- 1Department of Rehabilitation Medicine, The First Affiliated Hospital of Dalian Medical University, Dalian, China
- 2Institute (College) of Integrative Medicine, Dalian Medical University, Dalian, China
Background: Post-stroke depression (PSD) is not only a frequent neuropsychiatric manifestation secondary to stroke but is also associated with disability, poor rehabilitation outcomes, sleep disorders, cognitive impairment, and increased mortality. Transcranial direct current stimulation (tDCS), a primary modality of non-invasive brain stimulation (NIBS), has shown promising clinical results in the rehabilitation of patients with PSD recently. The primary aim of this systematic review is to assess the effects of tDCS on PSD.
Methods: PubMed and Cochrane databases were used for paper identification up to May 2022. Only English language studies and published data were taken into consideration. The methodological quality of selected studies was assessed according to the modified Sackett Scale, based on Physiotherapy Evidence Database (PEDro) scores.
Results: Six experimental studies were included for the PSD treatment of tDCS and all of them reported that, following the intervention of tDCS, the experimental group shows a statistically significant decrease in the depression level in accordance with different assessment scales.
Conclusion: This article simply aims at providing a comprehensive overview of the raw data reported in this field to date. Based on the current evidence, tDCS presents promising results for the treatment of PSD. Moreover, tDCS is also effective in PSD patients with aphasia or CPSP. However, an optimal stimulation protocol is needed to formulate. Thus, the development of robustly controlled, randomized, and high-quality clinical trials to further assess the utility of tDCS as a therapeutic tool for the treatment of PSD survivors is encouraged.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023322076, identifier: CRD42023322076.
Introduction
Stroke is a cerebrovascular disease with a high incidence worldwide. It is mainly manifested as a series of pathological reactions caused by ischemic or hemorrhagic injury of the brain tissue. Among the complications of a stroke, post-stroke depression (PSD), a source of suffering among stroke survivors (1), is the most frequent psychiatric problem. Persons with PSD are strongly associated with higher mortality rates (2, 3), higher rates of suicidal ideation (4), and lower quality of life compared with post-stroke patients without depression. Hence, it is vital to have knowledge of the principles of identification and effective treatment options for PSD.
The pathophysiology of PSD is complicated and still incompletely understood, which may include a result of the joint action of multiple mechanisms. One of the most widely accepted hypotheses is the monoamine neurotransmitter hypothesis, represented by low levels of expression. Other processes that may contribute to PSD include the reduction of brain-derived neurotrophic factor (BDNF) content, excess of inflammatory cytokines, dysfunction of the hypothalamic-pituitary-adrenal axis, neuroanatomical mechanism, and glutamate-mediated excitotoxicity (5–7). A recent study suggests that the gut microbiome may play a role in the development of PSD (8), which may be involved in the regulation of lipid metabolism.
The main symptoms of PSD include persistent low mood, lack of interest, apathy, slow thinking, pessimism, and even suicidal thoughts. The fifth US Diagnostic and Statistical Manual of Mental Disorders (DSM-5) is currently the most commonly used scale to diagnose PSD. However, when used in busy and resource-poor clinical settings, the DSM-5 may not be validated for use in stroke. As a result, it is often appropriate to use a self-completed depression screening scale, such as the 9-item Patient Health Questionnaire, the Hamilton Depression Rating Scale (HDRS), the Beck Depression Inventory (BDI), Hospital Anxiety and Depression Scale (HADS), and the Montgomery-Åsberg Depression Rating Scale (MADRS) (9).
With the standardized use of new antidepressants and the rapid development of psychotherapy pharmacological treatment (10), psychosocial interventions (11), traditional Chinese medicine (TCM), especially non-invasive Brain Stimulation (NIBS) technology, and the quality of life of patients with PSD has improved dramatically. Pharmacotherapy is still the first-line treatment of PSD with an improvement of cognitive impairment and long-term survival (1, 12), although with a controversial efficacy (13), frequently accompanied by a high risk of adverse outcomes (14). TCM may be a potential selection for patients with PSD who fail to afford high charges of psychotherapy or other PSD treatments or are unable to tolerate antidepressant side effects. Acupuncture, an effective form of practice of TCM, is a promising effective therapy that is gradually being accepted as a therapeutic option for neuropsychiatric disorders across the world (13, 15, 16).
Recently, the role of NIBS in the rehabilitation of cognitive impairments after stroke has attracted much attention (17). The main modalities of NIBS are repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS), which are emerging neuromodulation techniques that are beneficial to the recovery of dysfunction after stroke (18–21).
Transcranial direct current stimulation is mainly used to regulate the cortical excitability under the stimulated brain regions (22) through constant and low-intensity current (0.5–2.0 mA), which can effectively change the polarization state of the cell membrane and modulate the plasticity of synapses (23). The anode electrode is usually applied to area C3 or C4, while the cathode electrode is mostly positioned on the contralateral supraorbital area (24, 25). C3 or C4 is the reflex region of the primary motor cortex according to the 10–20 electroencephalography [EEG] system. Anode tDCS stimulation can increase stimulated cortical excitability, while cathode stimulation decreases it (23).
Transcranial direct current stimulation is currently widely used in neuropsychiatric disorders, such as depression, post-stroke aphasia, and Parkinson's disease (26). However, the neurobiological mechanisms underlying tDCS remain elusive, involving several pathological processes in the central nervous system, such as modulating the resting membrane potential of the targeted neuronal population (27), enhancing the functional connectivity between two brain regions (28, 29) and increase the synaptic plasticity, which can be achieved by inducing the release of neurotransmitters, modifying the activity of N-methyl D-aspartate (NMDA) receptor (30, 31) and inducing the occurrence of long-term potentiation (LTP) of cortical recombination (32). BDNF, which plays a key role in LTP formation, is modulated by tDCS based on some studies (33). TDCS also has long-lasting after effects (34). Research confirms that after 5 min tDCS anode stimulation, it can induce increased excitability of the motor cortex, which lasts for more than a few minutes (35). TDCS after effects is affected by a lot of factors, such as duration and frequency of stimulation, locations of anode/cathode electrode, current density, and co-administered treatments (31).
In view of the increasingly obvious disadvantages of various treatment methods for PSD, tDCS, as a novel treatment method, has attracted more and more scholars' attention. But the effectiveness is not yet well established. Therefore, we conducted a systematic review of the clinical studies on tDCS in the treatment of PSD in recent years to contribute to the standardized use of tDCS and improve the wellbeing of patients with PSD.
Methods
This systematic review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42023322076). The review was administrated in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.
Search strategies
The study search was to capture as many relevant clinical studies as possible. This article referred only to published data. PubMed and Cochrane databases were used for paper identification up to May 2022. Only English language studies and published data were taken into consideration. The search strategies combined medical subject heading (MeSH) with free-text terms, which were adjusted in terms of the requirements of a specific database. Our key search terms were stroke, depression, and transcranial direct current stimulation.
Two authors (WJH and YN) managed the literature searches and strictly screened eligible research according to inclusion and exclusion criteria. When it came to any disagreements, a third author (YL) was consulted to cope with inconsistencies. Two authors (WJH and XYG) were assigned to carry out the data extraction. Subsequently, all the authors assessed the methodological quality of each article and then crosschecked it to ensure accuracy. The search strings used in both databases are shown as Supplementary material.
Inclusion and exclusion criteria
Selected studies had to meet the following inclusion criteria: (a) the main intervention was tDCS; (b) the primary subject of the study is people; (c) the principal diagnosis for patients was PSD [patients diagnosed with stroke with brain neuroimaging, clinical history, and physical examination; diagnosis with depression mainly according to the Mini-International Neuropsychiatry Interview (MINI) questionnaire, the Beck Depression Inventory (BDI)], DSM-IV, or DSM-V; (d) 10 is the minimal amount of tDCS sessions; (e) Published in English; and (f) Peer-reviewed.
The reasons for excluding studies were as follows: (a) Relevant indexes were not reported; (b) Studies with the main diagnosis were anxiety, epilepsy, or other cognitive disorders; (c) Duplicate publications; and (d) Valid data were unavailable or data not completed.
Data extraction
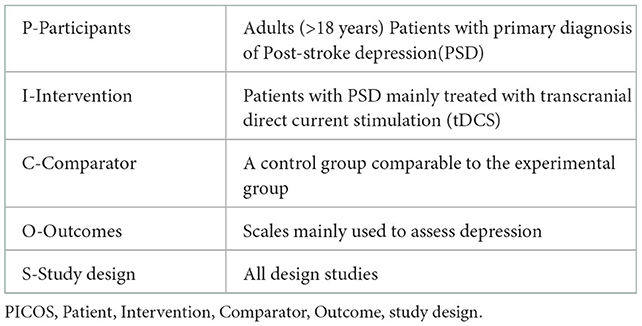
According to the PRISMA guidelines, we used the PICOS tool, which was more sensitive than other search tools such as SPIDER or PICO, and was recommended for current practice to ensure exhaustive literature searches for our research (36, 37). We paid particular attention to patients' features (gender, age, sample size, post-stroke time onset, diagnosis and diagnosis instruments, stroke type, and lesion position), intervention, machine type, comparator, outcomes, study design, and stimulation parameters. Then, we analyzed the similarities and differences among the selected articles. The specific PICOS model is shown in Table 1.
Study quality assessments
The methodological quality of selected studies was assessed according to the modified Sackett Scale, based on Physiotherapy Evidence Database (PEDro) scores (38). The PEDro has 11 items on study quality, each of the concepts answered with “yes” (score = 1) or “no” (score = 0). The PEDro has been shown to be a more comprehensive measure of the methodological quality for trials in the stroke rehabilitation literature compared with others such as the Jaded scale.
Results
From our literature search, 49 records were identified through databases. Besides 1 duplicate removed, 28 articles were excluded based on their titles and abstracts, and 14 articles were excluded owing to inconsistency with inclusion criteria. A total of three types of clinical research were screened in terms of the PICOS rule. However, due to the small number of selected research, three case reports were also included to fully discuss the research status of tDCS application in PSD. After the full-text assessment, six studies were included in this systematic review. Figure 1 is the flow diagram of the study selection process.
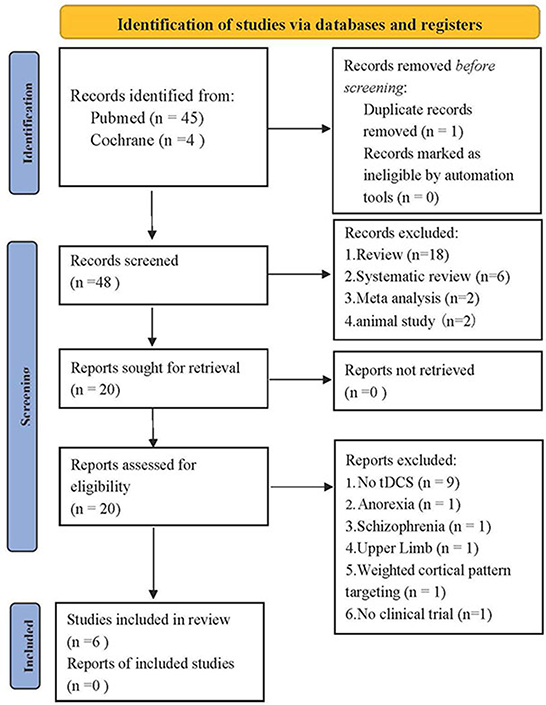
Figure 1. PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers (39).
Quality assessments
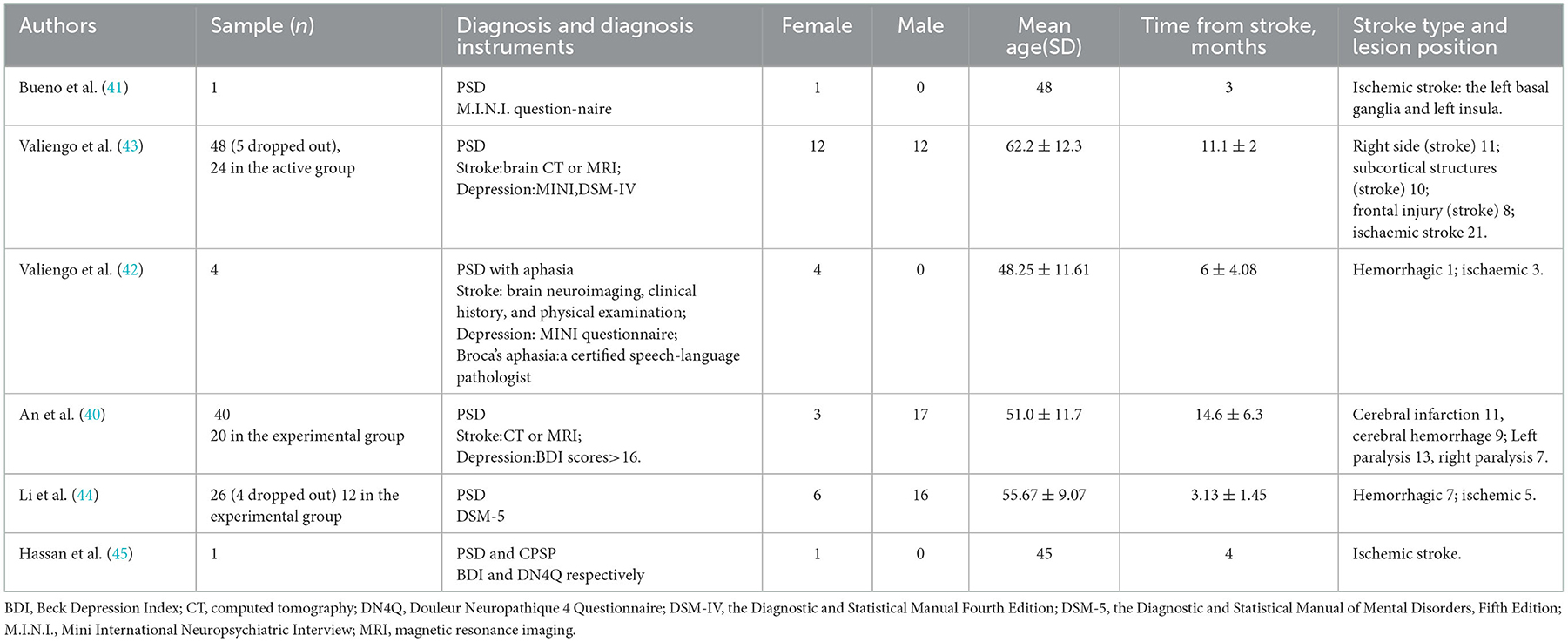
Six experimental studies of PSD were included in this systematic review (40–45) with tDCS treatment. The methodological quality of Valiengo's randomized, placebo-controlled clinical trial (RCT) was high, with a mean PEDro score of 8 out of 10 (level 1b evidence). Both Li et al. and An et al. had a control group and were rated as level 3 on the modified Sackett Scale. The case series study (42) and the case reports (41, 45) were considered as level 4 and 5 evidence, respectively.
Participants' characteristics
A total of 120 patients diagnosed with PSD were involved in this study. Following is a detailed description of homogeneous characteristics: (a) All the patients with PSD were diagnosed by physical examination, neuroimaging, and scales, specifically made for assessing the depression level. (b) Participants' age ranged between 32 and 74 years. (c) The time for stroke varies between 2 and 24 months. (d) Stroke types included hemorrhagic and ischemic stroke. Lesion position is particularly presented in Table 2. (e) All patients were antidepressant-free across six studies.
Stimulation protocols
In five studies (40–44), the anode electrode was placed on the scalp corresponding to the left DLPFC, while the cathode was attached to the right DLPFC according to the International 10–20 EEG System. However, in Hassan's study, the anode electrode was placed over the right DLPFC and the cathode on the contralateral supraorbital region.
Transcranial direct current stimulation was delivered at an intensity of 2 mA (current density = 0.80 A/m2) for 30 min in the active/experimental group in four studies (40–43), while in Li and Hassan's studies, the patients received anodal tDCS stimulation at an intensity of 2 mA for only 20 min.
The number of sessions in Valiengo's two studies is 12, comprising once daily on weekdays for 2 weeks as well as two additional sessions after 2 and 4 weeks. However, in Bueno and Hassan's studies, patients received only 10 sessions in contrast to the 20 sessions that patients received in An and Li's studies. In three studies (40, 43, 44), the stimulation was stopped at 15, 30, and 60 s after the application in the control/sham group, respectively, though the anode and cathode positions were the same as in the active/experimental group. A specific description is presented in Table 3.
Concomitant therapy/tasks
In Bueno and Li's studies, patients were treated with antidepressants (fluoxetine dose and sertraline hydrochloride of 50 mg qd, respectively) during tDCS stimulation. While in An's studies, conventional occupational therapy was used as the concomitant task. No other intervention or pharmacological treatment was mentioned in Hassan and Valiengo's studies (42, 43, 45) except for tDCS.
Placebo
Only in Valiengo's study in 2017, the authors used a randomized, sham-controlled, and double-blind trial design with the sham group consisting of only 60 s of stimulation.
Depression
Five scales were used to investigate the depression level of PSD across the six studies. HDRS was applied in two studies. In one study, HDRS was one of the moods and cognitive rating scales (41); in the other study, differently, HDRS-17 (the 17-item version) was the primary outcome (43). The MADRS has also been used in Bueno's and Valiengo's (43) studies. The BDI was administered to score the depression levels in patients before and after the intervention in Bueno's, Hassan's, and An's studies.
Two of the five scales were specific for aphasic patients with PSD in Valiengo et al.'s study (42). A 9-item interview, the Aphasic Depression Rating Scale (ADRS), used as the primary outcome in Valiengo et al.'s study (42), was executed at the baseline (before the tDCS treatment), Week 2, Week 4, and Week 6 to evaluate depression degree in patients with aphasia. In addition, the Stroke Aphasic Depression Questionnaire (SADQ), consisting of a 21-item questionnaire, was applied in Valiengo's study to detect low mood in stroke patients with aphasia.
Safety and adverse effects
Two studies (42, 43) assessed the safety with a tDCS adverse effects questionnaire, and both of them showed no adverse effects were observed. There were no side effects were reported in other included studies, and the treatment was well tolerated.
Outcome
Bueno et al. first analyzed the feasibility of tDCS in the treatment of patients with PSD in 2011. In this open-label case report, a 48-year-old woman, who was diagnosed with PSD, showed marked amelioration of significant mood and cognitive impairment in the HDRS, BDI, MADRS, MOCA, and MMSE following the combination of anodal stimulation over the left DLPFC with fluoxetine dose. These positive results were intended to encourage further controlled trials on the field. Subsequently, in 2017, Valiengo et al. first conducted a randomized, sham-controlled study to verify that tDCS was effective and safe for PSD. Prior to this, a preliminary, open-label study was conducted by Valiengo et al. (42) to assess the safety and efficacy of tDCS for PSD patients with aphasia. In this study, four drug-free female patients with PSD who, due to their aphasia, showed improvement in depression after 12 sessions as manifested by a decrease in the Aphasic Depression Rating Scale (ADRS). Moreover, this improvement was maintained for 4 weeks after the treatment.
One year later, in Valiengo's controlled trial, 48 antidepressant-free patients with PSD met the inclusion criteria, and 43 completed the study (five patients dropped out). With the similar stimulation protocol described in Valiengo et al.'s study (42), the active group showed greater improvement in depressive symptoms as shown in HDRS-17 and also presented higher response (categorical, defined as ≥50% reduction from the baseline HDRS score) and remission (categorical, defined as an endpoint HDRS score of <8) rates in the active vs. the sham group. The authors recommended that tDCS was a favorable and safe option for PSD.
To assess the effects of tDCS on depression and quality of life (QOL) in patients with stroke, 40 patients were confirmed to be severely depressed and completed the experiment in An's controlled study. The BDI was administered to score the depression levels in patients before and after the intervention. They drew the conclusion that tDCS intervention caused improvement in depression levels as well as QOL in the experimental group, which might introduce a new outcome measure for the evaluation of the efficacy of tDCS in the treatment of PSD. However, the small sample size limited the generalization of the positive result.
Li et al. used fNIRS to investigate the neural mechanism of tDCS in the treatment of PSD. With the semblance stimulation protocol described in Valiengo et al.'s study (42, 43), two tasks (an emotional face sex judgment task and a “1-back” working memory task) were arranged for 26 patients with PSD to evaluate reaction times and relative concentration changes of oxyhemoglobin (Oxy-Hb) in the prefrontal cortex (PFC). As shown in the result, there was no notable difference between the experimental and the control group in the first task. In the second task, there was a statistical difference between the two groups with shorter reaction times in the experiment group. As for relative Oxy-Hb, both the left and right PFC in the experimental group showed a significant result. This study provided insight into the mechanism by which tDCS improves patients with PSD, enhancing aerobic metabolism in the PFC.
Hassan et al. shared the latest case report about the effectiveness of using tDCS over the DLPFC with short inter-session intervals to reduce central pain and depression in a stroke survivor who presented with central post-stroke pain (CPSP) and depression, following a stroke. The BDI score declined from 25 to 7 after the intervention of 2 mA, and 20 min of anodal tDCS stimulations for 2 weeks. However, the BDI score returned to 25 at 3 weeks post-intervention. After the second protocol of stimulation (seven daily sessions of stimulations of 2 mA, 13 min, each with 20 min inter-session intervals for 1 week), the pain score turned to 0 immediately, while the BDI score improved to 18 at 3 weeks and later to 7 at 6 months post-intervention, which might be related to a higher tDCS dose and the aftereffects of tDCS.
Discussion
Post-stroke depression is a common neuropsychiatric complication after stroke (46). Although the pathophysiology or pathogenesis of PSD is complex and largely unknown, there are increasing treatments such as pharmacotherapy, psychotherapy, psychosocial–behavioral intervention, TCM, and especially NIBS to deal with PSD. In this review, we have included six studies to provide a relatively comprehensive summary of the raw data reported in this area so far. Although all the studies show marked improvement of depressive symptoms in patients with PSD, there are some questions needed to be further explored.
Biomarkers reflecting the effects of tDCS and predictors of PSD need to be further explored. In addition, there is no universal standard for the diagnosis of PSD. Therefore, it is difficult to make a comparison between different studies because the six included studies hold different diagnostic criteria for PSD.
Transcranial direct current stimulation seems to show a promising result in the treatment of patients with PSD and with aphasia according to Valiengo et al.'s study in 2016. Aphasia can be a frequent complication following a stroke. However, with a missing investigation of the potential changes in language deficits before the study, this study cannot provide strong evidence that tDCS is effective for aphasia. With a growing number of evidence supporting the enhancing effects of tDCS in the recovery of post-stroke aphasia (47, 48), controlled and randomized clinical trials to further verify the utility of tDCS for the treatment of PSD patients with aphasia is encouraged. Moreover, HDRS-17, MADRS, and BDI scales are the most commonly used scales to evaluate PSD. Nevertheless, when used to assess PSD patients with aphasia, the ADRS scale is more appropriate.
The pathological mechanism by which tDCS improves symptoms in PSD patients with CPSP in Hassan's study is attributed to a common target of chronic pain and depression: the DLPFC, whose cortical excitation is increased due to tDCS stimulation. However, due to only one case report and short follow-up time, more experiments are needed in the future to generalize the conclusion that tDCS can improve depression and pain in PSD patients with CPSP.
Transcranial direct current stimulation is an emerging neuromodulation technique that obsesses the benefits of convenience, safety profile, and lower cost compared with TMS (49). Only a few studies have reported transient skin irritation, itching, erythema, and tingling (50). Although the studies under consideration do not report any adverse events, it is necessary to validate the safety parameters of tDCS because the intensity and location of the current may vary depending on the local anatomy and lesion time (38). Unfortunately, the study only from Valiengo in 2017 is reported to conduct a tDCS adverse effects questionnaire for assessing safety. Consequently, safety has to be proven in further high-quality research with clinical assessments. In addition to better safety, tDCS allows a reliable sham condition for a controlled study, which is difficult to easily identify from active stimulation (51). These advantages facilitate the application of tDCS in both hospitals, and especially homes for bedridden patients, boosting the generalization of tDCS (52).
There are many factors that might influence the tDCS effects. TDCS dose has been taken into consideration recently. A meta-analysis shows that tDCS dose may be an independent predictor of better efficacy (53). Evidence suggests that the effects of tDCS are the results of cumulative effects (54). This phenomenon has been well confirmed in the study of Hassan et al., with better scores in BDI after increased tDCS dose. However, among the included studies, authors conducted that 20 is the longest session owing to a low rate of back to the clinical center. Therefore, patients who have trouble returning to the clinical center (e.g., physical disabilities, living in a remote area, and so on) are in pressing need of an alternative approach that they can use when they stay away from the clinic or research facility. The good news is that a comprehensive guide to operating tDCS safely in home settings and clinical use is provided recently. The guideline can facilitate further clinical research to a certain extent (54).
Another factor affecting the tDCS effects is stimulation protocols. For example, four studies (40–43) recorded the stimulation parameters were 2 mA for 30 min daily. However, two studies (44, 45) reported patients receiving sessions of anodal stimulations of 2 mA intensity for only 20 min. Moreover, in the sham/controlled group, a brief stimulation period, 60 s (43) or 30s (40), is conducted to mimic common skin effects experienced just after stimulation for 30 min, followed by no stimulation during the remaining period. While in the study of anodal stimulations of 2 mA for only 20 min, stimulation is stopped after 15 s in the control group. