- 1Department of Neurointervention, Beijing Neurosurgical Institute, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
Objective: To evaluate the safety and efficacy of stent-assisted coiling (SAC) using the Neuroform Atlas stent for aneurysms that recur after coil embolization.
Methods: We retrospectively reviewed patients who underwent SAC using the Neuroform Atlas stent to treat aneurysms that recurred after coil embolization from November 2020 to November 2021. Patient and aneurysm characteristics, procedural details, complications, and angiographic and clinical follow-up outcomes were recorded and analyzed.
Results: Eleven patients with 11 recurrent aneurysms were included for analysis. Atlas stent deployment was successful in all cases. Angiography immediately after the SAC procedure and at last follow-up showed complete occlusion in 10 patients (90.9%) and a residual neck in one (9.1%). Mean angiographic and clinical follow-ups were 9.2 and 10 months, respectively. A single procedure-related complication occurred, mildly blurred vision in the left eye, which recovered completely. No permanent morbidity or mortality occurred.
Conclusion: SAC using the Atlas stent to treat aneurysms that recur after coil embolization is safe and effective. Large-scale studies with long-term follow-up are warranted to confirm our results.
Introduction
Endovascular treatment of intracranial aneurysms is effective; however, recurrence is a known complication that requires re-treatment to reduce the risk of aneurysm growth and hemorrhage (1–3). The reported rates of aneurysm recurrence and re-treatment after coiling are 20% and 10%, respectively (4). Intracranial stents can reduce the incidence of recurrence by preventing coil protrusion into the parent artery, maintaining high coil density within the aneurysm sac, and creating a scaffold for endothelial coverage (5, 6). Several studies have shown that stent-assisted coiling (SAC) is associated with a lower recurrence rate than coiling alone (7).
Since the introduction of the Neuroform stent (Stryker Neurovascular, Fremont, CA, USA) in 2002, intracranial stents have been continually refined. New-generation stents designed with varying structures, lower profile, and improved delivery systems have been introduced to improve aneurysmal occlusion and reduce recurrence (8–10). The self-expanding Neuroform Atlas stent (Stryker Neurovascular) is the successor of the Neuroform stent. This laser-cut stent is made of nitinol and has a mixed open-cell/closed-cell design. Delivery is via microcatheter (0.0165–0.017 inch). The Atlas stent can be used in small distal vessels, which has increased the number of aneurysms amenable to endovascular treatment.
Although efficacy of the Atlas stent has been established in several multicenter studies, to the best of our knowledge, SAC using the Atlas stent for treatment of recurrent aneurysms after coil embolization has not been evaluated (11–13). This study reports our experience.
Materials and methods
Patient population
We retrospectively reviewed all patients with intracranial aneurysms treated using the Atlas stent between November 2020 and November 2021 at Beijing Tiantan Hospital. Patients who met the following criteria were included for analysis: (1) age 18 to 80 years; (2) intracranial aneurysm confirmed by digital subtraction angiography (DSA) and previously treated with coiling alone; (3) aneurysm recurrence diagnosed on initial follow-up DSA; (4) re-treatment with SAC using the Atlas stent; and (5) clinical and angiographic follow-up were available after re-treatment. Institutional review board approval was obtained and all patients provided written informed consent.
The following data regarding patient and aneurysm characteristics were recorded: age; sex; hypertension; diabetes mellitus; smoking and alcohol use; symptoms before treatment; history of subarachnoid hemorrhage (SAH); aneurysm location (including bifurcation); irregular aneurysm; aneurysm size; aneurysm neck width; dome/neck ratio; modified Rankin scale (mRS) score before treatment, at discharge, and at follow-up; immediate and follow-up angiographic results; number of stents placed; stent size; procedure-related complications; and interval between initial treatment and re-treatment.
Endovascular procedure and antiplatelet regimen
Patients with unruptured aneurysms were premedicated with a dual-antiplatelet regimen (clopidogrel 75 mg/d and aspirin 100 mg/d) for at least 5 days. For patients with ruptured aneurysms, we administered loading doses of clopidogrel 300 mg and aspirin 300 mg orally or through a stomach tube 4 h before the procedure. All SAC procedures were performed via the femoral approach under general anesthesia and full anticoagulation with heparin (targeted activated clotting time was two to three times above the patient's baseline value). A triaxial guide-catheter system using a 6-Fr Cook (Cook Medical, Bloomington, IN, USA) or 6-Fr Neuron MAX (Penumbra, Alameda, California, USA) long sheath, 5-Fr or 6-Fr Navien (Covidien, Irvine, California, USA) intermediate support catheter, and Excelsior SL-10 or XT-17 microcatheter (Stryker Neurovascular) was used to deploy the stent. Aneurysm morphology and parent arterial structure were assessed using three-dimensional rotational angiography and the proper working projection was selected. An Echelon-10 microcatheter (Medtronic, Dublin, Ireland) was then placed into the aneurysm lumen. An Excelsior SL-10 or XT-17 microcatheter was placed into the parent artery under microguidewire guidance. Aneurysm coiling was performed using the jailing technique. Clopidogrel 75 mg and aspirin 100 mg daily were continued for at least 3 months after the procedure, then aspirin alone for 6 months or life.
Clinical and angiographic evaluations
Procedure-related complications were categorized as ischemic or hemorrhagic. Ischemic complications were defined as thromboembolic events associated with re-treatment, namely persistent focal neurological deficit, transient ischemic attack, or cerebral infarction. Hemorrhagic complications were defined as visualization of contrast leakage from the aneurysm or ruptured vessel during the procedure or visualization of intracranial hemorrhage on an imaging study performed in the periprocedural period.
