
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Neurol., 19 August 2022
Sec. Multiple Sclerosis and Neuroimmunology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.979203
This article is part of the Research TopicMultiple Sclerosis and Neuroimmunology – Case Report Collection, Volume IIView all 35 articles
Objectives: The “hot cross bun sign” (HCBs) on magnetic resonance imaging (MRI) has been initially considered specific for multiple system atrophy with cerebellar features. However, a number of other conditions have since been described, which may be associated with this imaging sign. We herein describe a patient with anti-Ri and paraneoplastic cerebellar ataxia, and review the association of the HCBs on imaging with various neurological autoimmune conditions.
Methods: We report a 40-year-old woman with anti-Ri-associated paraneoplastic neurological syndrome and breast carcinoma, in whom brain MRI revealed the HCBs late in the disease course. We also reviewed similar cases reported in the literature.
Results: The patient presented with cerebellar ataxia, polyneuropathy, and pyramidal signs. Although brain MRI was initially unremarkable, the HCBs and T2-weighted hyperintensity of the bilateral middle cerebellar peduncles were observed at later follow-up. Anti-Ri was detected in the serum and cerebrospinal fluid. Breast adenocarcinoma was confirmed via an axillary lymph node biopsy. Her symptoms partially resolved after the first corticosteroid pulse. However, subsequent immunotherapy and tumor treatments were ineffective. Four autoimmune cerebellar ataxia cases with the HCBs (two paraneoplastic and two non-paraneoplastic) were identified in the literature.
Discussion: The HCBs can be associated with paraneoplastic and non-paraneoplastic cerebellar ataxia, which may reflect neurodegeneration secondary to autoimmune injury. Thus, the HCBs should not be considered a contraindication for autoimmune cerebellar syndrome.
The “hot cross bun sign” (HCBs) refers to a characteristic cruciform pontine T2-weighted hyperintensity evident on brain magnetic resonance imaging (MRI) and is suggestive of multiple system atrophy with cerebellar features (MSA-C). MSA-C is a neurodegenerative alpha-synucleinopathy and a common cause of adult-onset sporadic cerebellar ataxia (1). The diagnostic specificity of the HCBs and middle cerebellar peduncular hyperintensity on MRI for MSA-C is as high as 98.5% (2). However, the HCBs has also been reported in patients with neurological autoimmunity. To help improve the differential diagnosis spectrum of the HCBs, here, we present a patient with anti-Ri-related paraneoplastic neurological syndrome (PNS) and breast cancer who showed the HCBs. We also provide a review of patients with the HCBs associated with autoimmune etiologies.
A 40-year-old woman presented with paresthesia and weakness in all four limbs for 2 years and an unstable gait for 1 year. Electromyography conducted 7 months after the disease onset showed neurogenic change, while brain MRI performed was unremarkable. Ganglioside antibodies in the serum and cerebrospinal fluid (CSF) were both negative. Empirical corticosteroids significantly improved her symptoms. However, the numbness and quadriplegia reappeared during corticosteroid weaning 4 months later, and she gradually developed dizziness, an unsteady gait, and diplopia. Brain MRI performed at 16 months after the disease onset revealed T2 hyperintensity in the middle of the pons (Figure 1). There was no improvement with pulse glucocorticoid therapy and intravenous immunoglobulin, and she developed slurred speech and dysphagia.
