- 1Division of Headache & Facial Pain, Department of Neurology & Neurological Sciences, Stanford University School of Medicine, Stanford, CA, United States
- 2Independent Researcher, Los Angeles, CA, United States
- 3Headache and Neurological Pain Research Group, Department of Medicine, Vall d'Hebron Research Institute, Universitat Autònoma de Barcelona, Barcelona, Spain
Editorial on the Research Topic
Lifestyle modifications to manage migraine
We read with interest the Research Topic articles on “Lifestyle Modifications to Manage Migraine.” Here, we present summaries and insights on regular lifestyle behavior (RLB)—followed by an overview of possible molecular mechanisms implicated in the modulation of migraine through RLB.
Raucci et al.'s review on lifestyle-based childhood migraine management highlighted the landmark 24-week comparative effectiveness RCT (randomized controlled trial) in which 61% of children on placebo (involving RLB, regular sleep, hydration, mealtime, exercise) achieved a 50% monthly reduction in headache frequency compared to 55 and 52% on daily topiramate and amitriptyline, respectively (Raucci et al.) (1, 2). RLB preempted drug-induced adverse effects (Raucci et al.) (1, 2). RLB education in adolescence [migraine onset peak age (Raucci et al.) (3, 4)] helps inculcate anti-migraine behavioral habits. Dehydration [a migraine precipitant (5, 6)] is common in children in North America and Europe (Raucci et al.) (7, 8). Unfavorable lifestyle-related habits that increase migraine burden e.g., sedentariness/obesity, screen time, smoking/alcohol/psychoactive substance use, stress (e.g., school-related bullying), and caffeine/cola consumption are rising in adolescents (Raucci et al.). Addressing lifestyle factors is central in pediatric headache management (Raucci et al.).
Lisicki et al. conducted a two-phase real-world study to understand dietary migraine triggers. The first phase cross-sectional study examined whether food/drink avoidance differs between people with and without migraine. Although 64.3% of people with migraine reported avoiding a food/drink type, there was no significant group difference in consumption between those with and without migraine. In a follow-up 2-month prospective diary study, chocolate, wine, sweeteners, and cheese were frequently consumed before migraine onset. Food cravings and decreased appetite were reported before a migraine attack. The authors suggested that consumption of “attack-triggering” food items may be a migraine prodrome rather than a cause. However, published RCTs demonstrate the efficacy of elimination diets in migraine (9, 10). Intraindividual changes and absolute or partial (additive or potentiating) (11) triggers may confound the potentially bidirectional migraine-diet relationship (12) complex. Ensuring a balanced regular meal and regarding diet as just one component of lifestyle-based migraine management is generally recommended.
Grozeva et al. took advantage of the mandatory COVID-19 lockdowns to examine the impact of lifestyle changes (e.g., sleep, work) on migraine in pre-post longitudinal cohorts. During the first COVID-19 lockdown which lasted 6–8 weeks, there was a reduction in migraine burden. However, during the second lockdown, the migraine burden returned to its basal/higher levels. The authors posited that sudden short-term (6–8 weeks) lifestyle changes may benefit migraine patients. This article indicates how observational studies and RCTs complement each other. RCTs may not always be ideal to study complex lifestyle behaviors (sleep, exercise) due to known challenges e.g., self-selection bias, fidelity, blinding. Observational studies also have their share of problems e.g., confounders, endogeneity, selection bias. A recent large-scale RCT proved that H. pylori eradication reduces the risk of gastric cancer (13)—validating what is already known in observational studies. Do we need to wait for further evidence from multiple RCTs [or a natural disaster, as the authors phrased it (Grozeva et al.)] before we recommend adopting lifestyle changes (e.g., regular sleep, stress coping skills) shown by observational studies to reduce migraine burden? Not really.