Clinical outcome was assessed based on mRS score at last follow-up and was classified as favorable (mRS score 0–2) or poor (mRS score 3–6). Morbidity was defined as any procedure-related neurological deterioration that caused an increase in mRS score.
Angiographic outcomes were evaluated immediately and 6 and 12 months after the procedure using the Raymond–Roy (RR) occlusion classification system: class I, complete occlusion; class II, residual neck; class III, residual aneurysm (14). The outcomes were independently determined by two experienced neuro-interventionalists. Follow-up outcomes were categorized based on comparison with the outcomes immediately after the procedure: (1) improvement, decreased contrast filling in the aneurysm sac; (2) stable, no change in contrast filling; and (3) recurrence, increased contrast filling.
Results
Patient and aneurysm characteristics
Eleven patients met inclusion criteria. Median patient age was 49.1 years (range, 31–65) and 8 patients were women. Nine aneurysms presented initially with a rupture. Aneurysm location was anterior communicating artery in 5 patients, posterior communicating artery in 3, pericallosal artery in 2, and superior hypophyseal artery in 1. Mean aneurysm size and neck width was 4.8 ± 2.2 mm (range, 2.7–7.2) and 3.8 ± 1.2 mm (range, 2.5–4.9), respectively. Dome/neck ratio was <2 in all aneurysms and therefore considered wide-necked. Patient and aneurysm characteristics at the time of initial treatment are summarized in Table 1.
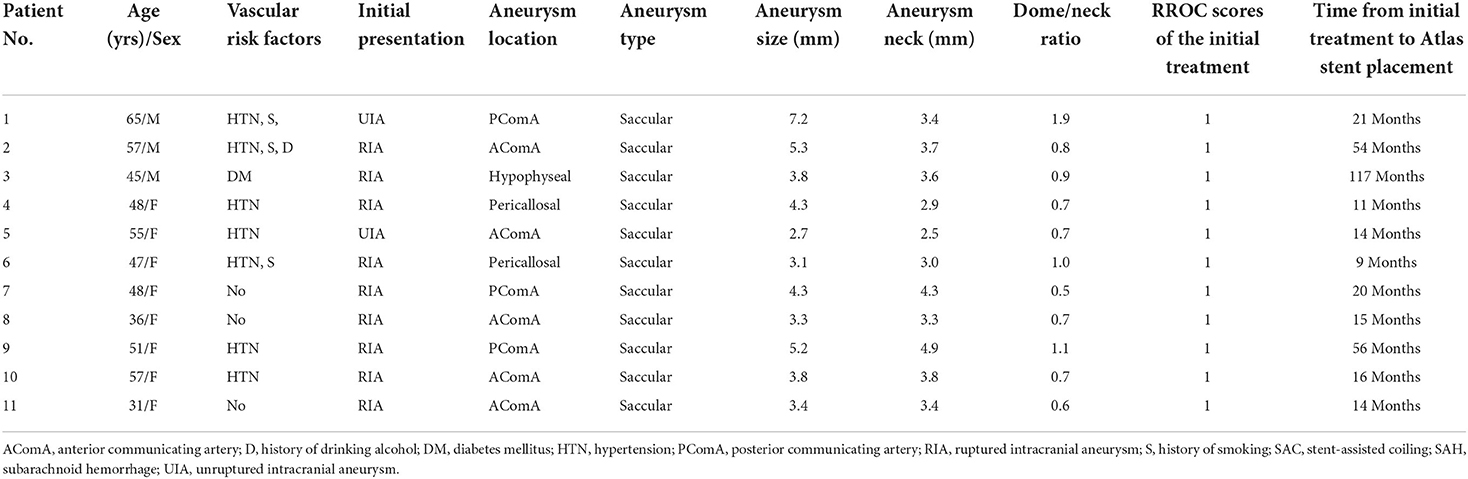
Table 1. Patient and aneurysm characteristics and procedural details at the time of initial endovascular treatment.
Technical and angiographic outcomes
Characteristics of the recurrent aneurysms, SAC procedural details, and follow-up outcomes are shown in Table 2. Among the 11 patients with recurrent aneurysms, only one patient (Case 9) presented with a ruptured aneurysm on admission. Atlas stent deployment was successful in all patients. Intraprocedural Dyna computed tomography (Siemens, Munich, and Germany) demonstrated satisfactory vessel wall apposition for all stents. Other than coils, no additional devices such as flow diverters or balloons were used during re-treatment. Immediate postprocedural angiographic showed that complete occlusion (RR class I) was achieved in 10 patients (90.9%), residual neck (RR class II) in 1 patient (9.1%). All patients underwent angiographic follow-up at least once. After a mean of 9.2 months of angiographic follow-up, 10 patients (90.9%) showed complete occlusion (RR class I), and 1 patient showed neck remnant (RR class II).