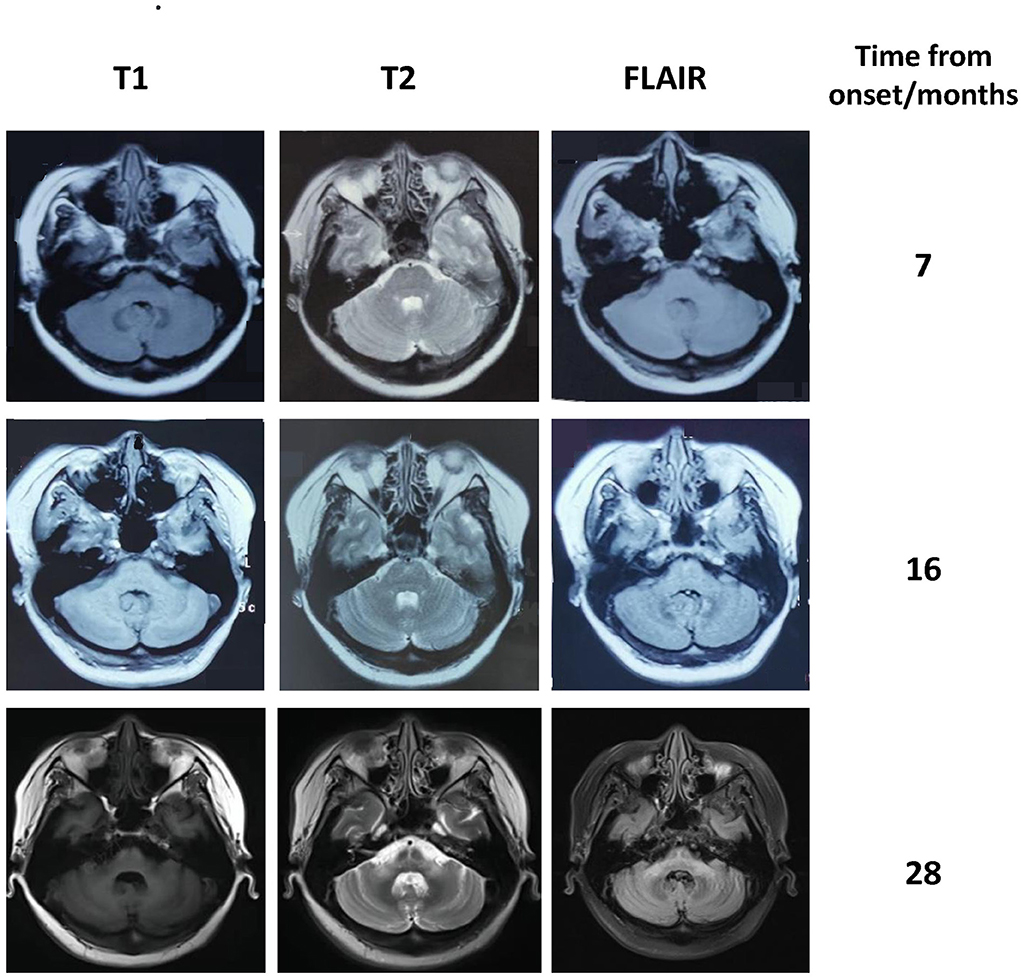
Figure 1. Brain MRI of our patient showing progressive atrophy of the cerebellum and the middle cerebellar peduncle. The hot cross bun sign and the abnormal signal in the middle cerebellar peduncles became more pronounced over time.
Physical examination revealed dysarthria, paresis of left eye adduction, diplopia, and nystagmus. The lower extremities exhibited weakness, hyporeflexia, and Babinski's signs. The finger-to-nose and knee-heel-shin tests revealed slowness and intentional tremors. She could not walk independently and was wheelchair-bound. Her sensation was intact. The Scale for the Assessment and Rating of Ataxia score was 33.5, and the modified Rankin Scale score was 4.
Complete blood count, biochemical tests, and screening for infection, toxins, and metabolic and systemic autoimmune diseases were unremarkable. There was an increased CSF white blood cell count (22 cells/μL) and positive oligoclonal bands. PNS autoantibody assays (for the Hu/Yo/Ri/Ma2/Ta/CV2/Tr/Zic4/SOX1/amphiphysin antibodies) revealed anti-Ri in the serum and CSF. Electromyography demonstrated sensory polyneuropathy. Spinal MRI was unremarkable. Hypermetabolism and enlargement of the left axillary lymph nodes were apparent on positron emission tomography-computed tomography. Biopsies revealed breast adenocarcinoma metastases [CK7 (+), ER (++), PR (–), HER-2 (+)]. No hypermetabolism in positron emission tomography-computed tomography was observed in the mammary glands, and pathological investigation after left mammectomy revealed no malignancy. 18F-fluorodeoxyglucose uptake of the cerebellum was decreased.
The patient was diagnosed with PNS with breast carcinoma. Endocrine therapy with goserelin and letrozole and plasma exchange were performed. However, her neurological symptoms worsened. Follow-up brain MRI revealed the HCBs, with signal change in the bilateral middle cerebellar peduncles and widened cerebellar sulci (Figure 1). Her symptom was stabilized by a repeated course of corticosteroid and mycophenolate mofetil treatment, after which CSF pleocytosis improved (white blood cell count, 2 cells/ul). However, CSF oligoclonal bands and anti-Ri in the serum and CSF remained positive. Her modified Rankin Scale score evaluated 34 months after the disease onset was 4. The clinical course of the patient is summarized in Figure 2.
Herein, we report a patient with paraneoplastic cerebellar ataxia and polyneuropathy in whom brain MRI showed the HCBs and bilateral middle cerebellar peduncle hyperintensities, imitating MSA-C. To our knowledge, this is the first report of the HCBs in a patient with PNS related to anti-Ri antibodies.