Rivera-Mancilla et al. examined the relationship between 3 common chronic conditions i.e., migraine (14.4% prevalence), obesity (13%), and diabetes mellitus (9.3%). The authors elucidated that the migraine-obesity relationship may be bidirectional due to shared lifestyle and biological risk factors, seen in clinicoepidemiological and interventional studies. RLB (e.g., exercise) is linked to the central and peripheral nervous system in migraine, obesity, and diabetes (Rivera-Mancilla et al.). Obesity can result from low physical activity following migraine disability and anti-migraine drug-induced weight gain (e.g., beta-blockers, antidepressants, anticonvulsants, calcium channel blockers) (Rivera-Mancilla et al.). The diabetes-migraine relationship is not clear (Rivera-Mancilla et al.). CGRP levels are high in migraine and obesity, while low in type-2 diabetes (Rivera-Mancilla et al.). These results do not add up considering that obesity (risk for migraine) causes diabetes. Does migraine (or migraine medications) mediate or moderate the risk obesity poses to diabetes/insulin resistance? Topiramate is the only anti-migraine drug resulting in fat loss among non-diabetic migraine patients without high BMI (Rivera-Mancilla et al.). Does topiramate have the same effect in diabetic and obese migraine patients? Weight loss interventions (behavioral, bariatric surgery) lead to migraine reduction (Rivera-Mancilla et al.). Given that CGRP modulates insulin release (Rivera-Mancilla et al.) (14), can long-term CGRP blockage in migraine patients influence diabetes (15)? Compared to obesity, migraine with aura has a stronger association with major cardiovascular or cerebrovascular diseases (16). More studies are needed to clarify the ischemia risk from CGRP blockade (17–19), particularly considering the complex migraine-obesity-diabetes triumvirate relationship.
Possible molecular mechanisms of regular lifestyle behavior for migraine control
RLB is attributed to adherence to three circadian-related aspects of daily life i.e., sleep-wake, mealtime, and exercise (20). Disrupted sleep, irregular mealtime, and sedentariness worsen migraine outcomes (20). These three arms of lifestyle can alter the epigenetic status of the genome, including modifications of histones, the DNA methylation status of specific genomic regions, and/or the expression of non-coding RNAs (such as microRNAs): three mechanisms that up- or down- regulate gene expression (21–24). A previous review summarizes the evidence showing the effects that sleep can have in modulating these epigenetic factors (22) and other studies have shown similar impacts for exercise (25) and regular mealtime (26, 27).
RLB adherence can be speculated to enhance migraine outcomes by modifying neuronal, epigenetic, and genetic factors. Deviation from RLB might induce macro and micro-environment changes, affecting neuronal epigenetics, that may get propagated through inter-cellular communication leading to neurovascular sensitization and neuroinflammation. Extra-cellular vesicles (EV) are harbingers of inter-cellular communication and they could be implicated in this process (28, 29). Pro-migraine inter-cellular information could be substituted by enriching the micro-environment with EV from a naïve exogenous source (e.g., stem cells) to trick cells into sensing the anti-migraine environment (30, 31). This might reverse epigenetic modifications caused by RLB deviation, leading to migraine control (Figure 1). Exogenous EV are systemically short-lived (28), their anti-migraine effect could be ephemeral against chronic RLB deviation. Stem cell-derived EV can best serve as adjunct migraine therapy along with RLB—enhancing RLB maintenance.
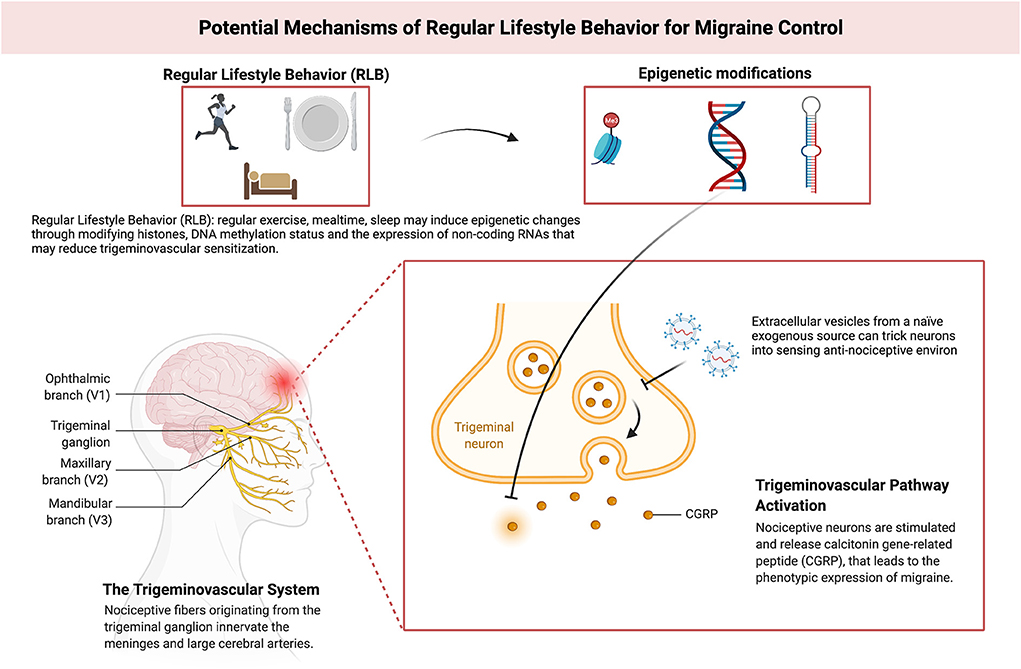
Figure 1. Model depicting putative mechanisms of Regular Lifestyle Behavior (RLB) for migraine control. Adapted from “The role of CGRP and the trigeminal system in migraine pathophysiology”, by BioRender.com (2022). Retrieved from https://app.Biorender.com/biorender-templates.
Besides epigenetics, other RLB-related molecular mechanisms in migraine include the strong link between the pathophysiology of headache and sleep (32); including several brainstem nuclei playing a role in both entities, the hypothalamus implicated both in regulating circadian rhythms and generating migraine attacks (33), and the modulation of sleep and headache by the same orexinergic systems (34). Another key point is the existing molecular link between appetite and migraine pathophysiology, where orexins also play a crucial role (35). Orexins are two hypothalamic neuropeptides linked to appetite regulation, wakefulness, and the perception and integration of pain (36), hence one could hypothesize whether these molecules may be involved in the increased susceptibility of migraine during RLB disruption.
Conclusion
This Research Topic articles provide an overview of how lifestyle modifications may affect migraine. Future studies on this topic can improve our understanding of this process and may unravel the precise molecular mechanisms involved, which eventually could lead to the development of new therapeutic targets.
Author contributions
YW drafted the first version of the manuscript. SS and MV-P prepared the molecular mechanisms section. All authors revised the final version, approved the manuscript and figure, and provided critical feedback and helped shape the research.
Funding
YW received research funding from the NINDS (National Institute of Neurological Disorders and Stroke), NIH (National Institutes of Health) (1K01NS124911-01). MV-P is a recipient of a Sara Borrell contract from the Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación, Spain (CD20/00019).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Powers SW, Coffey CS, Chamberlin LA Ecklund DJ Klingner EA Yankey JW, et al. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. (2017) 376:115–24. doi: 10.1056/NEJMoa1610384
2. Hershey AD, Powers SW, Coffey CS, Eklund DD, Chamberlin LA, Korbee LL, et al. Childhood and Adolescent Migraine Prevention (CHAMP) study: a double-blinded, placebo-controlled, comparative effectiveness study of amitriptyline, topiramate, and placebo in the prevention of childhood and adolescent migraine. Headache. (2013) 53:799–816. doi: 10.1111/head.12105
3. Victor T, Hu X, Campbell J, Buse D, Lipton R. Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia. (2010) 30:1065–72. doi: 10.1177/0333102409355601
4. Antonaci F, Voiticovschi-Iosob C, di Stefano AL, Galli F, Ozge A, Balottin U. The evolution of headache from childhood to adulthood: a review of the literature. J Headache Pain. (2014) 15:15. doi: 10.1186/1129-2377-15-15
5. Blau JN. Water deprivation: a new migraine precipitant. Headache. (2005) 45:757–9. doi: 10.1111/j.1526-4610.2005.05143_3.x
6. Spigt M, Weerkamp N, Troost J, van Schayck CP, Knottnerus JA. A randomized trial on the effects of regular water intake in patients with recurrent headaches. Family Practice. (2012) 29:370–5. doi: 10.1093/fampra/cmr112
7. Suh H, Kavouras SA. Water intake and hydration state in children. Eur J Nutr. (2019) 58:475–96. doi: 10.1007/s00394-018-1869-9
8. Kenney EL, Long MW, Cradock AL, Gortmaker SL. Prevalence of inadequate hydration among us children and disparities by gender and race/ethnicity: national health and nutrition examination survey, 2009–2012. Am J Public Health. (2015) 105:e113–8. doi: 10.2105/AJPH.2015.302572
9. Alpay K, Ertas M, Orhan EK, Ustay DK, Lieners C, Baykan B. Diet restriction in migraine, based on IgG against foods: a clinical double-blind, randomised, cross-over trial. Cephalalgia. (2010) 30:829–37. doi: 10.1177/0333102410361404
10. Özön AÖ, Karadaş Ö, Özge A. Efficacy of Diet Restriction on Migraines. Noro psikiyatri arsivi. (2018) 55:233–7. doi: 10.5152/npa.2016.15961
11. Spierings ELH, Donoghue S, Mian A, Wöber C. Sufficiency and necessity in migraine: how do we figure out if triggers are absolute or partial and, if partial, additive or potentiating? Curr Pain Headache Rep. (2014) 18:455. doi: 10.1007/s11916-014-0455-y
12. Gazerani P. A bidirectional view of migraine and diet relationship. Neuropsychiatr Dis Treat. (2021) 17:435–51. doi: 10.2147/NDT.S282565
13. Choi IJ, Kim CG, Lee JY, Kim Y, Kook M-C, Park B, et al. Family history of gastric cancer and helicobacter pylori treatment. N Engl J Med. (2020) 382:427–36. doi: 10.1056/NEJMoa1909666
14. Yamaguchi A, Chiba T, Morishita T, Nakamura A, Inui T, Yamatani T, et al. Calcitonin gene-related peptide and induction of hyperglycemia in conscious rats in vivo. Diabetes. (1990) 39:168–74. doi: 10.2337/diabetes.39.2.168
15. Halloran J, Lalande A, Zang M, Chodavarapu H, Riera CE. Monoclonal therapy against calcitonin gene-related peptide lowers hyperglycemia and adiposity in type 2 diabetes mouse models. Metabol Open. (2020) 8:100060. doi: 10.1016/j.metop.2020.100060
16. Kurth T, Rist PM, Ridker PM, Kotler G, Bubes V, Buring JE. Association of migraine with aura and other risk factors with incident cardiovascular disease in women. JAMA. (2020) 323:2281–9. doi: 10.1001/jama.2020.7172
17. Gomez J, Burish M, Savitz SI, McCullough LD, Ganduglia Cazaban C. Abstract P643: incidence of ischemic stroke among migraineurs on calcitonin gene-related peptide inhibitors. Stroke. (2021) 52(Suppl_1). doi: 10.1161/str.52.suppl_1.P643
18. Aradi S, Kaiser E, Cucchiara B. Ischemic stroke associated with calcitonin gene-related peptide inhibitor therapy for migraine: a case report. J Stroke Cerebrovasc Dis. (2019) 28:104286. doi: 10.1016/j.jstrokecerebrovasdis.2019.07.002
19. Mulder IA, Li M, de Vries T, Qin T, Yanagisawa T, Sugimoto K, et al. Anti-migraine calcitonin gene-related peptide receptor antagonists worsen cerebral ischemic outcome in mice. Ann Neurol. (2020) 88:771–84. doi: 10.1002/ana.25831
20. Woldeamanuel YW, Cowan RP. The impact of regular lifestyle behavior in migraine: a prevalence case–referent study. J Neurol. (2016) 263:669–76. doi: 10.1007/s00415-016-8031-5
21. Kresovich JK, Park Y-MM, Keller JA, Sandler DP, Taylor JA. Healthy eating patterns and epigenetic measures of biological age. Am J Clin Nutr. (2022) 115:171–9. doi: 10.1093/ajcn/nqab307
22. Gaine ME, Chatterjee S, Abel T. Sleep deprivation and the epigenome. Front Neural Circuits. (2018) 12:14. doi: 10.3389/fncir.2018.00014
23. Fitzgerald KN, Hodges R, Hanes D, Stack E, Cheishvili D, Szyf M, et al. Potential reversal of epigenetic age using a diet and lifestyle intervention: a pilot randomized clinical trial. Aging. (2021) 13:9419–32. doi: 10.18632/aging.202913
24. Barrón-Cabrera E, Ramos-Lopez O, González-Becerra K, Riezu-Boj JI, Milagro FI, Martínez-López E, et al. Epigenetic modifications as outcomes of exercise interventions related to specific metabolic alterations: a systematic review. Lifestyle Genom. (2019) 12:25–44. doi: 10.1159/000503289
25. Voisin S, Eynon N, Yan X, Bishop DJ. Exercise training and DNA methylation in humans. Acta Physiology. (2015) 213:39–59. doi: 10.1111/apha.12414
26. Selvaraji S, Efthymios M, Foo RSY, Fann DY, Lai MKP, Chen CLH, et al. Time-restricted feeding modulates the DNA methylation landscape, attenuates hallmark neuropathology and cognitive impairment in a mouse model of vascular dementia. Theranostics. (2022) 12:3007–23. doi: 10.7150/thno.71815
27. Hibler E, Huang L, Andrade J, Spring B. Impact of a diet and activity health promotion intervention on regional patterns of DNA methylation. Clin Epigenet. (2019) 11:133. doi: 10.1186/s13148-019-0707-0
28. Yáñez-Mó M, Siljander PR-M, Andreu Z, Zavec AB, Borràs FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. (2015) 4:27066. doi: 10.3402/jev.v4.27066
29. Hill AF. Extracellular vesicles and neurodegenerative diseases. J Neurosci. (2019) 39:9269–73. doi: 10.1523/JNEUROSCI.0147-18.2019
30. Park K-S, Bandeira E, Shelke G v, Lässer C, Lötvall J. Enhancement of therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther. (2019) 10:288. doi: 10.1186/s13287-019-1398-3
31. Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells. (2019) 8:1605. doi: 10.3390/cells8121605
32. Holland PR. Headache and sleep: shared pathophysiological mechanisms. Cephalalgia. (2014) 34:725–44. doi: 10.1177/0333102414541687
33. Schulte LH, May A. The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain. (2016) 139(Pt 7):1987–93. doi: 10.1093/brain/aww097
34. Holland PR. Biology of neuropeptides: orexinergic involvement in primary headache disorders. Headache. (2017) 57(Suppl. 2):76–88. doi: 10.1111/head.13078
35. Martins-Oliveira M, Tavares I, Goadsby PJ. Was it something I ate? Understanding the bidirectional interaction of migraine and appetite neural circuits. Brain Res. (2021) 1770:147629. doi: 10.1016/j.brainres.2021.147629
Keywords: migraine, lifestyle and behavior, headache, regular lifestyle behavior, lifestyle medicine
Citation: Woldeamanuel YW, Shrivastava S and Vila-Pueyo M (2022) Editorial: Lifestyle modifications to manage migraine. Front. Neurol. 13:966424. doi: 10.3389/fneur.2022.966424
Received: 10 June 2022; Accepted: 27 June 2022;
Published: 29 August 2022.
Edited and reviewed by: Pablo Irimia, University Clinic of Navarra, Spain
Copyright © 2022 Woldeamanuel, Shrivastava and Vila-Pueyo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yohannes W. Woldeamanuel, eXdvbGRlYW1Ac3RhbmZvcmQuZWR1
†ORCID: Yohannes W. Woldeamanuel orcid.org/0000-0003-4879-6098
Surya Shrivastava orcid.org/0000-0003-0021-2751
Marta Vila-Pueyo orcid.org/0000-0003-0652-2988
 Yohannes W. Woldeamanuel
Yohannes W. Woldeamanuel Surya Shrivastava
Surya Shrivastava Marta Vila-Pueyo
Marta Vila-Pueyo