
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 10 August 2022
Sec. Endovascular and Interventional Neurology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.967395
Objective: Nearly half of patients who undergo mechanical thrombectomy (MT) do not experience a favorable outcome. The association between blood pressure fluctuation and clinical outcomes after successful MT is controversial. We evaluated the influence of blood pressure variability (BPV) on the clinical outcomes of stroke patients with large vessel occlusion (LVO) who underwent successful recanalization after MT.
Methods: Patients with anterior circulation LVO stroke who underwent successful emergency MT (modified Thrombolysis in Cerebral Infarction, mTICI ≥ 2b) at the Shanghai Tenth People's Hospital of Tongji University from 2017 to 2021 were enrolled. Multivariate logistic models were used to investigate the association between BPV (mean arterial pressure [MAP] assessed using the standard deviation [SD]) and clinical outcomes. The primary outcome was 90-day modified Rankin Scale scores (mRS), and the secondary outcomes were 30-day mortality and symptomatic intracranial hemorrhage (sICH).
Results: A total of 458 patients (56.8% men), with a mean age of 72 ± 1 years, were enrolled. Among them, 207 (45.2%) patients had unfavorable functional outcomes (mRS score 3–6) at 90 days, 61 (13.3%) patients died within 30 days, and 20 (4.4%) patients had sICH. In a fully adjusted model, BPV was associated with a higher risk of a 90-day mRS score of 3–6 (P = 0.04), 30-day mortality (P < 0.01), and sICH (P < 0.01). A significant interaction between MAP SD and rescue futile recanalization treatment was observed (P < 0.01).
Conclusions: Among patients with LVO stroke who underwent successful recanalization, higher BPV was associated with worse functional outcomes, especially in those who underwent rescue treatment.
Large vessel occlusion (LVO) stroke is associated with a 4.5-fold increased risk of mortality compared to non-LVO stroke (1). Mechanical thrombectomy (MT) is the most effective treatment method for patients with LVO stroke and has a one-fold higher recanalization rate when compared to intravenous thrombolysis for patients with acute ischemic stroke caused by anterior circulation LVO. However, nearly half of patients with successful recanalization fail to achieve functional improvement (2).
Hypertension is one of the most common risk factors for stroke (3). Most observational studies have suggested that increased blood pressure (BP) after MT increases mortality and symptomatic intracranial hemorrhage (sICH) (4, 5). However, BP management after endovascular therapy remains a clinical challenge. The American Stroke Association (ASA) guidelines recommend maintaining a BP level of <180/105 mmHg for 24 h after MT, based on low-level evidence (6). In the DAWN trial, a systolic blood pressure (SBP) of <140 mmHg is maintained in the first 24 h in patients with successful reperfusion (defined as achieving a modified Thrombolysis in Cerebral Infarction (mTICI) grading system score of 2b-3) after MT (7). Contrarily, 62% of the stroke centers in the USA set a target SBP of <160 mmHg for patients with successful reperfusion (8). There were no significant differences in the efficacies between the intensive and non-intensive arms in the BP-TARGET trial, suggesting BP parameters other than SBP may be closely associated with clinical outcomes (9).
Blood pressure variability (BPV) has been reported to be a better predictor of all-cause and cardiovascular mortality and cardiac disease than other BP paraments (10). The influence of BPV on clinical outcomes in stroke patients has recently attracted growing attention (11, 12). However, conflicting conclusions have been reported regarding the association between BPV and clinical outcomes after MT. A secondary analysis of the prospective cohort study BEST revealed that higher BPV is associated with exacerbated 90-day outcomes (13), and yet post hoc analyses of the random clinical trial BP-TARGET suggested that BPV was not associated with functional outcomes (14).
Therefore, we performed a retrospective cohort study to investigate the association between BPV after successful recanalization and clinical outcomes in patients with LVO after MT.
This study was a retrospective cohort analysis conducted at a comprehensive stroke center (Shanghai Tenth People's Hospital, Tongji University). Consecutive patients with anterior circulation LVO stroke who underwent emergency MT between January 2017 and December 2021 were enrolled. The study was approved by the local ethics committee (No. SHSY-IEC-4.1/21-208/01).
Patients were registered if they met the following inclusion criteria: (1) aged ≥18 years; (2) time from stroke onset to puncture (OTP) was ≤6 h; (3) baseline National Institutes of Health Stroke Scale (NIHSS) score of ≥6, baseline Alberta Stroke Program Early computed tomography (ASPECT) score of ≥6 and pre-stroke modified Rankin Scale (mRS) score of <2; (4) occlusion of the internal carotid artery, proximal segment (M1/M2) of the middle cerebral artery confirmed by computed tomography angiography or digital subtraction angiography imaging; (5) successful recanalization, defined as an mTICI score of 2b or 3 after MT. Patients were not enrolled based on the following exclusion criteria: (1) insufficient BP data (recorded for <24 hours); (2) incomplete radiographic images; and (3) absence of follow-ups. The flow chart of the study population inclusion is shown in Figure 1.