As the targets of anti-Ri are intracellular RNA-binding proteins encoded by Nova-1 and Nova-2 genes, direct pathogenicity of anti-Ri antibodies is unlikely, and T lymphocyte-mediated neuronal damage is considered the main pathogenic mechanism (3). In pathological studies, lymphocytic infiltration was detected in the cerebellum, brainstem, and neocortex, with prominent Purkinje cell loss (4). Most patients with anti-Ri antibodies have unremarkable brain MRI findings, although some abnormalities have been reported, including signal changes in the brainstem, the medial temporal and insular lobes, and reversible lesions in the pontine tegmentum (3, 5). This seems in line with the multifocal neurological abnormalities involving cerebellar ataxia, opsoclonus-myoclonus syndrome, jaw dystonia, and Parkinsonism (3, 6, 7). As for the present case, peripheral neuropathy was frequently reported, although Ri is exclusively expressed in the central nervous system (3).
Historically, the HCBs was considered a marker of MSA-C (8). However, its diagnostic specificity is now being questioned because of its identification in a range of other diseases. The pathogenic conditions underlying the HCBs include infectious (e.g., progressive multifocal leukoencephalopathy, Creutzfeldt-Jakob disease) (9–11), hereditary (e.g., spinocerebellar ataxia type 1, spinocerebellar ataxia type 3, cerebrotendinous xanthomatosis, oculodentodigital dysplasia, fragile X tremor ataxia syndrome) (12–16) or inflammatory (17) disorders. The HCBs secondary to leptomeningeal metastasis and infarction was also reported (18–20). The sign is thought to reflect Wallerian degeneration of the transverse pontocerebellar fibers and neuronal loss in the pontine raphe, with preservation of the pontine tegmentum, superior ventral cerebellar peduncles, and the bilateral corticospinal tracts (19, 21, 22). Gliosis of the reticular formation in the middle of the pontine and the pontocerebellar fiber also contributes to the HCBs (23).
In our systematic review, we searched the PubMed and EMBASE databases using “hot cross bun sign” OR “cruciform” OR “cruciate” and “autoimmune” OR “encephalitis” OR “rhombencephalitis” OR “paraneoplastic” as keywords. We excluded patients with central nervous system inflammatory demyelinating diseases because of their distinct pathogenicity. Four autoimmune cerebellar ataxia patients with the HCBs were identified in the literature (Table 1). Two of the patients were paraneoplastic, and two were non-paraneoplastic with anti-Homer 3 antibodies (24–26). All four patients presented with cerebellar ataxia, with or without other neurological abnormalities, including diplopia, pyramidal sign, rapid eye movement sleep behavior disorder, and sensorineural hearing loss. Notably, the HCBs usually appeared later in the disease course, with or without middle cerebellar peduncle lesions, while brain MRI at presentation was typically unremarkable or showed cerebellar atrophy. The patients generally had a poor outcome despite a wide range of immunological and oncological treatments.
In patients with PNS, the HCBs was first reported in a patient with kelch-like protein 11 (KLHL11) antibody (25). Interestingly, that patient and the present patient both presented with occult malignancies and lymph node metastases. Thus, a propensity toward lymphatic metastasis may facilitate the presentation of tumor autoantigens to the immune system, triggering cross reaction with the nervous tissue. The strong immune response may also promote regression of the primary tumor (27).
The patients with Homer 3 antibodies could show rapid eye movement sleep behavior disorder and cerebellar ataxia, in addition to the HCBs. Thus, it can be difficult to differentiate this disorder from degenerative MSA-C, particularly when the onset is insidious or an inflammatory CSF profile is absent (24, 28). Antineuronal autoantibody testing is important for identifying such potentially treatable etiologies.
The HCBs usually appears late in the disease course, and patients generally experience poor outcomes despite immunological and oncological treatments. In our patient, immunotherapy and tumor treatment failed to improve the cerebellar syndrome. Nevertheless, stabilization of the ataxia and alleviation of CSF inflammation support an immune-mediated etiology and treatment efficacy. In these circumstances, irreversible neuronal loss secondary to autoimmune destruction of the cerebellum and related structures may have already occurred, as suggested by the prominent cerebellar atrophy and the HCBs on brain MRI. However, there is some evidence that the HCBs can be reversed after immunotherapy, such as in neuromyelitis optica spectrum disorders (1).
