
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 22 August 2022
Sec. Endovascular and Interventional Neurology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.957353
Background: Bilateral transverse sinus stenosis (BTSS) is associated with intracranial hypertension. Enlarged vertebral venous plexus (EVVP) refers to a compensation mechanism against elevated intracranial pressure (ICP) in patients with BTSS. This study aims to investigate the influencing factors of EVVP.
Methods: Patients with BTSS were prospectively recruited from the neurology department and neurosurgery department of Xuanwu Hospital Capital Medical University from January 2020 to December 2021.
Results: A total of 37 patients were enrolled with a mean age of 45.42 ± 15.64 years. Women tend to be more susceptible to BTSS. The most common co-morbid disease was hypertension. The most common clinical manifestations were visual disorders, headaches, and tinnitus. BMI and DBP were significantly higher in BTSS patients without EVVP than those with EVVP. Multivariate analysis revealed that diastolic blood pressure (DBP) was negatively correlated with EVVP. In addition, a positive correlation between DBP and the ICP was also observed. A DBP of 81.5 mmHg was calculated as the cutoff value for the presence of EVVP. BTSS patients with DBP ≤ 81.5 mmHg had a higher incidence of EVVP and a lower ICP compared to those with DBP > 81.5 mmHg.
Conclusions: DBP was identified as an independent predictor of EVVP. DBP was lower (≤81.5 mmHg) in patients with EVVP and therefore was associated with a lower ICP in patients with BTSS.
Transverse sinus stenosis is known as the narrowing of the unilateral or bilateral transverse sinus, with the prevalence of unilateral TSS and bilateral TSS (BTSS) in the general population of 33% and 5%, respectively (1). However, cases of symptomatic unilateral TSS are rarely reported (2); contrastively, BTSS is deemed as an independent cause of intracranial hypertension (IH), bringing about clinical symptoms, such as headache, tinnitus, and blurred vision (3, 4). Nonetheless, it is noteworthy that not every patient with BTSS experiences IH (5). Our previous study reported that the presence of enlarged vertebral venous plexus (EVVP) compensates for the elevated intracranial pressure (ICP) in patients with BTSS (6). However, no previous study has reported any influencing factor of EVVP. This study aims to find out the influencing factors of EVVP.
In this study, we prospectively recruited patients with BTSS who were admitted to the neurology department and neurosurgery department at Xuanwu Hospital Capital Medical University from January 2020 to December 2021. The inclusion criteria were as follows: symptomatic cases diagnosed as BTSS by magnetic resonance venography (MRV) and/or digital subtraction angiography (DSA). The representative images of BTSS and EVVP were as previously mentioned (6). The exclusion criteria were defined as follows: patients with (1) drug-related IH; (2) brain parenchymal lesions; (3) moderate to severe stenosis in intracranial, carotid, or vertebral arteries, (3) moderate to severe stenosis in internal jugular veins or intracranial sinuses other than transverse sinus. All patients signed the informed consent, and their anonymity was preserved. This study was approved by the Xuanwu Hospital ethics committee.
Since ICP measured by lumbar puncture agreed well with that measured intracranially (7), lumbar puncture was performed in the morning of the 2nd day of hospitalization to obtain the ICP of each patient. Age, gender, body mass index (BMI), past medical history, personal history, vital signs at admission, clinical manifestations, neuroimaging, and ICP were recorded.
All statistical analyses were conducted using SPSS Version 16 (SPSS, Inc., Chicago, Illinois, United States). Continuous data were expressed as mean ± standard deviation (SD) and processed by using student's t-test. Categorical data were reported as numbers (percentage) and processed by using the chi-square test. Linear regression was used to predict the relationship between two variables. Binary logistic regression analysis was conducted with the enter method to identify the odds ratio (OR) and the corresponding 95% CI. A cutoff point was calculated by using receiver operating characteristics (ROC) curves. A p-value of <0.05 was considered statistically significant.
A total of 37 eligible patients with BTSS were included in this study, with a mean age of 45.42 ± 15.64 years and an average body mass index (BMI) of 25.47 ± 4.26. A gender-specific prevalence of BTSS in women was 83.8%. The most common co-morbid disease was hypertension (29.7%). The smoking rate (5.4%), and alcohol rate (2.7%) in patients with BTSS were apparently lower than those in the general population in China (8). The vital signs were within the normal ranges. Moreover, visual disorder (62.2%), headache (56.8%), and tinnitus (37.8%) were the top three clinical manifestations of BTSS (Table 1).
Demographic characteristics of patients with BTSS with or without EVVP were summarized in Table 2. BMI (p = 0.011) and DBP (p = 0.01) were significantly higher in patients with BTSS without EVVP than those with EVVP (Table 2).
Univariate logistic regression analysis showed that BMI (OR = 0.785, p =0.014) and DBP (OR = 0.873, p = 0.005) were negatively correlated with EVVP (Table 3). Whereas, multivariate logistic regression analysis revealed that only DBP correlated with EVVP (OR = 0.891, p = 0.021, Table 3).
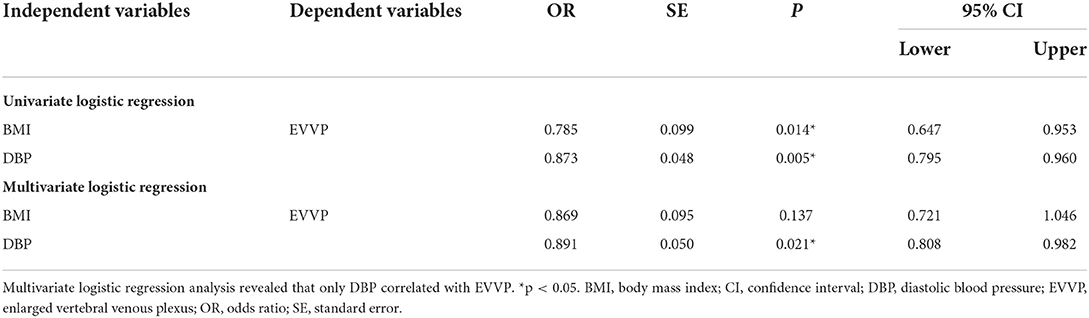
Table 3. Univariate logistic regression analysis showed that BMI and DBP negatively correlated with EVVP.
Subsequently, the impact of BMI and DBP on ICP was investigated. Univariate linear regression analysis showed that BMI (Coefficient = 6.451, p = 0.049, Table 4) and DBP (Coefficient = 3.968, p = 0.01, Table 4) positively correlated with ICP. However, multivariate linear regression analysis revealed that only DBP (Coefficient = 3.305, p = 0.04, Table 4) correlated with ICP.
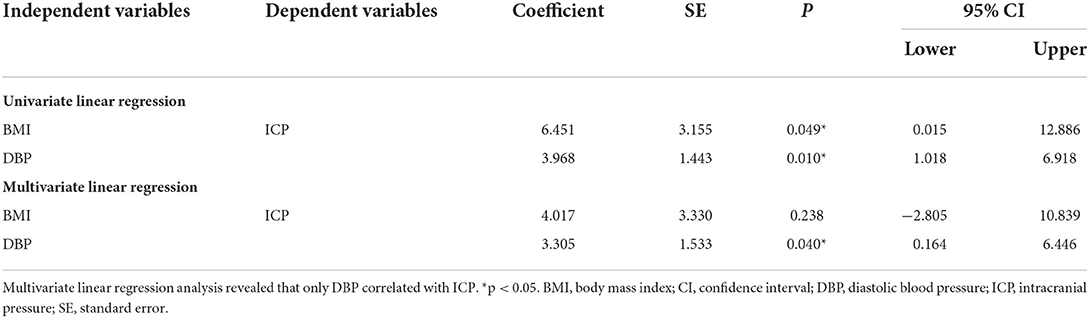
Table 4. Univariate linear regression analysis showed that BMI and DBP positively correlated with ICP.
In order to identify a cutoff value of DBP to predict the presence of EVVP, the ROC curve was performed. A DBP of 81.5 mmHg was calculated as the cutoff value for the presence of EVVP (AUC = 0.813, 95%CI = 0.6867–0.9394, p < 0.001, Figure 1A). DBP ≤ 81.5 mmHg indicated the presence of EVVP in patients with BTSS. Further analysis confirmed that the incidence of EVVP was significantly higher in patients with BTSS with DBP ≤ 81.5 mmHg than those with DBP > 81.5 mmHg (p = 0.002, Figure 1B). Moreover, ICP was significantly lower in patients with BTSS with DBP ≤ 81.5 mmHg than those with DBP > 81.5 mmHg (p = 0.008, Figure 1C).
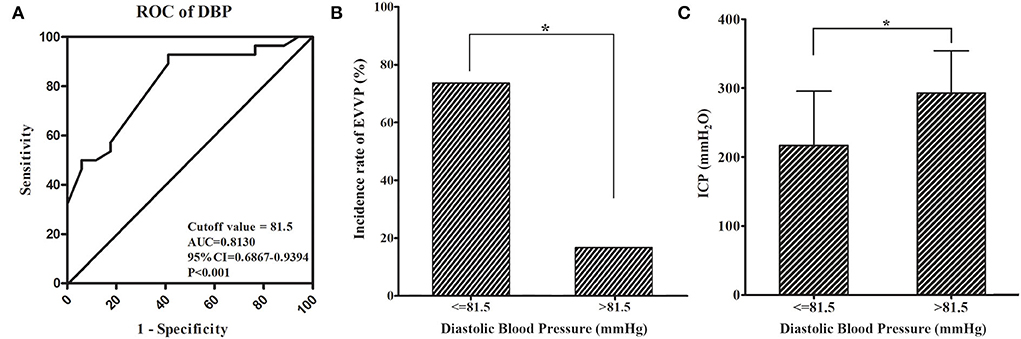
Figure 1. Receiver operating characteristic curve showed that DBP ≤ 81.5 mmHg indicated the presence of EVVP in BTSS patients (A). The incidence of EVVP was significantly higher (B) and ICP was significantly lower (C) in BTSS patients with DBP ≤ 81.5 mmHg than those with DBP > 81.5 mmHg. * p < 0.05. BTSS, bilateral transverse sinus stenosis; DBP, diastolic blood pressure; EVVP, enlarged vertebral venous plexus; ICP, intracranial pressure.
As the results above have clearly demonstrated, BTSS is more prevalent in women. Miyachi et al. (9) also report a female predominance in patients with BTSS. The possible explanation is that sex hormones are involved in the pathogenesis of BTSS. However, further investigations are required to prove this hypothesis. Low percentages of smoking and alcohol rates may be due to the low proportion of male patients.
It has been reported that BTSS is correlated with IH (3). Results from our previous study also supported this finding (6). A triad of IH which manifested as headache, tinnitus, and papilledema are confirmed as the most common symptoms in patients with BTSS in this study. Moreover, hypertension has been identified as the most common comorbid disease for maintaining cerebral perfusion pressure in the presence of IH.
However, the causality between BTSS and IH has not been completely elaborated. Markey et al. (3) supported that venous sinus stenosis disturbs the reflux of cerebrospinal fluid with a resultant rise in ICP. Whereas Volpe et al. (10) yield a seemingly inconsistent result that elevated ICP compresses the venous sinus and leads to secondary venous sinus segmental stenosis. Karahalios et al. demonstrated that venous sinus stenosis still exists even if ICP has been reduced to a normal level (11). From our perspective, there may be an enhanced loop between BTSS and IH, featuring the exacerbation of either BTSS or IH while the other one emerges.
The transverse sinus-sigmoid sinus-internal jugular vein and the vertebral venous plexus are two main outflow tracts of intracranial venous blood (12). In the presence of BTSS, it is predicted that the intracranial venous blood was mainly drained through the vertebral venous plexus (13). Therefore, the presence of EVVP would guarantee sufficient cerebral venous reflux. Our previous report has demonstrated that EVVP compensates for the elevated ICP in patients with BTSS (6).
Although BMI was significantly higher in patients with BTSS without EVVP than those with EVVP, BMI was not correlated with EVVP after adjusting for DBP. It has been reported that obesity is a risk factor for idiopathic IH (14, 15). However, we found that BMI was not correlated with ICP after being adjusted for DBP. It is suggested that BMI is incapable of predicting EVVP and ICP.
In the meanwhile, we also found that DBP was significantly higher in patients with BTSS without EVVP than in those with EVVP. DBP was identified as an independent predictor of EVVP and ICP after adjusting for BMI. ROC analysis showed that DBP ≤ 81.5 mmHg indicated the presence of EVVP in patients with BTSS. Patients with BTSS with DBP ≤ 81.5 mmHg had a higher incidence of EVVP and a lower ICP compared to those with DBP > 81.5 mmHg. It is suggested that DBP ≤ 81.5 mmHg indicates a higher incidence of EVVP and therefore predicts a lower ICP in patients with BTSS.
It has been demonstrated that elevated venous pressure promotes the contraction of arteriolar smooth muscle, thus resulting in an increase in peripheral vascular resistance, which in turn leads to elevated DBP (16, 17). Therefore, lower DBP may be correlated with lower central venous pressure (CVP). The higher pressure gradient between ICP and CVP increases shear stress in the vertebral venous plexus and thereby causing EVVP. However, this hypothesis requires further verification.
The limitation of this study was the small sample size. Further validation with a larger sample size will be conducted in the future.
Diastolic blood pressure was identified as an independent predictor of EVVP. DBP was lower (≤81.5 mmHg) in patients with EVVP and therefore was associated with a lower ICP in patients with BTSS.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was approved by the Xuanwu Hospital Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
ML contributed to study design, data collection, manuscript writing, and final approval of the manuscript. XG contributed to data analysis, manuscript writing, and final approval of the manuscript. FL and NX contributed to manuscript writing and final approval of the manuscript. JS contributed to data collection and final approval of the manuscript. RM contributed to acquisition of study funding, study design, critical revision of the manuscript, and final approval of the manuscript. XJ contributed to data interpretation, critical revision of the manuscript, and final approval of the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by Beijing Natural Science Foundation (7212047). The funding agencies had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Durst CR, Ornan DA, Reardon MA, Mehndiratta P, Mukherjee S, Starke RM, et al. Prevalence of dural venous sinus stenosis and hypoplasia in a generalized population. J Neurointerv Surg. (2016) 8:1173–7. doi: 10.1136/neurintsurg-2015-012147
2. Bono F, Lupo MR, Lavano A, Mangone L, Fera F, Pardatscher K, et al. Cerebral MR venography of transverse sinuses in subjects with normal CSF pressure. Neurology. (2003) 61:1267–70. doi: 10.1212/01.WNL.0000092021.88299.B4
3. Markey KA, Mollan SP, Jensen RH, Sinclair AJ. Understanding idiopathic intracranial hypertension: mechanisms, management, and future directions. Lancet Neurol. (2016) 15:78–91. doi: 10.1016/S1474-4422(15)00298-7