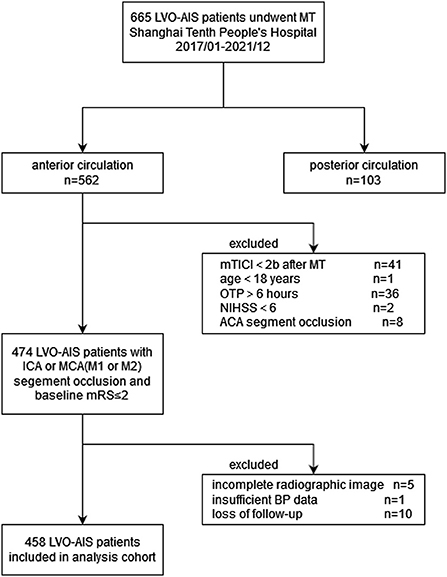
Figure 1. Flow chart of patient inclusion. LVO, Large vessel occlusion; AIS, acute ischemic stroke; MT, mechanical thrombectomy; mTICI, modified tissue thrombolysis in cerebral ischemia; OTP, onset to Puncture time (minute); NIHSS, national institutes of health stroke scale; ACA, anterior cerebral artery; ICA, internal carotid artery; MCA, middle cerebral artery; mRS, modified Rankin Scale; BP, blood pressure.
LVO in the anterior circulation was defined as occlusion of the internal carotid artery or middle cerebral artery segment M1 or proximal M2 (2). Rescue treatment was defined as balloon angioplasty and/or stenting (15). The recanalization status of the patients was evaluated based on the mTICI grading system. Successful recanalization was defined as an modified Thrombolysis in Cerebral Infarction (mTICI) score of 2b or 3 (16, 17).
Although the use of continuous intravenous antihypertensive agents had not been previously defined in the data, steady-state use was defined as long-term use. In the Chinese guidelines for endovascular treatment, recommendations for BP management include maintaining a BP of <140/90 mmHg while avoiding one of <100/60 mmHg.
According to previous studies (13, 14, 18, 19), demographic characteristics (sex and age), medical history (hypertension, diabetes, and atrial fibrillation), admission variables (baseline NIHSS score, ASPECT score, and level of occlusion), procedural variables (bridging therapy, puncture to recanalization [PTR] time, onset to recanalization [OTR] time, rescue treatment, and postoperative continuous intravenous therapy), and BP data 24 h after MT were collected.
Demographic characteristics, medical history, baseline NIHSS scores, and BP data (SBP/diastolic blood pressure, [DBP] were obtained from an electronic medical database. If the NIHSS score was not documented in the database, it was extrapolated from documented neurologic examinations using a validated method. The ASPECT score was calculated for these patients by a neurologist and radiologist who were blinded to the patient outcomes.
The procedural parameters were recorded by the operators, including PTR, OTR, occlusion site, bridging, rescue treatment, and continuous intravenous antihypertensive agents administered.
BP was measured at 1-h intervals from admission for the first 24 h after MT. If the patient was out for examination at the time of BP measurement, BP was measured 10 min after returning to the stroke unit. Nurses in the stroke and intensive care units recorded the blood pressure measurements into the electronic medical record. BP data were obtained from an electronic medical database in our study. The standard deviation (SD) of the mean arterial pressure (MAP) was used as the key indexes.
The primary outcome was futile recanalization, defined as a 90-day mRS score of 3–6 (20).
The secondary outcomes included 30-day mortality and sICH. sICH was defined as any intraparenchymal, subarachnoid, or intraventricular hemorrhage diagnosed by post-endovascular thrombectomy non-contrast head CT with a ≥4 point increase in the NIHSS score within 24 h after endovascular thrombectomy according to the European-Australasian Acute Stroke Study II criteria (21).
Outcome assessments were obtained through clinic or telephone follow-ups if specific documentation was not available in the electronic medical database.
Continuous variables are presented as means ± standard deviation (normal distribution) or median (interquartile range for skewed distribution). Categorical variables are presented as numbers (%). Continuous variables were analyzed using the Kruskal-Wallis test. Categorical variables were analyzed using the Chi-squared test or Fisher's exact test, as appropriate.