This present case provides further evidence that the HCBs is not specific for MSA-C but, rather, can also appear in paraneoplastic or non-paraneoplastic autoimmune cerebellar ataxia, including PNS with anti-Ri. When this imaging characteristic is associated with the subacute/acute disease onset, an inflammatory CSF profile, and the absence of autonomic dysfunction (which were atypical for MSA-C), clinicians should consider the potential for autoimmune and paraneoplastic etiologies, and CSF examinations, neuronal autoantibody assays, and malignancy screening should be considered.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was approved by the Ethics Committee of Peking Union Medical College Hospital (JS-891). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
ML and HR drafted the manuscript for intellectual content and collected and analyzed the data. NL and YT collected and analyzed the data and revised the manuscript for intellectual content. SF and HG revised the manuscript for intellectual content. All authors contributed to the article and approved the submitted version.
This study is funded by CAMS Innovation Fund for Medical Sciences (CIFMS #2021-I2M-1-003).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CSF, cerebrospinal fluid; HCBs, hot cross bun sign; MRI, magnetic resonance imaging; MSA-C, multiple system atrophy with cerebellar features; PNS, paraneoplastic neurological syndrome.
1. Zhu S, Li H, Deng B, Zheng J, Huang Z, Chang Z, et al. Various Diseases and Clinical Heterogeneity Are Associated With “Hot Cross Bun”. Front Aging Neurosci. (2020) 12:592212. doi: 10.3389/fnagi.2020.592212
2. Carré G, Dietemann JL, Gebus O, Montaut S, Lagha-Boukbiza O, Wirth T, et al. Brain MRI of multiple system atrophy of cerebellar type: a prospective study with implications for diagnosis criteria. J Neurol. (2020) 267:1269–77. doi: 10.1007/s00415-020-09702-w
3. Pittock SJ, Lucchinetti CF, Lennon VA. Anti-neuronal nuclear autoantibody type 2: paraneoplastic accompaniments. Ann Neurol. (2003) 53:580–7. doi: 10.1002/ana.10518
4. Brieva-Ruíz L, Diaz-Hurtado M, Matias-Guiu X, Márquez-Medina D, Tarragona J, Graus F. Anti-Ri-associated paraneoplastic cerebellar degeneration and breast cancer: an autopsy case study. Clin Neurol Neurosurg. (2008) 110:1044–6. doi: 10.1016/j.clineuro.2008.06.016
5. Kim H, Lim Y, Kim K-K. Anti-ri-antibody-associated paraneoplastic syndrome in a man with breast cancer showing a reversible pontine lesion on MRI. J Clin Neurol. (2009) 5:151–2. doi: 10.3988/jcn.2009.5.3.151
6. Takkar A, Mehta S, Gupta N, Bansal S, Lal V. Anti- RI antibody associated progressive supranuclear palsy like presentation in a patient with breast carcinoma. J Neuroimmunol. (2020) 347:577345. doi: 10.1016/j.jneuroim.2020.577345
7. Simard C, Vogrig A, Joubert B, Muñiz-Castrillo S, Picard G, Rogemond V, et al. Clinical spectrum and diagnostic pitfalls of neurologic syndromes with Ri antibodies. Neurol Neuroimmunol Neuroinflamm. (2020) 7:e699. doi: 10.1212/NXI.0000000000000699
8. Lin DJ, Hermann KL, Schmahmann JD. The Diagnosis and Natural History of Multiple System Atrophy, Cerebellar Type. Cerebellum. (2016) 15:663–79. doi: 10.1007/s12311-015-0728-y
9. Soares-Fernandes JP, Ribeiro M, Machado A. “Hot cross bun” sign in variant Creutzfeldt-Jakob disease. AJNR Am J Neuroradiol. (2009) 30:E37. doi: 10.3174/ajnr.A1335
10. Padmanabhan S, Cherian A, Iype T, Mathew M, Smitha S. Hot cross bun sign in HIV-related progressive multifocal leukoencephalopathy. Ann Indian Acad Neurol. (2013) 16:672–3. doi: 10.4103/0972-2327.120479
11. Jain RS, Nagpal K, Tejwani S. 'Hot-cross bun' and 'inverse trident sign' in progressive multifocal leukoencephalopathy with HIV seropositivity. Neurol India. (2014) 62:341–2. doi: 10.4103/0028-3886.137032
12. Constantinides VC, Paraskevas GP, Kalogera S, Yapijakis C, Kapaki E. Hot cross bun sign and prominent cerebellar peduncle involvement in a patient with oculodentodigital dysplasia. Neurol Sci. (2021) 42:343–5. doi: 10.1007/s10072-020-04569-1
13. Jain RS, Sannegowda RB, Agrawal A, Hemrajani D, Jain R, Mathur T. 'Hot cross bun' sign in a case of cerebrotendinous xanthomatosis: a rare neuroimaging observation. BMJ Case Rep. (2013) 2013:bcr2012006641. doi: 10.1136/bcr-2012-006641
14. Pedroso JL, Rivero RLM, Barsottini OGP. “Hot cross bun” sign resembling multiple system atrophy in a patient with Machado-Joseph disease. Arq Neuropsiquiatr. (2013) 71:824. doi: 10.1590/0004-282X20130132
15. Wang Y, Koh K, Takaki R, Shindo K, Takiyama Y. Hot cross bun sign in a late-onset SCA1 patient. Neurol Sci. (2016) 37:1873–4. doi: 10.1007/s10072-016-2635-5
16. Kamm C, Healy DG, Quinn NP, Wüllner U, Moller JC, Schols L, et al. The fragile X tremor ataxia syndrome in the differential diagnosis of multiple system atrophy: data from the EMSA Study Group. Brain: J Neurol. (2005) 128:1855–60. doi: 10.1093/brain/awh535
17. Muqit MM, Mort D, Miskiel KA, Shakir RA. “Hot cross bun” sign in a patient with parkinsonism secondary to presumed vasculitis. J Neurol Neurosurg Psychiatr. (2001) 71:565–6. doi: 10.1136/jnnp.71.4.565
18. Roh SY, Jang H-S, Kim YH. Hot cross bun sign following bilateral pontine infarction: a case report. J Mov Disord. (2013) 6:37–9. doi: 10.14802/jmd.13009
19. Pan Z, Yang G, Yuan T, Wang Y, Pang X, Gao Y, et al. 'Hot cross bun' sign with leptomeningeal metastases of breast cancer: a case report and review of the literature. World J Surg Oncol. (2015) 13:43. doi: 10.1186/s12957-015-0483-z
20. Zhang H, Tian Y, Jin T, Zhang H, Sun L. The “hot cross bun” sign in leptomeningeal carcinomatosis. Canad J Neurol Sci. (2013) 40:597–8. doi: 10.1017/S0317167100014736
21. Portet M, Filyridou M, Howlett DC. Hot cross bun sign. J Neurol. (2019) 266:2573–4. doi: 10.1007/s00415-019-09439-1
22. Schrag A, Kingsley D, Phatouros C, Mathias CJ, Lees AJ, Daniel SE, et al. Clinical usefulness of magnetic resonance imaging in multiple system atrophy. J Neurol Neurosurg Psychiatr. (1998) 65:65–71. doi: 10.1136/jnnp.65.1.65
23. Takao M, Kadowaki T, Tomita Y, Yoshida Y, Mihara B. 'Hot-cross Bun Sign' of Multiple System Atrophy. Intern Med. (2007) 46:1883. doi: 10.2169/internalmedicine.46.0514
24. Liu M, Ren H, Fan S, Zhang W, Xu Y, Zhao W, et al. Neurological autoimmunity associated with homer-3 antibody: a case series from China. Neurol Neuroimmunol Neuroinflamm. (2021) 8:e1077. doi: 10.1212/NXI.0000000000001077
25. Ishikawa H, Mandel-Brehm C, Shindo A, Cady MA, Mann SA, Niwa A, et al. Long-term MRI changes in a patient with Kelch-like protein 11-associated paraneoplastic neurological syndrome. Eur J Neurol. (2021) 28:4261–6. doi: 10.1111/ene.15120
26. Schlapakow E, Keil VC, Paus M, Kornblum C, Hattingen E, Klockgether T. Multiple system atrophy mimicry in MRI: Watch out for paraneoplastic rhombencephalitis. J Clin Neurosci. (2020) 76:238–40. doi: 10.1016/j.jocn.2020.04.052
27. Ishikawa H, Kawada N, Taniguchi A, Odachi K, Mizutani A, Asahi M, et al. Paraneoplastic neurological syndrome due to burned-out testicular tumor showing hot cross-bun sign. Acta Neurol Scand. (2016) 133:398–402. doi: 10.1111/ane.12469
Keywords: hot cross bun sign, anti-Ri antibody, paraneoplastic neurological syndrome (PNS), autoimmune cerebellar ataxia, case report
Citation: Liu M, Ren H, Lin N, Tan Y, Fan S and Guan H (2022) The “hot cross bun sign” in patients with autoimmune cerebellar ataxia: A case report and literature review. Front. Neurol. 13:979203. doi: 10.3389/fneur.2022.979203
Received: 27 June 2022; Accepted: 25 July 2022;
Published: 19 August 2022.
Edited by:
Robert Weissert, University of Regensburg, GermanyReviewed by:
Lotfi Hacein-Bey, Sutter Medical Foundation, United StatesCopyright © 2022 Liu, Ren, Lin, Tan, Fan and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongzhi Guan, Z3Vhbmh6QDI2My5uZXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.