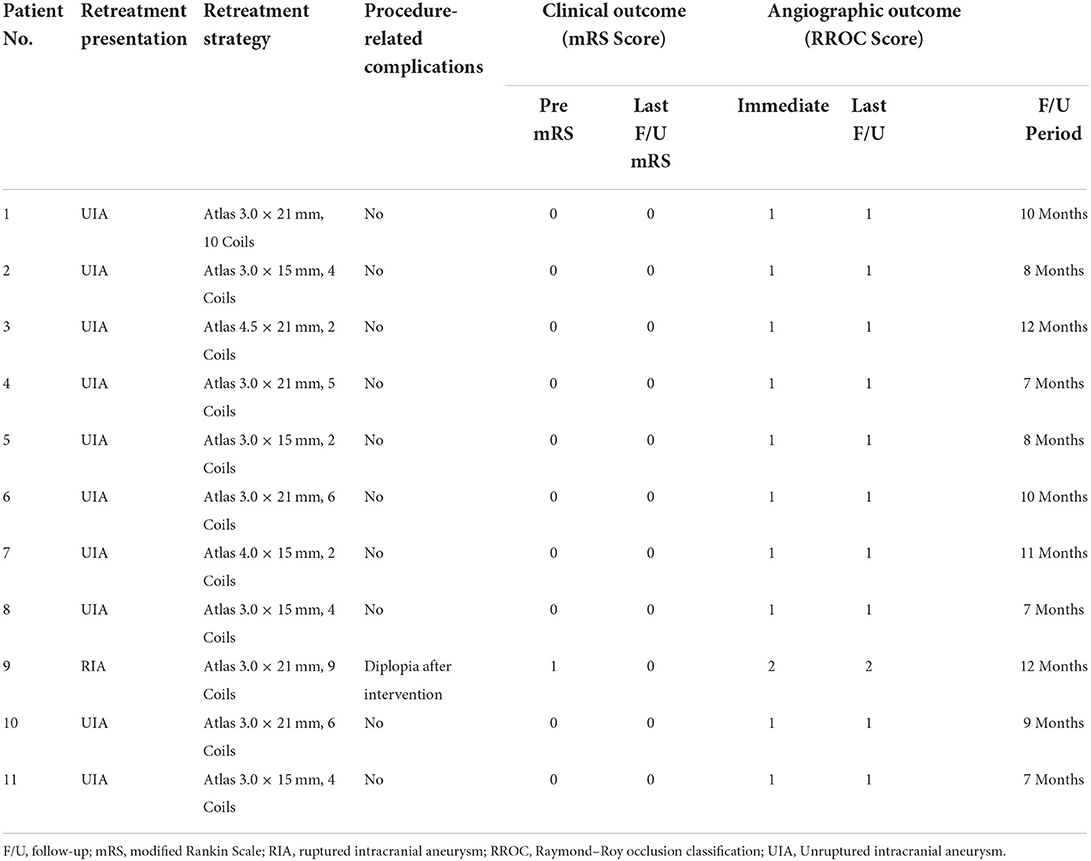
Table 2. Characteristics of recurrent aneurysms, procedural details of stent-assisted coiling, and follow-up outcomes.
Complications and clinical outcomes
The only complication was minor visual impairment in one patient who developed mildly blurred vision in the left eye after the procedure (mRS score 1). Therefore, clinical outcome was favorable (mRS score 0–2) in all patients at discharge. Mean clinical follow-up was 10 months (range, 6–15). During follow-up, the patient who experienced blurred vision recovered completely (mRS score 0) and the others reported no new neurologic deficits. Follow-up clinical outcome was favorable in all patients without morbidity or mortality.
Case presentations
Case 1
A 65-year-old man presented to an outside hospital with a 1-month history of dizziness and headache. Magnetic resonance angiography showed a left posterior communicating artery aneurysm and he was transferred to our hospital. DSA confirmed the aneurysm (4.8 × 6.7 mm) and RR class I occlusion was achieved with coil embolization (Figures 1A,B). Aneurysm recurrence was diagnosed 21 months later (Figure 1C) and SAC using the Atlas stent (3.0 × 21 mm) was performed without complications. Intraprocedural angiography showed the three radiopaque markers at the proximal and distal ends of the Atlas stent (Figure 1D). Angiography immediately after the procedure revealed RR class I occlusion and dense coil packing within the aneurysm (Figure 1E). Follow-up DSA 10 months later still showed complete aneurysmal occlusion and a patent posterior communicating artery (Figure 1F).
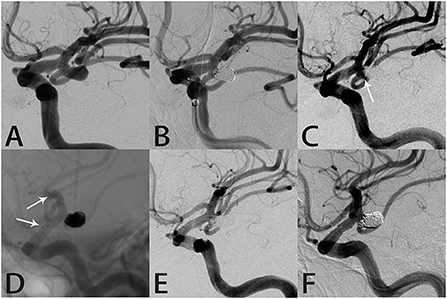
Figure 1. Images from a 65-year-old man with a left posterior communicating artery aneurysm (case 1). (A) Preoperative angiography showed a left posterior communicating artery aneurysm. (B) The aneurysm was occluded completely after coil embolization. (C) Follow-up angiography 21 months after the procedure revealed a mild recurrence in the aneurysm neck (white arrow). (D) Angiography during re-treatment showed the deployed Atlas stent (3.0 × 21 mm) covering the aneurysm neck and coils densely packed within the sac (white arrow indicates the end of the stent). (E) Angiography immediately after the procedure showed the aneurysm was occluded completely. (F) Follow-up angiography 10 months later showed complete aneurysmal occlusion and parent artery patency.
Case 2
A 57-year-old man was admitted to the hospital with a severe headache. Computed tomography showed SAH in the longitudinal and right Sylvian fissures as well as around the brainstem (Figure 2A). DSA showed a saccular anterior communicating artery aneurysm (Figure 2B). Coil embolization was performed, which achieved RR class I occlusion and relieved the headache (Figure 2C). Follow-up angiography 54 months later showed contrast within the aneurysm neck (Figure 2D). During re-treatment, an Echelon 10 microcatheter (Medtronic, Dublin, Ireland) was delivered into the aneurysm sac to place the coils with stent assistance (Figure 2E). Intraprocedural angiography showed the three radiopaque markers at the proximal and distal ends of the Atlas stent (3.0 × 15 mm) and coils within the sac (Figure 2F). Angiography immediately after embolization showed RR class I occlusion (Figure 2G). The aneurysm remained completely occluded and the parent artery was patent on follow-up angiography 8 months later (Figure 2H).
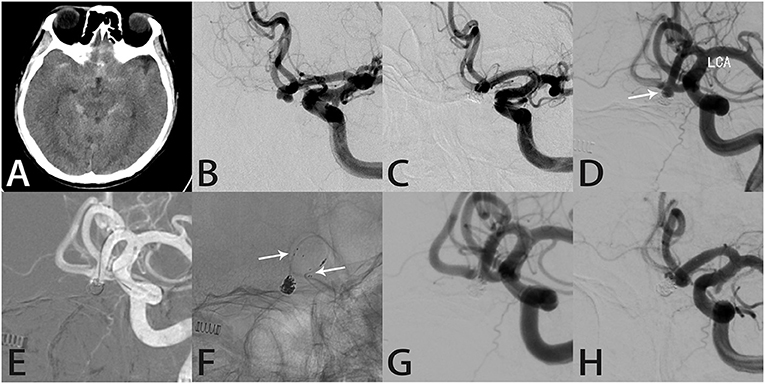
Figure 2. Images from a 57-year-old man with an anterior communicating artery aneurysm (case 2). (A) Computed tomography showed subarachnoid hemorrhage in the longitudinal and right Sylvian fissures as well as around the brainstem. (B) Preoperative angiography showed an anterior communicating artery aneurysm. (C) The aneurysm was occluded completely after coil embolization. (D) Follow-up angiography 54 months later showed an obvious recurrence in the aneurysm neck (white arrow). (E) During re-treatment, an Echelon 10 microcatheter (Medtronic, Dublin, Ireland) was delivered into the aneurysm sac to place the coils. (F) Intraprocedural angiography showed the three radiopaque markers (white arrows) at the proximal and distal ends of the Atlas stent (3.0 × 15 mm) and the coils within the aneurysm sac. (G) Angiography immediately after the procedure showed the aneurysm was occluded completely. (H) Follow-up angiography 8 months later showed complete aneurysmal occlusion and parent artery patency.
Case 4
A 48-year-old woman presented with severe headache. Computed tomography showed SAH in the longitudinal fissure (Figure 3A). DSA showed a right pericallosal aneurysm (Figure 3B). Coil embolization was performed (Figure 3C). RR class I occlusion was shown on angiography performed immediately after treatment (Figure 3D). Aneurysm recurrence was diagnosed 11 months later on follow-up angiography (Figure 3E). Because of the complexity of the aneurysm, we elected to perform SAC using the Atlas stent, which was successful without complications. Intraprocedural angiography showed the three radiopaque markers at the proximal and distal ends of the Atlas stent (3.0 × 21 mm) and coils within the sac (Figure 3F). Post-embolization angiography showed RR class I occlusion (Figure 3G). Seven months later, the aneurysm remained completely occluded and the parent artery was patent (Figure 3H).
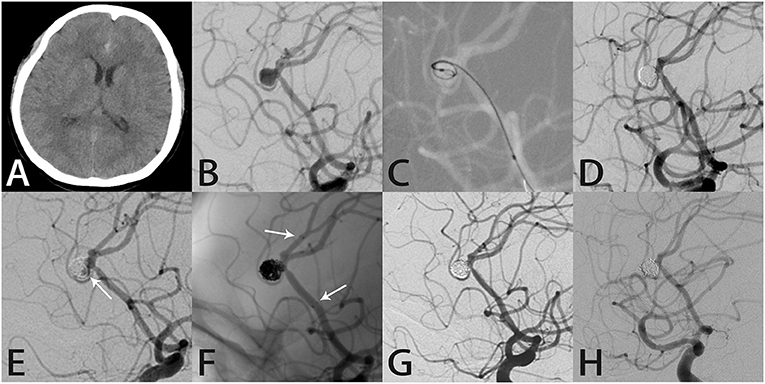
Figure 3. Images from a 48-year-old woman with a right pericallosal aneurysm (case 4). (A) Computed tomography showed subarachnoid hemorrhage in the longitudinal fissure. (B) Angiography showed a right pericallosal aneurysm. (C) During treatment, an Echelon 10 microcatheter (Medtronic, Dublin, Ireland) was delivered into the aneurysm sac to place the coils. (D) The aneurysm was occluded completely after coil embolization. (E) Follow-up angiography 11 months later showed recurrence in the aneurysm neck (white arrow). (F) Intraprocedural angiography showed the deployed Atlas stent (3.0 × 21 mm) covering the aneurysmal neck and coils densely packed within the sac (white arrows indicate the ends of the stent). (G) Angiography immediately after the procedure showed the aneurysm was occluded completely. (H) Follow-up angiography 7 months later showed complete aneurysmal occlusion with the coils densely packed within the aneurysm.
Case 9
A 51-year-old woman suddenly developed a severe headache with nausea and vomiting, followed by loss of consciousness, and was conscious for about 1 h later. Computed tomography performed in our hospital showed SAH in the brain basal cistern, lateral fissure cistern, longitudinal fissure cistern, and ambient cistern (Figure 4A). DSA showed a left posterior communicating artery aneurysm (Figure 4B). We performed coil embolization of the aneurysm, and immediate post-procedural angiographic showed that the aneurysm achieved RR class I occlusion (Figure 4C). The patient recovered well after the procedure, and the headache symptoms recovered at the time of discharge. However, the patient was re-admitted with severe headache 56 months after the initial coil embolization. Computed tomography confirmed that the patient was re-bleeding, and the SAH involved the lateral fissure cistern and sulcus (Figure 4D). The patient had Fisher score of grade 3 and a WFNS score of grade 1. DSA showed recurrence of the left posterior communicating artery aneurysm (Figure 4E). Considering that this patient was re-bleeding and led to recurrence 56 months after the initial coil embolization, after the discussion of several experts in our group, we finally decided to adopt the Atlas stent-assisted coiling treatment strategy. Atlas stents can reduce the incidence of recurrence by preventing coil protrusion into the parent artery, and the low metal coverage rate of the stent may also reduce the incidence of thromboembolic complications. Thus, we performed SAC using the Atlas stent (3.0 × 21 mm), and angiography immediately after re-treatment showed RR class II occlusion (Figure 4F), and the patient developed mildly blurred vision in the left eye after the procedure. Twelve months later, the follow-up angiography showed the aneurysm remained RR class II occlusion and the Atlas stent was stable (Figures 4G,H). Notably, the patient's vision was completely recovered.