Logistic regression models were used to investigate any BPV correlations and to calculate the odds ratios between BPV and each outcome. Associations between BPV and outcomes were assessed using continuous variables with BPV as a continuous variable (per SD). According to the recommendations of the STROBE statement (22), we simultaneously show the results of unadjusted (Crude Model), minimally adjusted (model I, adjusted for demographic characteristics, medical history, and admission variables), and fully adjusted analyses (model II, adjusted for model I and procedural variables). We also used smooth curve fitting (penalized spline method) to identify the nonlinear relationships between the continuous variables (age, baseline NIHSS, PTR time, OTR time, MAP mean) and outcomes. Nonlinear regression (curve fit) was used for the variables that did not fit the linear model. To evaluate the robustness of this study, a subgroup analysis using a logistic model was performed as a sensitivity test. We converted it to a categorical variable according to the clinical cut-off point and then performed an interaction test effect modification for subgroup indicators using the likelihood ratio test.
Data were analyzed using the R statistical packages (The R Foundation; http://www.r-project.org; version 3.4.3) and EmpowerStats (www.empowerstats.com; X&Y Solutions Inc.). Statistical significance was defined as a two-sided P < 0.05.
From January 2017 to December 2021, 665 consecutive patients were enrolled in the thrombectomy cohort. Among them, 562 (84.5%) were ischemic stroke in the anterior circulation. We enrolled 474 patients in the initial cohort according to the inclusion criteria. In total, 16 patients (3.4%) were excluded from the final analysis because of incomplete radiographic images (n = 5), insufficient BP data (n = 1, this patient died within 24 h after thrombectomy) and lost to follow-up (n = 10). Consequently, 458 patients were analyzed (Figure 1).
The baseline characteristics of all participants according to MAP SD quartiles are shown in Table 1. The mean age of the participants was 71.7 ± 10.7 years, and 56.8% were men. Among them, 74.2% had hypertension, 28.2% had diabetes mellitus, and 46.7% had atrial fibrillation. The mean baseline NIHSS score was 16.0 ± 4.1, PTR time was 47.0 min (30.0–70.0), and onset-to-reperfusion time was 270.1 ± 83.5 min.
Participants with higher BPV tended to be older, men, had lower ASPECT scores, higher baseline NIHSS scores, hypertension, and showed higher usage of continuous intravenous antihypertensive agents.
A total of 207 (45.2%) patients had unfavorable functional outcomes (mRS score 3–6) at 90 days; 61 (13.3%) patients died within 30 days, and 20 (4.4%) patients experienced sICH (Table 1). Patients with higher BPV tended to have a higher prevalence of futile recanalization (Q1: 34.8%; Q2: 37.7%; Q3: 45.6%; Q4: 62.6%; P < 0.001), mortality (Q1: 5.2%; Q2: 9.6%; Q3: 10.5%; Q4: 27.8%; P < 0.001), and sICH (Q1: 1.7%; Q2:2.6%; Q3: 5.3%; Q4: 7.8%; P = 0.101) (Table 1 and Figures 2–4).
The results of the univariate analyses are summarized in Supplementary Table S1. Univariate analyses revealed an increased incidence for each outcome in patients with higher BPV (P < 0.05). Each 1 mmHg increment of MAP SD was associated with the following increases: 17% in futile recanalization (95% confidence interval [CI: 1.09 to 1.26); 35% in 30 day-mortality (95% CI: 1.22 to 1.48); and 23% in symptomatic intracerebral hemorrhage (95% CI: 1.10 to 1.37). No significant correlation was found between higher MAP means and each outcome (P > 0.05).
Smooth curve fitting plots (Supplementary Figures S1–S3) suggest that age, NIHSS score, PTR time, and OTR time were linearly related to all outcomes. While, MAP mean was linearly related to futile recanalization and symptomatic intracerebral hemorrhage instead of 30-day mortality. Thus, nonlinear regression (curve fit) was used for MAP mean in the multivariable analysis between 30-day mortality.
According to the above results, we performed a multivariate logistic analysis to further explore MAP SD as a prognostic marker. In the multivariable analysis, and the data with and without adjustment are listed in Table 2. The MAP SD level was an independent risk factor for poor functional outcome at 90 days in Model I (OR adj = 1.37, per 1-SD increase, 95% CI: 1.08–1.72, P = 0.009) and Model II (OR adj = 1.31, per 1-SD increase, 95% CI: 1.01–1.68, P = 0.039). Multivariable logistic regression also revealed a significant association between MAP SD level and 30-day mortality (OR adj = 1.80, per 1-SD increase, 95% CI: 1.29–2.50, P = 0.001, Model II) and sICH (OR adj = 1.69, per 1-SD increase, 95% CI: 1.15–2.47, P = 0.007, Model II).
To evaluate other potentially influencing factors, we conducted a sub-analysis by stratifying patients according to age, sex, hypertension, diabetes mellitus, atrial fibrillation, and level of occlusion; ASPECT score, baseline NIHSS score, PTR time, OTR time, rescue treatment, continuous intravenous antihypertensive agents, bridging therapy, and mean MAP, as presented in Figures 5, 6. The number of patients with sICH was low; therefore, subgroup analysis was not performed. Notably, all subgroups demonstrated a similar relationship (all OR>1) between MAP SD level and post-operative futile recanalization and 30 day-mortality.