4. Biousse V, Bruce BB, Newman NJ. Update on the pathophysiology and management of idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry. (2012) 83:488–94. doi: 10.1136/jnnp-2011-302029
5. Kelly LP, Saindane AM, Bruce BB, Ridha MA, Riggeal BD, Newman NJ, et al. Does bilateral transverse cerebral venous sinus stenosis exist in patients without increased intracranial pressure? Clin Neurol Neurosurg. (2013) 115:1215–9. doi: 10.1016/j.clineuro.2012.11.004
6. Li M, Bai C, Sun J, Xia N, Meng R, Ji X. Enlarged vertebral venous plexus alleviated intracranial hypertension-related symptoms in patients with bilateral transverse sinus stenosis. Cerebrovasc Dis. (2022) 51:525–31. doi: 10.1159/000519716
7. Johannesson G, Eklund A, Linden C. Intracranial and intraocular pressure at the lamina cribrosa: gradient effects. Curr Neurol Neurosci Rep. (2018) 18:25. doi: 10.1007/s11910-018-0831-9
8. Lee YH, Wang Z, Chiang TC, Liu CT. Beverage intake, smoking behavior, and alcohol consumption in contemporary China-a cross-sectional analysis from the 2011 China health and nutrition survey. Int J Environ Res Public Health. (2017) 14:493. doi: 10.3390/ijerph14050493
9. Miyachi S, Hiramatsu R, Ohnishi H, Takahashi K, Kuroiwa T. Endovascular treatment of idiopathic intracranial hypertension with stenting of the transverse sinus stenosis. Neurointervention. (2018) 13:138–43. doi: 10.5469/neuroint.2018.00990
10. Volpe NJ. Idiopathic intracranial hypertension: important questions answered with more to come. JAMA Neurol. (2014) 71:678–80. doi: 10.1001/jamaneurol.2014.444
11. Karahalios DG, Rekate HL, Khayata MH, Apostolides PJ. Elevated intracranial venous pressure as a universal mechanism in pseudotumor cerebri of varying etiologies. Neurology. (1996) 46:198–202. doi: 10.1212/WNL.46.1.198
12. Jayaraman MV, Boxerman JL, Davis LM, Haas RA, Rogg JM. Incidence of extrinsic compression of the internal jugular vein in unselected patients undergoing CT angiography. AJNR Am J Neuroradiol. (2012) 33:1247–50. doi: 10.3174/ajnr.A2953
13. Doepp F, Schreiber SJ, von Munster T, Rademacher J, Klingebiel R, Valdueza JM. How does the blood leave the brain? A systematic ultrasound analysis of cerebral venous drainage patterns. Neuroradiology. (2004) 46:565–70. doi: 10.1007/s00234-004-1213-3
14. Pollak L, Zohar E, Glovinsky Y, Huna-Baron R. The laboratory profile in idiopathic intracranial hypertension. Neurol Sci. (2015) 36:1189–95. doi: 10.1007/s10072-015-2071-y
15. Baykan B, Ekizoglu E, Altiokka Uzun G. An update on the pathophysiology of idiopathic intracranial hypertension alias pseudotumor cerebri. Agri. (2015) 27:63–72. doi: 10.5505/agri.2015.22599
16. Iida N, Mitamura Y. Effects of venous pressure elevation on myogenic vasoconstrictive responses to static and dynamic arterial pressures. Jpn J Physiol. (1989) 39:811–23. doi: 10.2170/jjphysiol.39.811
Keywords: diastolic blood pressure, intracranial pressure, enlarged vertebral venous plexus, transverse sinus stenosis, venous sinus stenosis
Citation: Li M, Gao X, Liu F, Sun J, Xia N, Meng R and Ji X (2022) Diastolic blood pressure predicts enlarged vertebral venous plexus and intracranial pressure in patients with bilateral transverse sinus stenosis. Front. Neurol. 13:957353. doi: 10.3389/fneur.2022.957353
Received: 07 June 2022; Accepted: 06 July 2022;
Published: 22 August 2022.
Edited by:
Aynur Özge, Mersin University, TurkeyReviewed by:
Babür Dora, Akdeniz University Hospital, TurkeyCopyright © 2022 Li, Gao, Liu, Sun, Xia, Meng and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ran Meng, cmFubWVuZzIwMTFAMTI2LmNvbQ==; Xunming Ji, aml4bUBjY211LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.