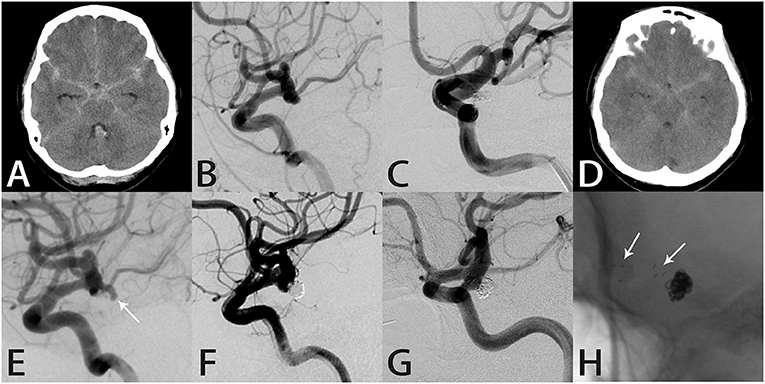
Figure 4. Images from a 51-year-old woman with a left posterior communicating artery aneurysm (case 9). (A) Computed tomography showed subarachnoid hemorrhage in the brain basal cistern, lateral fissure cistern, longitudinal fissure cistern, and ambient cistern. (B) Angiography showed a left posterior communicating artery aneurysm. (C) The aneurysm was occluded completely after coil embolization. (D) Computed tomography showed subarachnoid hemorrhage in the lateral fissure cistern and sulcus. (E) Angiography showed recurrence of the left posterior communicating artery aneurysm. (F) Angiography immediately after re-treatment showed RR class II occlusion. (G) Follow-up angiography 12 months later showed the aneurysm remained RR class II occlusion. (H) Intraprocedural angiography showed the Atlas stent remained stable and the three radiopaque markers (white arrows) could be seen at the proximal and distal ends of the Atlas stent (3.0 × 21 mm).
Discussion
The effectiveness of the Atlas stent in assisting coil embolization to achieve aneurysm occlusion with low rates of recurrence and morbidity has made it an appealing adjunct when treating intracranial aneurysms (12, 15, 16). As the incidence of aneurysm recurrence has increased in conjunction with increased use of endovascular treatment, so has the use of SAC with the Atlas stent to treat these recurrences. Currently, few data are available regarding SAC using the Atlas stent for treatment of recurrent aneurysms. Thus, in this study, we describe our experience spanning 1 year at a single center with the safety and efficacy of SAC using the Atlas stent to treat recurrent aneurysms after coil embolization.
Since the publication of the International Subarachnoid Aneurysm Trial, the paradigm for intracranial aneurysm treatment has gradually shifted from microsurgical clipping to endovascular intervention (17). Endovascular coil embolization is now widely used and is well-known for its low morbidity and mortality (18). However, risk of recurrence remains a major concern. A systematic review reported that 10% to 33.6% of all cerebral aneurysms treated with endovascular coil embolization recur and re-treatment rates range between 4.7 and 12.3% (19). In addition, recurrent aneurysms are associated with a higher rate of intracranial hemorrhage. Slob et al. (20) reported a 6.9% hemorrhage rate in patients with incompletely occluded aneurysms after initial coiling who were not re-treated; however, the rate was zero in those who underwent additional coiling. Therefore, re-treatment of recurrent aneurysms appears to reduce the risk of future hemorrhage.
Several endovascular treatment modalities are available to manage recurrent aneurysms, including re-coiling, SAC, and placement of a flow diverter. Previous studies have found that re-embolization using coils alone is associated with a higher rate of recurrence (21, 22). Stent assistance stabilizes the inserted coils, maintains parent artery patency, and provides protection against recurrence (22). Therefore, SAC may be a better alternative. Daou et al. (23) reported an 86.7% complete or near-complete occlusion rate after placement of the Pipeline embolization device (Medtronic, Dublin, and Ireland) in previously coiled recurrent aneurysms, demonstrating the efficacy of flow diversion. However, another study reported high rates of complications (17.2%) and permanent morbidity (6.9%) with this approach (24). Therefore, neurointerventionalists should carefully consider the appropriate treatment modality on an individual basis when managing recurrent aneurysms.
Re-treating recurrent aneurysms is frequently technically challenging. Accurate measurement of the recurrent lumen may be difficult owing to the previously inserted coils (25). Furthermore, if thrombus has formed within the lumen, it may dislodge into the parent artery and cause a serious thromboembolic complication (25). Moreover, many recurrent aneurysms have a wide neck and relatively shallow depth because of coil compaction (22). Therefore, the procedure may require complex manipulations through multiple microcatheters and stent assistance may be necessary to prevent coil protrusion and migration. Our technical success rate for SAC using the Atlas stent was 100%, which is comparable to previously reported rates for treatment of naïve aneurysms (5, 26). The Atlas stent can be successfully used for SAC of previously coiled aneurysms.
Our rates of RR class I and II occlusion immediately after the procedure were 90.9 and 9.1%, respectively, and these rates remained stable at the last follow-up. We attribute the favorable angiographic outcomes to our use of Atlas stent assistance. These rates compare favorably with other multicenter studies that have evaluated Atlas stent performance in SAC of naïve aneurysms (12, 16, 27). The rate of complete occlusion in these studies ranged from 81.3 to 86.7%. In addition, our results are comparable to those achieved in a multicenter study of SAC using the Acclino stent (Acandis GmbH, Pforzheim, Germany) to treat recurrent and residual aneurysms; this study reported a 94.7% complete occlusion rate immediately after the procedure that decreased to 76.9% at last angiographic follow-up (28). Previous studies of SAC using the LVIS Jr (MicroVention, Aliso Viejo, California, USA) and LEO Baby (Balt Extrusion, Montmorency, France) stents have focused on the treatment of naïve aneurysms (8, 29); they have not been examined yet for treatment of recurrent aneurysms.
Procedure-related complications occurred in only one patient (9.1%), a 51-year-old woman with a recurrent left posterior communicating artery aneurysm who developed mildly blurred vision in the left eye. Angiography immediately after re-treatment showed RR class II occlusion. Although her vision completely recovered, follow-up angiography remained RR class II occlusion. We believe her blurred vision may be attributed to oculomotor nerve palsy (ONP). Unilateral ONP occurs in approximately 25% of patients with a posterior communicating artery aneurysm (30) and often occurs at the time of aneurysm rupture. The cause may be rupture-related trauma to the nerve or the presence of localized hematoma and/or subarachnoid blood (31). However, ONP may also be observed in association with unruptured aneurysms because of direct mechanical compression from the aneurysm sac and/or aneurysm pulsatility (32). Considering that this patient's aneurysm was relatively small and unruptured, we presume that the most likely cause was aneurysm pulsatility. A previous study reported that coiling can eliminate aneurysmal pulsations, which allows more complete nerve recovery (31). This patient's vision recovery supports this hypothesis.
Two previous studies of SAC using the Atlas stent for treatment of naïve aneurysms have reported thromboembolic complication rates of 3.8 and 2.3%, respectively, and hemorrhagic complication rates of 0.8 and 0.8%, respectively (33, 34). Reported thromboembolic complication rates in SAC studies using the Neuroform and Enterprise (Codman Neurovascular, Raynham, MA, USA) stents were 8.8 and 8.7%, respectively (35, 36). The Atlas stent appears to be associated with a lower rate of thromboembolic complications than conventional stents. One possible explanation is that its miniaturized design and delivery system reduces exposure of the metal-covered surfaces to blood flow, which reduces thrombus formation.
All patients who initially presented with SAH in our study were treated using coil embolization alone, suggesting that most neurointerventionalists are reluctant to use stent assistance when embolizing acutely ruptured aneurysms. This is understandable because the body is in a hypercoagulable state in the acute stage of SAH and introduction of a stent into the cerebral vasculature may increase the risk of thromboembolism. Furthermore, the antiplatelet therapy that is generally associated with stent placement may increase the risk of re-hemorrhage, especially in patients with an intraventricular catheter. In patients with a ruptured aneurysm, the rate of ventriculostomy-related re-hemorrhage is 3.4 times higher in those who undergo SAC than in those who undergo coiling alone (37). Therefore, stent use should be considered with caution in ruptured aneurysm patients.
Although the results of this study are encouraging, future large-scale studies with long-term follow-up are needed to fully evaluate the efficacy of SAC using the Atlas stent for recurrent aneurysms.
Limitations
This study has several limitations. Its retrospective single-center design lacked a control group and selection bias may have been introduced. In addition, its sample size was small and the follow-up period was short; therefore, our reported occlusion rates may not accurately reflect the true rates.
Conclusion
SAC using the Atlas stent to treat aneurysms that recur after coil embolization is safe and effective. However, large-scale studies with long-term follow-up are necessary to validate our results.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was reviewed and approved by the Ethics Committee of Beijing Tiantan Hospital. Written informed consent to participate in this study was provided by the patients or their legal guardian/next of kin.
Author contributions
JW, ML and PL: conception and design. XC, LZ, ZZ, QP, and ZJ: data analysis and interpretation. LD and JW: manuscript writing. The final version was approved by ML on behalf of all authors. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Youth Program of National Natural Science Foundation of China (Grant No. 81901197) and National Key Research and Development Program of the 14th Five-Year Plan (Grant No. 2021YFC2501100).
Acknowledgments
We thank Liwen Bianji (Edanz) (https://www.liwenbianji.cn) for editing the language of a draft of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer YW declared a shared parent affiliation with the authors to the handling editor at the time of review.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhang Q, Jing L, Liu J, Wang K, Zhang Y, Paliwal N, et al. Predisposing factors for recanalization of cerebral aneurysms after endovascular embolization: a multivariate study. J Neurointerv Surg. (2018) 10:252–7. doi: 10.1136/neurintsurg-2017-013041
2. Chalouhi N, Jabbour P, Singhal S, Drueding R, Starke RM, Dalyai RT, et al. Stent-assisted coiling of intracranial aneurysms: predictors of complications, recanalization, and outcome in 508 cases. Stroke. (2013) 44:1348–53. doi: 10.1161/STROKEAHA.111.000641
3. Lecler A, Raymond J, Rodriguez-Régent C, Al Shareef F, Trystram D, Godon-Hardy S, et al. Intracranial aneurysms: recurrences more than 10 years after endovascular treatment-a prospective cohort study, systematic review, and meta-analysis. Radiology. (2015) 277:173–80. doi: 10.1148/radiol.2015142496
4. Ferns SP, Sprengers ME, van Rooij WJ, Rinkel GJ, van Rijn JC, Bipat S, et al. Coiling of intracranial aneurysms: a systematic review on initial occlusion and reopening and retreatment rates. Stroke. (2009) 40:e523–e29. doi: 10.1161/STROKEAHA.109.553099
5. Caragliano AA, Papa R, Pitrone A, Limbucci N, Nappini S, Ruggiero M, et al. The low-profile Neuroform Atlas stent in the treatment of wide-necked intracranial aneurysms–immediate and midterm results: an Italian multicenter registry. J Neuroradiol. (2020) 47:421–7. doi: 10.1016/j.neurad.2019.03.005
6. Wanke I, Forsting M. Stents for intracranial wide-necked aneurysms: more than mechanical protection. Neuroradiology. (2008) 50:991–8. doi: 10.1007/s00234-008-0460-0
7. Zhang X, Zuo Q, Tang H, Xue G, Yang P, Zhao R, et al. Stent assisted coiling versus non-stent assisted coiling for the management of ruptured intracranial aneurysms: a meta-analysis and systematic review. J Neurointerv Surg. (2019) 11:489–96. doi: 10.1136/neurintsurg-2018-014388
8. Luecking H, Struffert T, Goelitz P, Engelhorn T, Brandner S, Kuramatsu JB, et al. Stent-assisted coiling using leo+ baby stent: immediate and mid-term results. Clin Neuroradiol. (2021) 31:409–16. doi: 10.1007/s00062-020-00904-3
9. Fiorella D, Boulos A, Turk AS, Siddiqui AH, Arthur AS, Diaz O, et al. The safety and effectiveness of the LVIS stent system for the treatment of wide-necked cerebral aneurysms: final results of the pivotal US LVIS trial. J Neurointerv Surg. (2019) 11:357–61. doi: 10.1136/neurintsurg-2018-014309
10. Vollherbst DF, Berlis A, Maurer C, Behrens L, Sirakov S, Sirakov A, et al. Periprocedural Safety and feasibility of the new LVIS EVO device for stent-assisted coiling of intracranial aneurysms: an observational multicenter study. AJNR Am J Neuroradiol. (2021) 42:319–26. doi: 10.3174/ajnr.A6887
11. Sweid A, Herial N, Sajja K, Chalouhi N, Velagapudi L, Doermann A, et al. Early multicenter experience with the neuroform atlas stent: feasibility, safety, and efficacy. Neurosurgery. (2020) 87:E321–35. doi: 10.1093/neuros/nyaa143
12. Zaidat OO, Hanel RA, Sauvageau EA, Aghaebrahim A, Lin E, Jadhav AP, et al. Pivotal trial of the neuroform atlas stent for treatment of anterior circulation aneurysms: one-year outcomes. Stroke. (2020) 51:2087–94. doi: 10.1161/STROKEAHA.119.028418
13. Jankowitz BT, Jadhav AP, Gross B, Jovin TG, Alhajeri AA, Fraser JF, et al. Pivotal trial of the neuroform atlas stent for treatment of posterior circulation aneurysms: one-year outcomes. J Neurointerv Surg. (2022) 14:143–8. doi: 10.1136/neurintsurg-2020-017115
14. Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. (2001) 32:1998–2004. doi: 10.1161/hs0901.095600
15. Ten Brinck MFM, De Vries J, Bartels RHMA, Grotenhuis JA, Boogaarts HD. NeuroForm atlas stent-assisted coiling: preliminary results. Neurosurgery. (2019) 84:179–89. doi: 10.1093/neuros/nyy048
16. Arslan G, Maus V, Weber W, Berlis A, Maurer C, Fischer S, et al. Two-center experience with neuroform atlas stent-assisted coil occlusion of broad-based intracranial aneurysms. Neuroradiology. (2021) 63:1093–101. doi: 10.1007/s00234-020-02602-w
17. Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. (2005) 366:809–17. doi: 10.1016/S0140-6736(05)67214-5
18. Pouratian N, Oskouian RJ, Jensen ME, Kassell NF, Dumont AS. Endovascular management of unruptured intracranial aneurysms. J Neurol Neurosurg Psychiatry. (2006) 77:572–8. doi: 10.1136/jnnp.2005.078469
19. Ries T, Siemonsen S, Thomalla G, Grzyska U, Zeumer H, Fiehler J, et al. Long-term follow-up of cerebral aneurysms after endovascular therapy prediction and outcome of retreatment. AJNR Am J Neuroradiol. (2007) 28:1755–61. doi: 10.3174/ajnr.A0649
20. Slob MJ, Sluzewski M, Van Rooij WJ, Roks G, Rinkel GJ. Additional coiling of previously coiled cerebral aneurysms: clinical and angiographic results. AJNR Am J Neuroradiol. (2004) 25:1373–6.