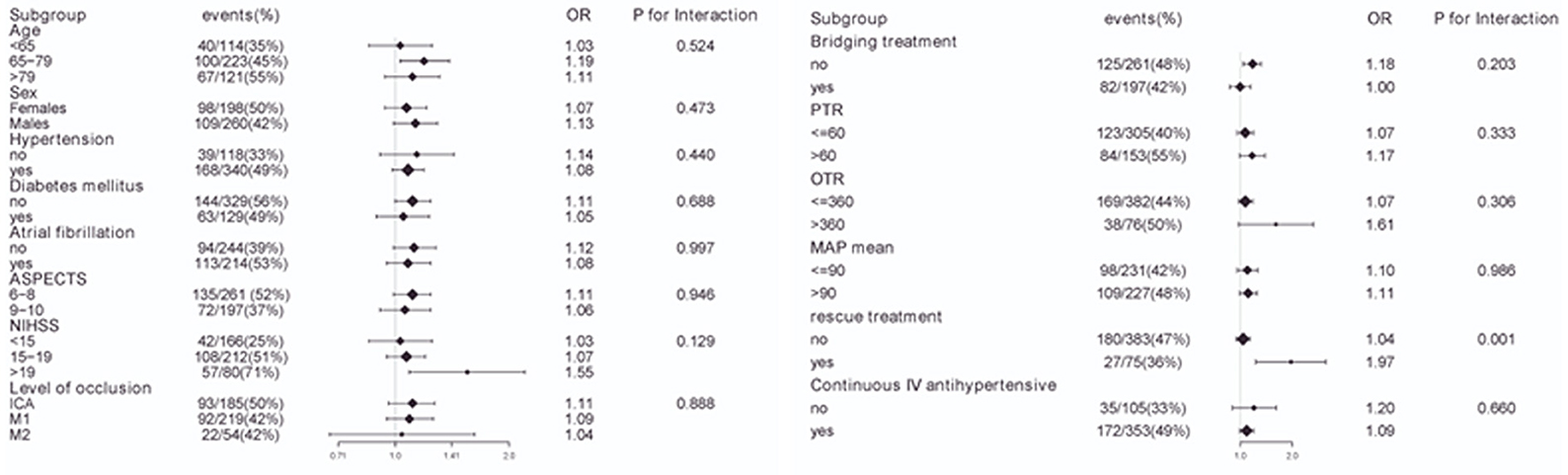
Figure 5. Interaction test for the effect of MAP SD on futile recanalization in different subgroups after adjusting for confounding factors. The above model adjusted for confounding factors in Table 2 (Model II). In each case, the model is not adjusted for the stratification variable.
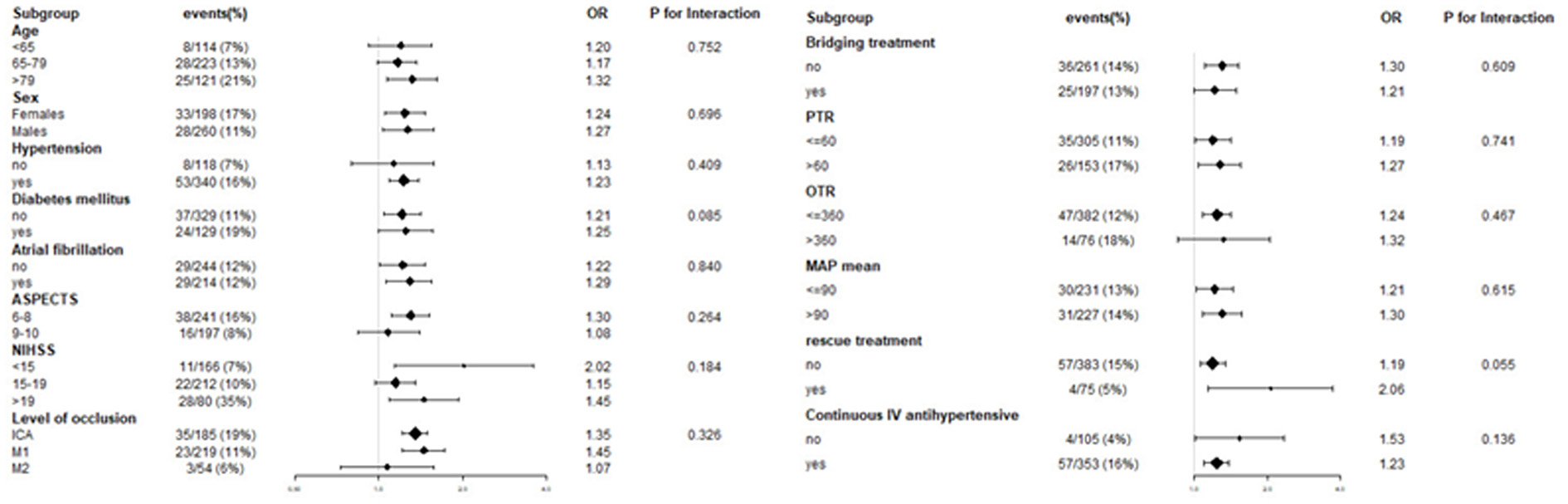
Figure 6. Interaction test for the effect of MAP SD on 30-day mortality in different subgroups after adjusting for confounding factors. The above model adjusted for confounding factors in Table 2 (Model II). In each case, the model is not adjusted for the stratification variable.
The stratified analyses of futile recanalization between each subgroup are shown in Figure 5. The interaction analysis revealed that rescue treatment played an interactive role in the association between BPV and futile recanalization (Figure 7). The patients who underwent rescue treatment had a higher odds ratio (OR) between BPV and futile recanalization (OR = 1.97; 95% CI, 1.23–3.14; P = 0.001) than those without rescue treatment (OR = 1.04; 95% CI, 0.95–1.14). The stratified analyses of 30-day mortality between each subgroup are shown in Figure 6. Logistic regression analysis did not demonstrate a significant relationship for 30 day-mortality between BPV and all subgroups (P > 0.05).
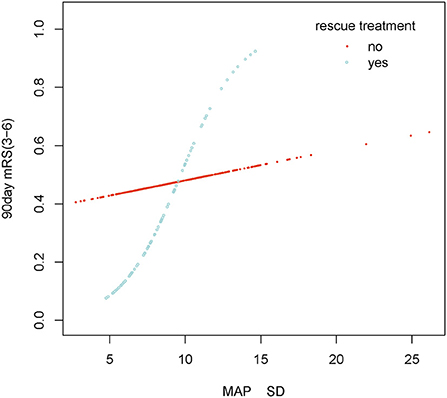
Figure 7. Smooth curve fitting of MAP SD and futile recanalization in rescue treatment subgroup. Adjustment variables: Male, Age, Hypertension, Diabetes mellitus, Atrial fibrillation, Level of occlusion on DSA imaging, ASPECTS, Baseline NIHSS, Puncture to recanalization, Onset to recanalization, rescue treatment, Continuous intravenous antihypertensive agents, Bridging treatment, MAP mean.
Our study showed that the successful recanalization (TICI 2b-3) rate of patients with LVO treated with MT was above 94%. The lower futile recanalization rate in our study compared to that in the HERMES study (2) (45.2 vs. 54.0%, respectively) may be attributed to the different periods in which the two studies were conducted (2017 vs. 2014), considering the advances in surgical devices and change in choice of surgical procedures [enhanced rescue treatment may improve neurological outcomes (23)]. Our study demonstrated similar 30-day mortality (13.3 vs. 15.3%, respectively) and sICH (4.4 vs. 4.4%, respectively) to the HERMES study. In this study, short-term BPV was significantly associated with adverse clinical outcomes.
BP is one of the strongest clinical decision tools (24, 25). The ASA guidelines recommended a BP target of 180/105 mmHg after MT as a reasonable extrapolation from intravenous thrombolysis literature (6). However, recanalization rates with MT are much higher than those of intravenous thrombolysis alone, and it remains unclear whether BP targets for patients who undergo MT should be the same. Given the considerable interindividual differences in hypertension history and collateral circulation, an individualized optimal BP may be more suitable than an absolute BP level (26).
Mechanisms such as exhausted collateral circulation, steal effects, and impaired cerebral autoregulation are more pronounced in patients with LVO stroke. Within a certain range, an almost linear relationship exists between cerebral blood flow and BP. The effects of inapt BP are more likely to be felt to a greater extent in the brain, which can lead to detrimental consequences (27). The 24-h postoperative MAP in this study was 89.9 mmHg, which is similar to the MAP target recommended by previous animal studies, clinical studies, and meta-analyses (28–30). However, the optimal mean MAP was not associated with favorable outcomes in our study.
Increased BPV is detrimental to functional outcomes after ischemic stroke (10); however, 24-h short-term BPV (assessed using ambulatory BP monitoring) shows a trend toward favorable outcomes after MT (31). MAP, a combination of SBP and DBP, is a more reliable biomarker for perfusion pressure in the brain (32). Considering that SBP or DBP is vulnerable to short fluctuations, we chose MAP as a predictive marker instead.
