difference did not reach statistical significance, the trend is clear. This may be due to the limitation of our data. As previously reported, the cytokine-driven imbalance in endogenous anticoagulant levels and hepatic dysfunction, especially in COVID patients, may contribute to coagulopathy with elevation in prothrombin time, and thrombocytopenia (18, 39). In addition to inflammation and coagulopathy, endothelial dysfunction with increased blood-brain barrier permeability after COVID-19 infection may also lead to more cases of sICH. A detailed assessment of coagulation to determine the risk: benefit ratio prior to recanalization therapy is necessary.
According to our subgroup analysis, we found that especially among studies including patients with admission NIHSS < 15, COVID 19 infection was associated with lower rates of functional independence, lower rates of successful recanalization, and higher in-hospital mortality rates. This might indicate that non-severe stroke patients who received recanalization treatments had poorer outcomes when infected by the SARS-CoV-2 virus.
This is the first meta-analysis to evaluate the efficacy and safety of recanalization therapy for AIS patients with COVID-19. Particular attention needs to be paid to the treatments for these patients, especially those non-severe stroke patients, to better improve their prognosis and decrease mortality. However, our study has some limitations. We acknowledge that the small sample size is a major limitation of the study. Most studies did not report the patients' long-term follow-up data. Also, the mechanisms of the poor outcomes of recanalization therapy for COVID-19 patients are not yet clear. We must admit that COVID-19 itself usually causes more severe strokes, and increases the risk of multisystemic complications, which might lead to poor outcomes in COVID-19 patients with AIS. In addition, whether COVID-19 causes AIS or whether it is a coincidental consequence of COVID-19 is difficult to determine, and the time from COVID-19 infection to AIS was not uniform across the included studies. Additionally, the results may change according to different variants, vaccinations and available COVID-19 treatments. Thus, further investigations are needed to better understand recanalization therapy for AIS patients with COVID-19.
Conclusions
Our meta-analysis suggests that AIS patients with COVID-19 who received recanalization treatments had poorer outcomes than those without COVID-19. We need to pay more attention to the treatments for these patients to decrease mortality and morbidity. Further trials with long-term follow-up periods are necessary to evaluate the recanalization treatments for these AIS patients with COVID-19.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
ZiW and HT was the principal investigator and contributed in writing of the article. ZiW, HT, and ZC designed the study and developed the analysis plan. ZC, XW, and XY analyzed the data and performed meta-analysis. YQ and HC revised the manuscript and polish the language. ZC, ZhW, and GC supervised the project. All authors read and approved the final submitted paper.
Funding
This work was supported by the Natural Science Foundation of Jiangsu Province (Grants No. BK20200203) and the National Natural Science Foundation of China (Grant No. 81873741).
Acknowledgments
We appreciate the valuable and constructive suggestions and assistance from our Team of Neurosurgical study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.984135/full#supplementary-material
References
1. Li J, Lai S, Gao GF, Shi W. The emergence, genomic diversity and global spread of SARS-CoV-2. Nature. (2021) 600:408–18. doi: 10.1038/s41586-021-04188-6
2. Nannoni S, de Groot R, Bell S, Markus HS. Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke. (2021) 16:137–49. doi: 10.1177/1747493020972922
3. Li Y, Li M, Wang M, Zhou Y, Chang J, Xian Y, et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. (2020) 5:279–84. doi: 10.1136/svn-2020-000431
4. Collaborators GS. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
5. Merkler AE, Parikh NS, Mir S, Gupta A, Kamel H, Lin E, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs. patients with influenza. JAMA Neurol. (2020) 77:1–7. doi: 10.1001/jamaneurol.2020.2730
6. Umapathi T, Kor AC, Venketasubramanian N, Lim CC, Pang BC, Yeo TT, et al. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS). J Neurol. (2004) 251:1227–31. doi: 10.1007/s00415-004-0519-8
7. Ntaios G, Michel P, Georgiopoulos G, Guo Y, Li W, Xiong J, et al. Characteristics and outcomes in patients with COVID-19 and acute ischemic stroke: the global COVID-19 stroke registry. Stroke. (2020) 51:e254–8.
8. Bach I, Surathi P, Montealegre N, Abu-Hadid O, Rubenstein S, Redko S, et al. Stroke in COVID-19: a single-centre initial experience in a hotspot of the pandemic. Stroke Vasc Neurol. (2020) 5:331–6. doi: 10.1136/svn-2020-000525
9. Mathew T, John SK, Sarma G, Nadig R, Kumar RS, Murgod U, et al. COVID-19-related strokes are associated with increased mortality and morbidity: a multicenter comparative study from Bengaluru, South India. Int J Stroke. (2021) 16:429–36. doi: 10.1177/1747493020968236
10. Siow I, Lee KS, Zhang JJY, Saffari SE, Ng A, Young B. Stroke as a neurological complication of COVID-19: a systematic review and meta-analysis of incidence, outcomes and predictors. J Stroke Cerebrovasc Dis. (2021) 30:105549. doi: 10.1016/j.jstrokecerebrovasdis.2020.105549
11. Sagris D, Papanikolaou A, Kvernland A, Korompoki E, Frontera JA, Troxel AB, et al. COVID-19 and ischemic stroke. Eur J Neurol. (2021) 28:3826–36. doi: 10.1111/ene.15008
12. Hess DC, Eldahshan W, Rutkowski E. COVID-19-related stroke. Transl Stroke Res. (2020) 11:322–5. doi: 10.1007/s12975-020-00818-9
13. Safouris A, Magoufis G, Tsivgoulis G. Emerging agents for the treatment and prevention of stroke: progress in clinical trials. Expert Opin Investig Drugs. (2021) 30:1025–35. doi: 10.1080/13543784.2021.1985463
14. Warner JJ, Harrington RA, Sacco RL, Elkind MSV. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke. Stroke. (2019) 50:3331–2. doi: 10.1161/STROKEAHA.119.027708
15. Malik P, Anwar A, Patel R, Patel U. Expansion of the dimensions in the current management of acute ischemic stroke. J Neurol. (2021) 268:3185–202. doi: 10.1007/s00415-020-09873-6
16. Gu S, Dai Z, Shen H, Bai Y, Zhang X, Liu X, et al. Delayed stroke treatment during COVID-19 pandemic in China. Cerebrovasc Dis. (2021) 50:715–21. doi: 10.1159/000517075
17. July J, Pranata R. Impact of the coronavirus disease pandemic on the number of strokes and mechanical thrombectomies: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. (2020) 29:105185. doi: 10.1016/j.jstrokecerebrovasdis.2020.105185
18. Wang Z, Yang Y, Liang X, Gao B, Liu M, Li W, et al. COVID-19 Associated ischemic stroke and hemorrhagic stroke: incidence, potential pathological mechanism, and management. Front Neurol. (2020) 11:571996. doi: 10.3389/fneur.2020.571996
19. Al Kasab S, Almallouhi E, Alawieh A, Levitt MR, Jabbour P, Sweid A, et al. International experience of mechanical thrombectomy during the COVID-19 pandemic: insights from STAR and ENRG. J Neurointerv Surg. (2020) 12:1039–44. doi: 10.1136/neurintsurg-2020-016671
20. Escalard S, Maïer B, Redjem H, Delvoye F, Hébert S, Smajda S, et al. Treatment of acute ischemic stroke due to large vessel occlusion with COVID-19: experience from Paris. Stroke. (2020) 51:2540–3. doi: 10.1161/STROKEAHA.120.030574
21. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
22. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
23. de Havenon A, Yaghi S, Mistry EA, Delic A, Hohmann S, Shippey E, et al. Endovascular thrombectomy in acute ischemic stroke patients with COVID-19: prevalence, demographics, and outcomes. J Neurointerv Surg. (2020) 12:1045–8. doi: 10.1136/neurintsurg-2020-016777
24. Genchi A, Semerano A, Schwarz G, Dell'Acqua B, Gullotta GS, Sampaolo M, et al. Neutrophils predominate the immune signature of cerebral thrombi in COVID-19 stroke patients. Acta Neuropathol Commun.(2022) 10:14. doi: 10.1186/s40478-022-01313-y
25. Pezzini A, Grassi M, Silvestrelli G, Locatelli M, Rifino N, Beretta S, et al. Impact of SARS-CoV-2 on reperfusion therapies for acute ischemic stroke in Lombardy, Italy: the STROKOVID network. J Neurol. (2021) 268:3561–8. doi: 10.1007/s00415-021-10497-7
26. Qureshi AI, Baskett WI, Huang W, Ishfaq MF, Naqvi SH, French BR, et al. Utilization and outcomes of acute revascularization treatments in ischemic stroke patients with SARS-CoV-2 infection. J Stroke Cerebrovasc Dis. (2022) 31:106157. doi: 10.1016/j.jstrokecerebrovasdis.2021.106157
27. Sasanejad P, Afshar Hezarkhani L, Arsang-Jang S, Tsivgoulis G, Ghoreishi A, Kristian B, et al. Safety and outcomes of intravenous thrombolytic therapy in ischemic stroke patients with COVID-19: CASCADE initiative. J Stroke Cerebrovasc Dis. (2021) 30:106121. doi: 10.1016/j.jstrokecerebrovasdis.2021.106121
28. Sawczyńska K, Wrona P, Kesek T, Wnuk M, Chrzan R, Homa T, et al. Mechanical thrombectomy in COVID-19-associated ischaemic stroke: patient characteristics and outcomes in a single-centre study. Neurol Neurochir Pol. (2022) 56:163–70. doi: 10.5603/PJNNS.a2022.0026
29. Sobolewski P, Antecki J, Brola W, Fudala M, Bieniaszewski L, Kozera G. Systemic thrombolysis in ischaemic stroke patients with COVID-19. Acta Neurol Scand. (2022) 145:47–52. doi: 10.1111/ane.13520
30. Jabbour P, Dmytriw AA, Sweid A, Piotin M, Bekelis K, Sourour N, et al. Characteristics of a COVID-19 cohort with large vessel occlusion: a multicenter international study. Neurosurgery. (2022) 90:725–33. doi: 10.1227/neu.0000000000001902
31. Sluis WM, Linschoten M, Buijs JE, Biesbroek JM, den Hertog HM, Ribbers T, et al. Risk, clinical course, and outcome of ischemic stroke in patients hospitalized with COVID-19: a multicenter cohort study. Stroke. (2021) 52:3978–86. doi: 10.1101/2021.03.11.21253189
32. Sriram K, Insel PA. Inflammation and thrombosis in COVID-19 pathophysiology: proteinase-activated and purinergic receptors as drivers and candidate therapeutic targets. Physiol Rev. (2021) 101:545–67. doi: 10.1152/physrev.00035.2020
33. Zhou Y, Hong C, Chang J, Xia Y, Jin H, Li Y, et al. Intravenous thrombolysis for acute ischaemic stroke during COVID-19 pandemic in Wuhan, China: a multicentre, retrospective cohort study. J Neurol Neurosurg Psychiatry. (2021) 92:226–8. doi: 10.1136/jnnp-2020-324014
34. Chen Y, Nguyen TN, Wellington J, Mofatteh M, Yao W, Hu Z, et al. Shortening door-to-needle time by multidisciplinary collaboration and workflow optimization during the COVID-19 pandemic. J Stroke Cerebrovasc Dis. (2022) 31:106179. doi: 10.1016/j.jstrokecerebrovasdis.2021.106179
35. Richter D, Krogias C, Eyding J, Bartig D, Grau A, Weber R. Comparison of stroke care parameters in acute ischemic stroke patients with and without concurrent COVID-19. A nationwide analysis. Neurol Res Pract. (2020) 2:48. doi: 10.1186/s42466-020-00095-9
36. Yamakawa M, Kuno T, Mikami T, Takagi H, Gronseth G. Clinical characteristics of stroke with COVID-19: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. (2020) 29:105288. doi: 10.1016/j.jstrokecerebrovasdis.2020.105288
37. de Havenon A, Ney JP, Callaghan B, Delic A, Hohmann S, Shippey E, et al. Impact of COVID-19 on outcomes in ischemic stroke patients in the United States. J Stroke Cerebrovasc Dis. (2021) 30:105535. doi: 10.1016/j.jstrokecerebrovasdis.2020.105535
38. Alvarez-Sabin J, Maisterra O, Santamarina E, Kase CS. Factors influencing haemorrhagic transformation in ischaemic stroke. Lancet Neurol. (2013) 12:689–705. doi: 10.1016/S1474-4422(13)70055-3
Keywords: acute ischemic stroke, COVID-19, intravenous thrombolysis, mechanical thrombectomy, meta-analysis, recanalization therapy
Citation: Wang Z, Teng H, Wu X, Yang X, Qiu Y, Chen H, Chen Z, Wang Z and Chen G (2022) Efficacy and safety of recanalization therapy for acute ischemic stroke with COVID-19: A systematic review and meta-analysis. Front. Neurol. 13:984135. doi: 10.3389/fneur.2022.984135
Received: 01 July 2022; Accepted: 10 August 2022;
Published: 30 August 2022.
Edited by:
Simone Beretta, San Gerardo Hospital, ItalyReviewed by:
Marialuisa Zedde, IRCCS Local Health Authority of Reggio Emilia, ItalyJoão Pedro Marto, Centro Hospitalar de Lisboa Ocidental, Portugal
Copyright © 2022 Wang, Teng, Wu, Yang, Qiu, Chen, Chen, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhouqing Chen, enFjaGVuNkAxNjMuY29t; Zhong Wang, d2FuZ3pob25nNzYxQDE2My5jb20=
†These authors have contributed equally to this work
 Zilan Wang1†
Zilan Wang1† Zhouqing Chen
Zhouqing Chen Zhong Wang
Zhong Wang Gang Chen
Gang Chen