21. Lee J, Lim JW, Cho YD. Follow-up outcomes after re-embolization for recanalized aneurysms after initial coiling: further recurrence rates and related risk factors. World Neurosurg. (2018) 114:e508–17. doi: 10.1016/j.wneu.2018.03.017
22. Cho YD, Lee JY, Seo JH, Lee SJ, Kang HS, Kim JE, et al. Does stent implantation improve the result of repeat embolization in recanalized aneurysms? Neurosurgery. (2012) 71:253–9. doi: 10.1227/NEU.0b013e3182647a97
23. Daou B, Starke RM, Chalouhi N, Tjoumakaris S, Khoury J, Hasan D, et al. The use of the pipeline embolization device in the management of recurrent previously coiled cerebral aneurysms. Neurosurgery. (2015) 77:692–7. doi: 10.1227/NEU.0000000000000901
24. Benaissa A, Januel AC, Herbreteau D, Berge J, Aggour M, Kadziolka K, et al. Endovascular treatment with flow diverters of recanalized and multitreated aneurysms initially treated by endovascular approach. J Neurointerv Surg. (2015) 7:44–9. doi: 10.1136/neurintsurg-2013-011046
25. Li YD, Li MH, Gao BL, Fang C, Cheng YS, Wang W, et al. Endovascular treatment of recurrent intracranial aneurysms with re-coiling or covered stents. J Neurol Neurosurg Psychiatry. (2010) 81:74–9. doi: 10.1136/jnnp.2009.171967
26. Hanel RA, Yoon N, Sauvageau E, Aghaebrahim A, Lin E, Jadhav AP, et al. Neuroform atlas stent for treatment of middle cerebral artery aneurysms: 1-year outcomes from neuroform atlas stent pivotal trial. Neurosurgery. (2021) 89:102–8. doi: 10.1093/neuros/nyab090
27. Jankowitz BT, Hanel R, Jadhav AP, Loy DN, Frei D, Siddiqui AH, et al. Neuroform atlas stent system for the treatment of intracranial aneurysm: primary results of the atlas humanitarian device exemption cohort. J Neurointerv Surg. (2019) 11:801–6. doi: 10.1136/neurintsurg-2018-014455
28. Pflaeging M, Goertz L, Smyk MA, Turowski B, Mpotsaris A, Pennig L, et al. Treatment of recurrent and residual aneurysms with the low-profile acandis acclino stent: multi-center review of 19 patients. J Clin Neurosci. (2021) 90:199–205. doi: 10.1016/j.jocn.2021.05.051
29. McEachern J, Iancu D, Van Adel B, Drake B, Kaderali Z, Spirou M, et al. Long term safety and effectiveness of LVIS Jr for treatment of intracranial aneurysms-a Canadian Multicenter registry. Int Neuroradiol. (2022) 83:267–73. doi: 10.1177/15910199221077588
30. Hall S, Sadek AR, Dando A, Grose A, Dimitrov BD, Millar J, et al. The resolution of oculomotor nerve palsy caused by unruptured posterior communicating artery aneurysms: a cohort study and narrative review. World Neurosurg. (2017) 107:581–7. doi: 10.1016/j.wneu.2017.07.123
31. McCracken DJ, Lovasik BP, McCracken CE, Caplan JM, Turan N, Nogueira RG, et al. Resolution of oculomotor nerve palsy secondary to posterior communicating artery aneurysms: comparison of clipping and coiling. Neurosurgery. (2015) 77:931–9. doi: 10.1227/NEU.0000000000000965
32. Hassan T, Hamimi A. Successful endovascular management of brain aneurysms presenting with mass effect and cranial nerve palsy. Neurosurg Rev. (2013) 36:87–97. doi: 10.1007/s10143-012-0404-3
33. Kwon O, Chung J. Outcomes of stent-assisted coiling using the neuroform atlas stent in unruptured wide-necked intracranial aneurysms. J Korean Neurosurg Soc. (2021) 64:23–9. doi: 10.3340/jkns.2020.0054
34. Burkhardt JK, Srinivasan V, Srivatsan A, Albuquerque F, Ducruet AF, Hendricks B, et al. Multicenter postmarket analysis of the neuroform atlas stent for stent-assisted coil embolization of intracranial aneurysms. AJNR Am J Neuroradiol. (2020) 41:1037–42. doi: 10.3174/ajnr.A6581
35. Fiorella D, Albuquerque FC, Woo H, Rasmussen PA, Masaryk TJ, McDougall CG, et al. Neuroform stent assisted aneurysm treatment: evolving treatment strategies, complications and results of long term follow-up. J Neurointerv Surg. (2010) 2:16–22. doi: 10.1136/jnis.2009.000521
36. Kadkhodayan Y, Rhodes N, Blackburn S, Derdeyn CP, Cross DT, Moran CJ. Comparison of enterprise with neuroform stent-assisted coiling of intracranial aneurysms. Am J Roentgenol. (2013) 200:872–8. doi: 10.2214/AJR.12.8954
Keywords: Neuroform Atlas stent, recurrent aneurysms, stent-assisted coiling, endovascular treatment, previously coiled
Citation: Dong L, Wang J, Chen X, Zhang L, Zhao Z, Peng Q, Jin Z, Wu J, Lv M and Liu P (2022) Stent-assisted coiling using the Neuroform Atlas stent for treatment of aneurysms that recur after coil embolization. Front. Neurol. 13:967942. doi: 10.3389/fneur.2022.967942
Received: 13 June 2022; Accepted: 29 August 2022;
Published: 27 September 2022.
Edited by:
Osama O. Zaidat, Northeast Ohio Medical University, United StatesCopyright © 2022 Dong, Wang, Chen, Zhang, Zhao, Peng, Jin, Wu, Lv and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Wu, d3VqdW5zbGZAMTI2LmNvbQ==; Ming Lv, ZHJhZ29udGlnZXJAMTYzLmNvbQ==; Peng Liu, c2tlbGV0b25saXUxOTg3QDE2My5jb20=
†These authors have contributed equally to this work
 Linggen Dong
Linggen Dong Jiejun Wang
Jiejun Wang Xiheng Chen
Xiheng Chen Longhui Zhang
Longhui Zhang Zhiqiang Zhao1
Zhiqiang Zhao1 Qichen Peng
Qichen Peng Zeping Jin
Zeping Jin Ming Lv
Ming Lv Peng Liu
Peng Liu