After adjusting for critical prognostic covariates of stroke, our study found that BPV was overall associated with unfavorable functional outcomes, which is consistent with the results of two recent studies. Secondary analysis of the BEST Study (a prospective, multicenter cohort study) indicated that patients with an increase in BPV are predisposed to poorer outcomes (90-day mRS 3–6) (13). Another prospective cohort study from Portugal showed that BPV was independently associated with poor functional outcomes at 90 days (19). However, our findings are inconsistent with a post hoc analysis of the BP TARGET trial, which showed that BPV was not associated with functional outcomes or sICH (19).
This discrepancy may be due to the following: (1) The number of BP measurements in the BP TARGET trial (15–16) was less than that in the other studies (24–37). The SBP SD/DBP SD (3.8–5.3/4.4–4.7 mmHg) in the BP TARGET trial were lower than those in the BEST study (13.9/10.4 mmHg), Portugal (13 /9 mmHg), and our study (12.5 /9.1 mmHg). Therefore, the data from the BP TARGET trial may fail to reflect actual BP fluctuations. (2) There were differences in the statistical methods (different adjusted covariates) and study populations among these studies. (3) The rate of internal carotid artery (ICA) occlusion in the BP TARGET trial (26%) was lower than that in our study (40%). Our subgroup analysis found that BPV after MT had a more substantial effect on patients with occlusion of the extracranial internal carotid artery when compared to occlusion of the intracranial middle cerebral artery. The differences in curvature and hemodynamic changes between the extracranial and intracranial arteries are the reason for this difference. Therefore, BP after recanalization has a more significant impact on the extracranial internal carotid artery (33). However, this explanation should be interpreted cautiously as our study was not a randomized trial to investigate the efficacy of BPV-lowering therapy.
Our study found that rescue treatment was an interaction factor between BPV and futile recanalization. Some published studies found that the BPV of patients with carotid artery stenting (CAS) before surgery was higher than that after surgery due to the compression of the stent on the carotid artery which affected the pressure. Therefore, it can be deduced that CAS could reduce BPV (34). A similar finding was made in our study, where the postoperative BPV and futile recanalization rates in the rescue treatment group (8.7 ± 2.4 vs. 9.1 ± 3.0 mmHg and 36 vs. 47%, respectively) were lower than that in the other group. Additionally, a Korean multicenter retrospective cohort study indicated that rescue stenting was independently associated with enhanced outcomes without an increase in sICH or mortality. This study found that patients in a rescue treatment group were younger, had lower NIHSS scores, more ICA occlusion, and had less arterial fibrillation than those in a non-rescue treatment group (35). In our study, the effect size remained unchanged after adjusting for confounders. Therefore, patients with higher BPV may have poorer outcomes, especially those who undergo rescue treatment.
Compared with previous studies, although our sample size was not significantly large, we used curve fitting and sensitive analysis. This lends credence to the association between BPV and functional outcomes and accuracy in identifying BPV vulnerable subgroups. We also demonstrated a linear association between BPV and each outcome.
Our study had several limitations. First, as this was a single-center study, our findings may not be generalizable to all settings. For the treatment of thrombectomy, SWIM (Solitaire stent retriever in combination with intracranial support catheter aspiration for MT) technology is the preferred choice at our center (36), while other centers may adopt conventional procedures, leading to differences in study results. Second, we measured BPV only after successful recanalization. BPV measurements before and during thrombectomy operations might be of great value for evaluating the association between BPV and outcomes. Third, our findings are based on a retrospective observational study. Additional randomized studies are warranted to confirm the causality of these observational results.
Our results showed that increased BPV after MT was associated with postoperative adverse events. Identification of patients with higher BPV has the potential to inform clinical managements, including transfer to the NICU, closer observation, earlier imaging follow-up for the detection of sICH, and decompressive hemicraniectomy surgery. Stricter management of BPV is required for patients who undergo rescue treatment.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Ethics Committee of Shanghai Tenth People's Hospital. The patients/participants provided their written informed consent to participate in this study.
QZ and JH: conception, design, and administrative support. YL and RS: provision of study materials or patients. RS: collection and assembly of data. XZ and JH: adjudicated the radiological findings. WL: data analysis and interpretation. All authors: manuscript writing and final approval of manuscript.
This study is supported by the Shanghai Municipal Key Clinical Specialty (No. shslczdzk06102).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.967395/full#supplementary-material
1. Smith WS, Lev MH, English JD, Camargo EC, Chou M, Johnston SC, et al. Significance of large vessel intracranial occlusion causing acute ischemic stroke and TIA. Stroke. (2009) 40:3834–40. doi: 10.1161/STROKEAHA.109.561787
2. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
3. O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. (2010) 376:112–23. doi: 10.1016/S0140-6736(10)60834-3
4. Goyal N, Tsivgoulis G, Pandhi A, Chang JJ, Dillard K, Ishfaq MF, et al. Blood pressure levels post mechanical thrombectomy and outcomes in large vessel occlusion strokes. Neurology. (2017) 89:540–7. doi: 10.1212/WNL.0000000000004184
5. Malhotra K, Goyal N, Katsanos AH, Filippatou A, Mistry EA, Khatri P, et al. Association of blood pressure with outcomes in acute stroke thrombectomy. Hypertension. (2020) 75:730–9. doi: 10.1161/HYPERTENSIONAHA.119.14230
6. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
7. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. (2018) 378:11–21. doi: 10.1056/NEJMoa1706442
8. Mistry EA, Mayer SA, Khatri P. Blood pressure management after mechanical thrombectomy for acute ischemic stroke: a survey of the strokenet sites. J Stroke Cerebrovasc Dis. (2018) 27:2474–8. doi: 10.1016/j.jstrokecerebrovasdis.2018.05.003
9. Mazighi M, Richard S, Lapergue B, Sibon I, Gory B, Berge J, et al. Safety and efficacy of intensive blood pressure lowering after successful endovascular therapy in acute ischaemic stroke (BP-TARGET): a multicentre, open-label, randomised controlled trial. Lancet Neurol. (2021) 20:265–74. doi: 10.1016/S1474-4422(20)30483-X
10. Gosmanova EO, Mikkelsen MK, Molnar MZ, Lu JL, Yessayan LT, Kalantar-Zadeh K, et al. Association of systolic blood pressure variability with mortality, coronary heart disease, stroke, renal disease. J Am Coll Cardiol. (2016) 68:1375–86. doi: 10.1016/j.jacc.2016.06.054
11. Schonenberger S, Henden PL, Simonsen CZ, Uhlmann L, Klose C, Pfaff JAR, et al. Association of general anesthesia vs procedural sedation with functional outcome among patients with acute ischemic stroke undergoing thrombectomy: a systematic review and meta-analysis. JAMA. (2019) 322:1283–93. doi: 10.1001/jama.2019.11455
12. Manning L, Hirakawa Y, Arima H, Wang X, Chalmers J, Wang J, et al. Blood pressure variability and outcome after acute intracerebral haemorrhage: a post-hoc analysis of INTERACT2, a randomised controlled trial. Lancet Neurol. (2014) 13:364–73. doi: 10.1016/S1474-4422(14)70018-3
13. Mistry EA, Mehta T, Mistry A, Arora N, Starosciak AK. F. Blood pressure variability and neurologic outcome after endovascular thrombectomy: a secondary analysis of the BEST study. Stroke. (2020) 51:511–8. doi: 10.1161/STROKEAHA.119.027549
14. Maier B, Gory B, Lapergue B, Sibon I, Escalard S, Kyheng M, et al. Effect of blood pressure variability in the randomized controlled BP TARGET trial. Eur J Neurol. (2021). doi: 10.1111/ene.15194
15. Lee JS, Lee SJ, Hong JM, Alverne F, Lima FO, Nogueira RG. Endovascular treatment of large vessel occlusion strokes due to intracranial atherosclerotic disease. J Stroke. (2022) 24:3–20. doi: 10.5853/jos.2021.01375
16. Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Cerebral Angiographic Revascularization Grading, Sgroup Rw, and STiForce CIT Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. (2013) 44:2650–63. doi: 10.1161/STROKEAHA.113.001972
17. Fugate JE, Klunder AM, Kallmes DF. What is meant by “TICI”? AJNR Am J Neuroradiol. (2013) 34:1792–7. doi: 10.3174/ajnr.A3496
18. Huang X, Guo H, Yuan L, Cai Q, Zhang M, Zhang Y, et al. Blood pressure variability and outcomes after mechanical thrombectomy based on the recanalization and collateral status. Ther Adv Neurol Disord. (2021) 14:1756286421997383. doi: 10.1177/1756286421997383
19. Castro P, Ferreira F, Nguyen CK, Payabvash S, Ozan Tan C, Sorond F, et al. Rapid assessment of blood pressure variability and outcome after successful thrombectomy. Stroke. (2021) 52:e531–5. doi: 10.1161/STROKEAHA.121.034291
20. Bustamante A, Ning M, Garcia-Berrocoso T, Penalba A, Boada C, Simats A, et al. Usefulness of ADAMTS13 to predict response to recanalization therapies in acute ischemic stroke. Neurology. (2018) 90:e995-e1004. doi: 10.1212/WNL.0000000000005162
21. Yaghi S, Willey JZ, Cucchiara B, Goldstein JN, Gonzales NR, Khatri P, et al. Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2017) 48:e343–61. doi: 10.1161/STR.0000000000000152
22. von Elm A. Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. (2007) 147:573–7. doi: 10.7326/0003-4819-147-8-200710160-00010
23. Luo G, Gao F, Zhang X, Jia B, Huo X, Liu R, et al. Intracranial stenting as rescue therapy after failure of mechanical thrombectomy for basilar artery occlusion: data from the ANGEL-ACT registry. Front Neurol. (2021) 12:739213. doi: 10.3389/fneur.2021.739213
24. Venema E, Mulder M, Roozenbeek B, Broderick JP, Yeatts SD, Khatri P, et al. Selection of patients for intra-arterial treatment for acute ischaemic stroke: development and validation of a clinical decision tool in two randomised trials. BMJ. (2017) 357:j1710. doi: 10.1136/bmj.j1710
25. Venema E, Roozenbeek B, Mulder M, Brown S, Majoie C, Steyerberg EW, et al. Prediction of outcome and endovascular treatment benefit: validation and update of the Mr Predicts decision tool. Stroke. (2021) 52:2764–72. doi: 10.1161/STROKEAHA.120.032935
26. Bosel J, Optimal blood pressure for stroke thrombectomy: high time for prospective data! Stroke. (2019) 50:2648–2649. doi: 10.1161/STROKEAHA.119.026550
27. Claassen J, Thijssen DHJ, Panerai RB, Faraci FM. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev. (2021) 101:1487–559. doi: 10.1152/physrev.00022.2020
28. Skare C, Karlsen H, Strand-Amundsen RJ, Eriksen M, Skulberg VM, Sunde K, et al. Cerebral perfusion and metabolism with mean arterial pressure 90 vs. 60 mmHg in a porcine post cardiac arrest model with and without targeted temperature management. Resuscitation. (2021) 167:251–60. doi: 10.1016/j.resuscitation.2021.06.011
29. Rasmussen M, Schonenberger S, Henden PL, Valentin JB, Espelund US, Sorensen LH, et al. Blood pressure thresholds and neurologic outcomes after endovascular therapy for acute ischemic stroke: an analysis of individual patient data from 3 randomized clinical trials. JAMA Neurol. (2020) 77:622–31. doi: 10.1001/jamaneurol.2019.4838
30. Sekhon MS, Gooderham P, Menon DK, Brasher PMA, Foster D, Cardim D, et al. The burden of brain hypoxia and optimal mean arterial pressure in patients with hypoxic ischemic brain injury after cardiac arrest. Crit Care Med. (2019) 47:960–9. doi: 10.1097/CCM.0000000000003745
31. Parati G, Stergiou GS, Dolan E, Bilo G. Blood pressure variability: clinical relevance and application. J Clin Hypertens (Greenwich). (2018) 20:1133–7. doi: 10.1111/jch.13304
32. Henry JB, Miller MC, Kelly KC, Champney D. Mean arterial pressure (MAP): an alternative and preferable measurement to systolic blood pressure (SBP) in patients for hypotension detection during hemapheresis. J Clin Apher. (2002) 17:55–64. doi: 10.1002/jca.10022
33. Lokossou A, Metanbou S, Gondry-Jouet C, Baledent O. Extracranial versus intracranial hydro-hemodynamics during aging: a PC-MRI pilot cross-sectional study. Fluids Barriers CNS. (2020) 17:1. doi: 10.1186/s12987-019-0163-4
34. He GY, Li YH, Wei JJ, Xiao JD, Chen Y, Fan BL, et al. Effect of perioperative blood pressure variability on cerebral hyperperfusion syndrome after carotid artery stenting: a retrospective study. Interv Neuroradiol. (2021) 15910199211065198. doi: 10.1177/15910199211065198
35. Chang Y, Kim BM, Bang OY, Baek JH, Heo JH, Nam HS, et al. Rescue stenting for failed mechanical thrombectomy in acute ischemic stroke: a multicenter experience. Stroke. (2018) 49:958–64. doi: 10.1161/STROKEAHA.117.020072
Keywords: blood pressure variability, recanalization, large vessel occlusion, stroke, mechanical thrombectomy
Citation: Lu Y, Shen R, Lin W, Zhou X, Hu J and Zhang Q (2022) Association between blood pressure variability and clinical outcomes after successful recanalization in patients with large vessel occlusion stroke after mechanical thrombectomy. Front. Neurol. 13:967395. doi: 10.3389/fneur.2022.967395
Received: 12 June 2022; Accepted: 18 July 2022;
Published: 10 August 2022.
Edited by:
Tianxiao Li, Henan Provincial People's Hospital, ChinaReviewed by:
Yingkun He, Henan Provincial People's Hospital, ChinaCopyright © 2022 Lu, Shen, Lin, Zhou, Hu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Quanbin Zhang, cXVhbmJpbnpoYW5nQGFsaXl1bi5jb20=; Jian Hu, ZG9jdG9yaHVqaWFuQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.