
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 13 September 2022
Sec. Neurorehabilitation
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.955893
Background: Progressive supranuclear palsy (PSP) is a parkinsonian-like progressive neurodegenerative syndrome. Key clinical features include ocular motor dysfunction, postural instability, and cognitive dysfunction. Maintaining and improving balance function and gait function are very important for patients with PSP with severe postural dysfunction and repeated falls. In addition, patients with PSP have a poor response to pharmacological treatment; hence, rehabilitation is a key approach in dealing with this syndrome. However, no conclusion on the beneficial effects of rehabilitation for patients with PSP have been established in the literature.
Objectives: The effectiveness of multiple therapeutic exercise program with probable or possible PSP according to the Movement Disorder Society criteria for the clinical diagnosis of PSP was validated.
Methods: Participants underwent multiple therapeutic exercise program customized for each participant, including resistance training, balance training, and walking exercises that were performed for 60–80 minutes a day, 5 days a week for 4 weeks. The outcomes measured were as follows: pull test, Berg Balance Scale (BBS), timed up and go test (TUG), and gait speed test.
Results: A total of 117 patients with PSP were enrolled and the analysis was performed on 20 patients with probable PSP. Four-week rehabilitation significantly improved pull test (p = 0.034) and BBS scores (p = 0.001). There were no significant differences both TUG (p = 0.502) and gait speed (p = 0.813).
Conclusion: The multiple therapeutic exercise program had beneficial effects on balance performance in patients with PSP in 4 weeks and could be an essential element in their rehabilitation. Although this pilot study was conducted without a control group, it provided valuable information for future prospective randomized controlled trials.
Progressive supranuclear palsy (PSP) is a parkinsonian-like progressive neurodegenerative syndrome. Key clinical features include ocular motor dysfunction, postural instability, akinesia, and cognitive dysfunction (1, 2). In addition, mobility problems are the most common early feature in PSP, and onset of falls within 1 year has been associated with a worse prognosis (3).
Maintaining and improving balance function and gait function are very important for patients with PSP with severe postural dysfunction and repeated falls. Rehabilitation for idiopathic Parkinson's disease (PD) was proven to be effective for improving the motor and balance function (4–6). Furthermore, rehabilitation combined with pharmacotherapy is known to improve motor symptoms, gait, and quality of life of patients with PD (7, 8). However, patients with PSP respond poorly to pharmacological treatment (2); consequently, rehabilitation could be highly relevant for maintaining the motor function and activities of daily living (ADL) in PSP (9).
Several studies related to rehabilitation interventions for patients with PSP used therapies designed for them. These therapies included balance and eye movement training (10, 11), harness-supported treadmill training (12–14), weighted vests during ambulation (15), and robot-assisted gait training (12, 16), of these, balance exercise (10, 11) and gait training (12–14, 16) indicated potential benefit. However, a recent systematic review concluded that the effects of structured physical activity for patients with advanced PSP remain unknown (17).
Moreover, the impact on patients with PSP of different multiple therapeutic exercise program (including resistance training, balance training, walking exercises) effective for motor and balance function in the elderly and patients with PD has not been investigated in a sufficient manner yet.
Our study aimed to determine whether multiple patient-specific customized therapeutic exercise program are effective in improving balance and motor functions of patients with PSP.
We conducted a pre–post study between January 2016 and December 2021 that included probable or possible patients with PSP according to the Movement Disorder Society criteria for the clinical diagnosis of PSP (2) in the National Hospital Organization Higashinagoya National Hospital. The exclusion criteria were: (1) a score of the modified Rankin Scale (mRS) ≥ 5, (2) patients with subtypes except Richardson syndrome (RS), (3) patients who underwent rehabilitation treatment for 4 consecutive weeks in the past, (4) inability to walk without assistance for at least 10 meters, (5) patients who were discharged within 4 weeks, and (6) patients with missing values in the outcome data. Patients were hospitalized for the purpose of rehabilitation. The medication of all participants was not changed to maintain a stable condition during the rehabilitation period.
The age, disease duration, sex, subtype, frontal assessment battery (FAB), mini-mental state examination (MMSE), mRS, and the PSP rating scale (PSPRS) scores of all patients were evaluated (18). The balance and basic motor functions were evaluated using the Berg Balance Scale (BBS) (19), timed up and go test (TUG) (20), and gait speed. All patients were evaluated for balance and basic motor functions within 2 days before and 4 weeks after rehabilitation. Participants underwent the following tests:
(1) PSPRS (18): The PSPRS was developed to assess disease severity in patients with PSP. Furthermore, PSPRS assesses characteristic symptoms associated with PSP, including behavioral change, ocular-motor, gait, and balance disfunctions. The maximum total score is 100 points. Higher scores indicate high disease severity. The PSPRS subitem scores and total score were evaluated as baseline, and the scores of V: limb movements and VI: gait and midline were evaluated pre and before interventions.
(2) Pull test (18, 21, 22): The pull test is used for evaluating postural stability (0–4 points) as a component of PSPRS. The examiner stands behind the patient and applies a strong pull on the shoulders with the patient erect with eyes open and feet comfortably apart.
(3) BBS (19): This evaluated the individual's balance abilities during the performance of 14 items (0–4 points per item), such as sitting, standing, and one leg standing, and positional changes. The maximum total score is 56 points. Higher scores indicate good balance ability.
(4) TUG (20): This evaluation consisted of the participant standing up from a sitting position in the chair with a seat height of 40 cm, walking a distance of 3 m, then passing around a cone, returning, and sitting back down in the chair. Comfortable and maximum gait speeds were both measured once each. To assess comfortable speed walking, participants were instructed to walk at their normal comfortable (natural) speed. To assess maximum walking speed, they were asked to walk as fast as possible without running.
(5) Gait speed: Comfortable and maximum gait speed were both measured once each. Participants were required to accelerate and decelerate 2 m before and after the 10 m test distance. To assess comfortable speed walking, participants were instructed to walk at their normal comfortable speed. To assess maximum walking speed, they were asked to walk as fast as possible without running.
This study was not considered about an assessment bias, therefore it included cases that the evaluators are the same professionals in charge of performing the therapies.
The multiple therapeutic exercise program consisted of balance training, resistance training, range of motion (ROM) exercises, stretching, walking exercises, and ADL training and was customized for each patient by physical and occupational therapists. More details about multiple therapeutic exercise program are provided in Supplementary Table 1. The program was performed for 60–80 mins a day, 5 days a week for 4 weeks.
We evaluated the normality of the distribution of all variables using the Shapiro–Wilk test. For comparison before and after the rehabilitation, we used the Wilcoxon signed-rank test or paired t-tests. In addition, we calculated the effect size (r) by the test statistic. Data were reported as mean ± standard deviation for normally distributed data and number for discrete variables. We performed the statistical analysis using the SPSS software, version 26 (IBM Inc., Armonk, NY, USA). A p-value of <0.05 was considered statistically significant.
We enrolled 117 probable or possible patients with PSP. We excluded the following patients who met the exclusion criteria: (1) a score of mRS ≥ 5, n = 53; (2) patients with subtypes except RS, n = 2; (3) patients who underwent rehabilitation treatment for 4 weeks in the past, n = 2; (4) inability to walk without assistance for at least 10 m, n = 12; (5) patients who were discharged within 4 weeks, n = 11; and (6) patients with missing values in the outcome data, n = 17. Consequently, the analysis was performed on 20 patients with probable PSP (Figure 1).
Table 1 lists the participants' demographic and clinical characteristics.
Table 2 shows the results of PSPRS V: Limb motor and VI: Gait and midline pre (0W) and post (4W) rehabilitation. Four-week rehabilitation significantly improved V: Gait and midline total scores (p = 0.004, r = 0.645). Similarly, significant improvements were observed in the VI subitems of arising from chair (p = 0.007, r = 0.607), and postural stability (p = 0.034, r = 0.474).
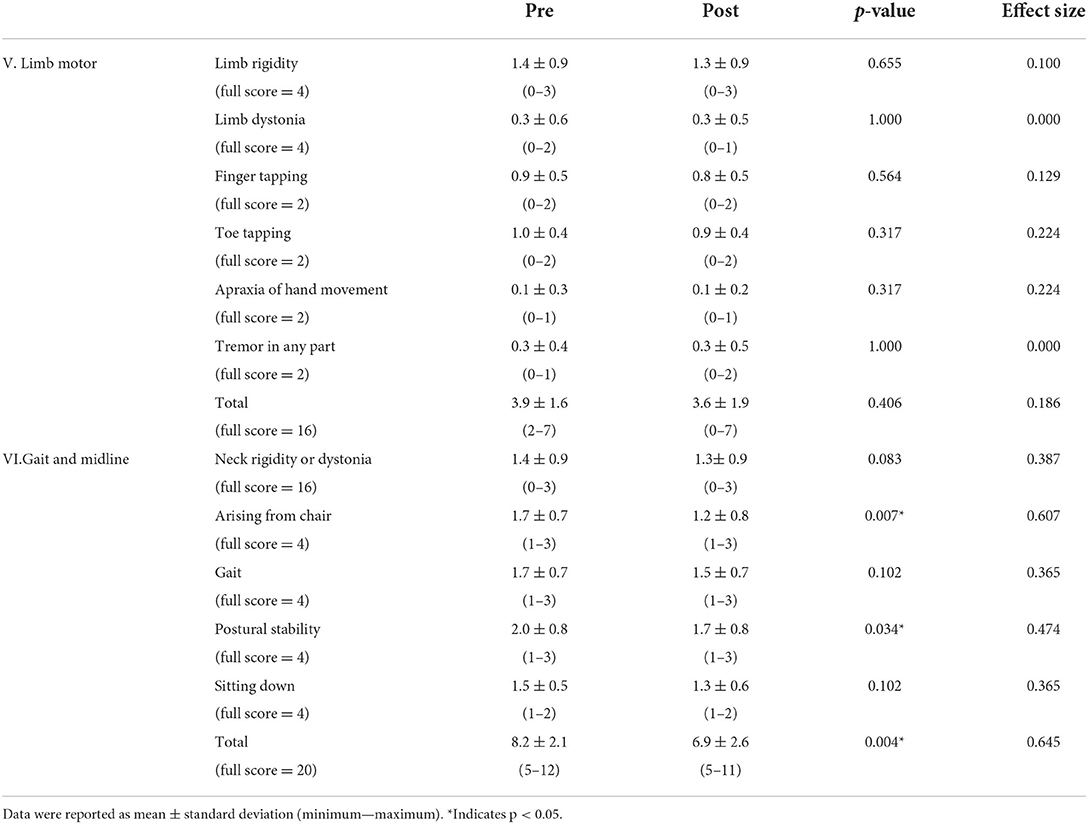
Table 2. Each subitem (V: Limb motor and VI: Gait and midline) score of PSPRS pre (0W) and post (4W) rehabilitation.
Table 3 shows the results of the motor function pre (0W) and post (4W) rehabilitation, and the plot data are shown in Supplementary Figure 1 in the Supplemental material. Four-week rehabilitation significantly improved pull test results (p = 0.034, r = 0.474, Supplementary Figure 1A) and BBS total scores (p = 0.001, r = 0.679, Supplementary Figure 1B). There were no significant differences between pre and post in TUG (comfortable: p = 0.502, r = 0.150; maximum: p = 0.956, r = 0.013, Supplementary Figure 1C) and gait speed (comfortable: p = 0.813, r = 0.055; maximum: p = 0.566, r = 0.133, Supplementary Figure 1D). These results suggest that 4-week rehabilitation improved postural control and balance function in PSP.
Table 4 shows the results of each subitem of BBS pre (0W) and post (4W) rehabilitation, and the plot data are shown in Supplementary Figure 2 in the Supplemental material. There were significant improvements in the items of reaching forward with outstretched arm (p = 0.011, r = 0.566), turning to look behind (p = 0.039, r = 0.461), turning 360 degrees (p = 0.046, r = 0.447), standing with one foot in front (p = 0.047, r = 0.445), and standing on one foot (p = 0.009, r = 0.588).
The present study shows that multiple therapeutic exercise program can improve the balance function in patients with PSP. As no standard pharmacological treatment them has been established yet (recall that PSP has poor response to Levodopa) (9, 23), therapeutic exercise is a key non-pharmacological approach for maintaining their motor function. However, the eventual positive effects of therapeutic exercise in patients with PSP have been insufficiently documented yet (17); therefore, our investigation is among the first to reveal the effectiveness of therapeutic exercise for these patients. This study excluded a control group. Therefore, this limitation hinders the applicability of the results. However, this pilot study provided valuable information for future prospective randomized controlled trials.
In PSP with similar severity, rehabilitation interventions improve PSPRS items V: Limb motor and VI: Gait and midline (12, 13). In this study, multiple therapeutic exercises improved items VI and VI subitems; arising from chair and postural stability post-intervention. Therefore, rehabilitation was expected to enhance the stability of gait and basic movements such as standing, sitting, etc., in patients with PSP.
The pull tests and BBS provided useful measures for changes that are related to balance function. The pull test easily evaluates postural instability and predicts falls in PD (21, 24). The BBS is a wellaccepted, comprehensive evaluation of balance that has excellent reliability and validity with older adults (25). It can also continuously monitor balance function and predict falls in patients with PD and neurodegenerative diseases (26, 27).
In the pre-intervention evaluation of balance function, the pull test showed that the participants had moderate postural instability. The total BBS score of participants who had moderate balance dysfunction was 40.6. In PD, the fall risk cutoff score on BBS reported by Dibble was 54 of 56 (28), that reported by Landers was 44 (29); the participants of this study had a higher fall risk. The results of the BBS subitems (Table 4) showed particularly low values for turning 360 degrees, placing alternate foot on a stool, standing with one foot in front, and standing on one foot. These results suggest that participants have particularly impaired anticipatory postural adjustments (APA), reactive postural adjustments (RPA) and the more challenging balance items. The main lesions of PSP are the substantia nigra, subthalamic nucleus, brain stem, globus pallidus, tegmental portion pons, subthalamic nucleus, cerebellar dentate nucleus, and frontal lobe (1, 30). Postural instability is the primary symptom of PSP, which impairs the ability to maintain balance while standing, rising, seated, changing direction, and walking, making the patient susceptible to falls. The balance dysfunction observed in patients with PSP is primarily a disruption of reactive and anticipatory postural coordination and is associated with dysfunction related to the basal ganglia and brainstem. In addition, cerebellar disturbances may potentiate postural instability.
A few recent reports have assessed the effect of several rehabilitation programs (12–14, 31, 32) for balance function in PSP. In the post-intervention assessment of balance function, our multiple therapeutic exercise program were found to improve balance function in patients with PSP, similar to earlier studies.
Several subsystems contribute to postural stability, such as the functional level of motor systems, anticipatory postural control, dynamic stability, static stability, sensory integration, functional stability limits, reactive postural control, cognitive influences, and verticality (33). Balance training was conducted, including exercise to cope with postural changes when moving the body voluntarily as an intervention for APA disorders and exercise to control posture against external disturbances as an intervention for RPA disorders. In addition, ROM exercises adjusted the participants' optimal postural alignment by improving ROM of limited joints, and strength training focusing on antigravity muscles was performed to maintain posture. These multiple approaches may have improved the balance function such as RPA, APA, static stability, functional stability limits in patients with PSP.
The TUG and gait speed provided useful measures for changes that are related to motor function and the risk of fall. A previous study reported fall risk cutoff score for TUG comfortable time to be 11.5 s in PD (34), whereas another reported it to be 13.5 s in elderly persons (35); the score in this study was 15.3 s. The fall risk cutoff score reported for comfortable 10-m gait speed was 1.1–1.2 m/sec in PD (36) and 1.0 m/sec in elderly persons (37), whereas the score in the present study was 56.6 m/min (converted value 0.94 m/sec). In the pre-intervention evaluation of motor function, participants' motor function was found to be lower than both patients with PD and elderly persons, who were at a higher risk for falls.
In patients with PD, therapeutic exercise, including ROM, stretching, balance training, resistance training, and treadmill walking, was effective for improving motor function, muscle strength, balance function, and gait speed (4, 8). The effect of the same multiple therapeutic exercise program was found to be beneficial for balance function in patients with PSP as well; however, their gait function did not improve as expected.
The fall risk cutoff score for both TUG and gait speed suggests that greater speed is associated with a lower risk of falls in patients with PD and the elderly. In contrast, as postural instability appears in the early stages in patients with PSP, it leads to difficulties in balance control and thereby to an increased risk of falls. As the disease progresses, gait instability due to cerebellar damage is observed in addition to symptoms of parkinsonism, such as rush symptoms and frozen gait (38). Therefore, we argue that primary rehabilitation for patients with PSP should focus on improving their gait stability instead of their gait speed. This study did not assess gait stability; therefore, evidence on the effects of gait stability on fall reduction needs to be validated in the future.
This study has several limitations. First is the fact that it was a retrospective study in a single facility involving a small sample size, the lack of a control group, and absence of follow-ups. Further prospective multicenter studies with a larger sample size, randomized controlled trial setting, and follow-up of long-term rehabilitation can help validate and support our findings. Second, in some cases, the intervention and evaluation were conducted by the same therapists, and there might be concerns about the assessment bias. Therefore, pre-determination and arrangement are needed in which intervention and evaluation are not performed by the same therapists in further prospective studies. Lastly, we lacked the assessment of the quality of life (QoL). This study mainly focused on motor and balance functions of PSP, but the impact of the therapeutical intervention on patients' QoL should be known. In the future, the effect of rehabilitation on QoL of patients with PSP by PSP-QoL (39) or EQ-5D (40) should be investigated.
In this pre–post study, multiple 4-week long therapeutic exercise program known to improve several functions of patients suffering from PD were shown to induce beneficial effects on balance function in patients with PSP.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Ethics Committee of the National Hospital Organization Higashinagoya Hospital (approval number 30-11). Written informed consent from the patients/participants or patients/participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements. All participants were verbally informed of the study and their consent was obtained.
NM and YT prepared and repeatedly revised the manuscript. IA is responsible for the ethics application and contributed to the development, procedure, and funding for research. YT and IA revised the manuscript. All authors reviewed the manuscript and provided final approval for the manuscript.
This work was supported by Grants-in Aid from the Research Committee of CNS Degenerative Diseases, Research on Policy Planning and Evaluation for Rare and Intractable Diseases, Health, Labor, and Welfare Sciences Research Grants, the Ministry of Health, and Labor and Welfare, Japan (20FC1049 to IA).
The authors thank the patients and their families for their contributions. We would also like to thank the doctors (Department of Neurology) and physical therapists (Department of Rehabilitation) at the National Hospital Organization Higashinagoya National Hospital for their support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.955893/full#supplementary-material
1. Steele JC, Richardson JC, Olszewski J. Progressive supranuclear palsy. a heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol. (1964) 10:333–59. doi: 10.1001/archneur.1964.00460160003001
2. Hoglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord. (2017) 32:853–64. doi: 10.1002/mds.26987
3. Nath U, Ben-Shlomo Y, Thomson RG, Lees AJ, Burn DJ. Clinical features and natural history of progressive supranuclear palsy: a clinical cohort study. Neurology. (2003) 60:910–6. doi: 10.1212/01.WNL.0000052991.70149.68
4. Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis. Mov Disord. (2008) 23:631–40. doi: 10.1002/mds.21922
5. Keus SH, Munneke M, Nijkrake MJ, Kwakkel G, Bloem BR. Physical therapy in Parkinson's disease: evolution and future challenges. Mov Disord. (2009) 24:1–14. doi: 10.1002/mds.22141
6. Yitayeh A, Teshome A. The effectiveness of physiotherapy treatment on balance dysfunction and postural instability in persons with Parkinson's disease: a systematic review and meta-analysis. BMC Sports Sci Med Rehabil. (2016) 8:17. doi: 10.1186/s13102-016-0042-0
7. Frazzitta G, Maestri R, Bertotti G, Riboldazzi G, Boveri N, Perini M, et al. Intensive rehabilitation treatment in early Parkinson's disease: a randomized pilot study with a 2-year follow-up. Neurorehabil Neural Repair. (2015) 29:123–31. doi: 10.1177/1545968314542981
8. Radder DLM, Ligia Silva de Lima A, Domingos J, Keus SHJ, van Nimwegen M, Bloem BR, et al. Physiotherapy in Parkinson's disease: a meta-analysis of present treatment modalities. Neurorehabil Neural Repair. (2020) 34:871–80. doi: 10.1177/1545968320952799
9. Litvan I. Parkinsonian features: when are they Parkinson disease? JAMA. (1998) 280:1654–5. doi: 10.1001/jama.280.19.1654
10. Zampieri C, Di Fabio RP. Balance and eye movement training to improve gait in people with progressive supranuclear palsy: quasi-randomized clinical trial. Phys Ther. (2008) 88:1460–73. doi: 10.2522/ptj.20070302
11. Zampieri C, Di Fabio RP. Improvement of gaze control after balance and eye movement training in patients with progressive supranuclear palsy: a quasi-randomized controlled trial. Arch Phys Med Rehabil. (2009) 90:263–70. doi: 10.1016/j.apmr.2008.07.024
12. Clerici I, Ferrazzoli D, Maestri R, Bossio F, Zivi I, Canesi M, et al. Rehabilitation in progressive supranuclear palsy: effectiveness of two multidisciplinary treatments. PLoS One. (2017) 12:e0170927. doi: 10.1371/journal.pone.0170927
13. Di Pancrazio L, Bellomo RG, Franciotti R, Iodice P, Galati V, D'Andreagiovanni A, et al. Combined rehabilitation program for postural instability in progressive supranuclear palsy. NeuroRehabilitation. (2013) 32:855–60. doi: 10.3233/NRE-130909
14. Suteerawattananon M, MacNeill B, Protas EJ. Supported treadmill training for gait and balance in a patient with progressive supranuclear palsy. Phys Ther. (2002) 82:485–95. doi: 10.1093/ptj/82.5.485
15. Wallace R, Abbott C, Gibson-Horn C, Skubic M. In-home measurement of the effect of strategically weighted vests on ambulation. Annu Int Conf IEEE Eng Med Biol Soc. (2013) 2013:949–52. doi: 10.1109/EMBC.2013.6609659
16. Sale P, Stocchi F, Galafate D, De Pandis MF, Le Pera D, Sova I, et al. Effects of robot assisted gait training in progressive supranuclear palsy (PSP): a preliminary report. Front Hum Neurosci. (2014) 8:207. doi: 10.3389/fnhum.2014.00207
17. Slade SC, Finkelstein DI, McGinley JL, Morris ME. Exercise and physical activity for people with progressive supranuclear palsy: a systematic review. Clin Rehabil. (2020) 34:23–33. doi: 10.1177/0269215519877235
18. Golbe LI, Ohman-Strickland PA. A clinical rating scale for progressive supranuclear palsy. Brain. (2007) 130:1552–65. doi: 10.1093/brain/awm032
19. Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health. (1992) 83 Suppl 2:S7-11.
20. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. (1991) 39:142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x
21. Hunt AL, Sethi KD. The pull test: a history. Mov Disord. (2006) 21:894–9. doi: 10.1002/mds.20925
22. Munhoz RP, Li JY, Kurtinecz M, Piboolnurak P, Constantino A, Fahn S, et al. Evaluation of the pull test technique in assessing postural instability in Parkinson's disease. Neurology. (2004) 62:125–7. doi: 10.1212/WNL.62.1.125
23. Lamb R, Rohrer JD, Lees AJ, Morris HR. Progressive supranuclear palsy and corticobasal degeneration: pathophysiology and treatment options. Curr Treat Options Neurol. (2016) 18:42. doi: 10.1007/s11940-016-0422-5
24. Munhoz RP, Teive HA. Pull test performance and correlation with falls risk in Parkinson's disease. Arq Neuropsiquiatr. (2014) 72:587–91. doi: 10.1590/0004-282X20140082
25. Berg KO, Maki BE, Williams JI, Holliday PJ, Wood-Dauphinee SL. Clinical and laboratory measures of postural balance in an elderly population. Arch Phys Med Rehabil. (1992) 73:1073–80.
26. Steffen T, Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with parkinsonism. Phys Ther. (2008) 88:733–46. doi: 10.2522/ptj.20070214
27. Qutubuddin AA, Pegg PO, Cifu DX, Brown R, McNamee S, Carne W. Validating the berg balance scale for patients with Parkinson's disease: a key to rehabilitation evaluation. Arch Phys Med Rehabil. (2005) 86:789–92. doi: 10.1016/j.apmr.2004.11.005
28. Dibble LE, Christensen J, Ballard DJ, Foreman KB. Diagnosis of fall risk in Parkinson disease: an analysis of individual and collective clinical balance test interpretation. Phys Ther. (2008) 88:323–32. doi: 10.2522/ptj.20070082
29. Landers MR, Backlund A, Davenport J, Fortune J, Schuerman S, Altenburger P. Postural instability in idiopathic Parkinson's disease: discriminating fallers from nonfallers based on standardized clinical measures. J Neurol Phys Ther. (2008) 32:56–61. doi: 10.1097/NPT.0b013e3181761330
30. Kovacs GG, Lukic MJ, Irwin DJ, Arzberger T, Respondek G, Lee EB, et al. Distribution patterns of tau pathology in progressive supranuclear palsy. Acta Neuropathol. (2020) 140:99–119. doi: 10.1007/s00401-020-02158-2
31. Nicolai S, Mirelman A, Herman T, Zijlstra A, Mancini M, Becker C, et al. Improvement of balance after audio-biofeedback. a 6-week intervention study in patients with progressive supranuclear palsy. Z Gerontol Geriatr. (2010) 43:224–8. doi: 10.1007/s00391-010-0125-6
32. Croarkin E, Robinson K, Stanley CJ, Zampieri C. Training high level balance and stepping responses in atypical progressive supranuclear palsy: a case report. Physiother Theory Pract. (2022) 1-12. doi: 10.1080/09593985.2022.2032509
33. Sibley KM, Beauchamp MK, Van Ooteghem K, Straus SE, Jaglal SB. Using the systems framework for postural control to analyze the components of balance evaluated in standardized balance measures: a scoping review. Arch Phys Med Rehabil. (2015) 96:122–32 e29. doi: 10.1016/j.apmr.2014.06.021
34. Nocera JR, Stegemoller EL, Malaty IA, Okun MS, Marsiske M, Hass CJ, et al. Using the Timed Up & Go test in a clinical setting to predict falling in Parkinson's disease. Arch Phys Med Rehabil. (2013) 94:1300–5. doi: 10.1016/j.apmr.2013.02.020
35. Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. (2000) 80:896–903. doi: 10.1093/ptj/80.9.896
36. Lindholm B, Nilsson MH, Hansson O, Hagell P. The clinical significance of 10-m walk test standardizations in Parkinson's disease. J Neurol. (2018) 265:1829–35. doi: 10.1007/s00415-018-8921-9
37. Imms FJ, Edholm OG. Studies of gait and mobility in the elderly. Age Ageing. (1981) 10:147–56. doi: 10.1093/ageing/10.3.147
38. Takamatsu Y, Matsuda N, Aiba I. The combination of short-step and wide-based gait is a gait characteristic in progressive supranuclear palsy: a retrospective, cross-sectional study. Eur Geriatr Med. (2019) 10:809–15. doi: 10.1007/s41999-019-00211-2
39. Schrag A, Selai C, Quinn N, Lees A, Litvan I, Lang A, et al. Measuring quality of life in PSP: the PSP-QoL. Neurology. (2006) 67:39–44. doi: 10.1212/01.wnl.0000223826.84080.97
Keywords: progressive supranuclear palsy, exercise, rehabilitation, rehabilitation research, balance
Citation: Matsuda N, Takamatsu Y and Aiba I (2022) Effect of therapeutic exercise on the balance of patients with progressive supranuclear palsy: A pilot study. Front. Neurol. 13:955893. doi: 10.3389/fneur.2022.955893
Received: 29 May 2022; Accepted: 01 August 2022;
Published: 13 September 2022.
Edited by:
Ina M. Tarkka, University of Jyväskylä, FinlandReviewed by:
Farwa Ali, Mayo Clinic, United StatesCopyright © 2022 Matsuda, Takamatsu and Aiba. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Naomi Matsuda, bmFvbWF0c3UxMTIwQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.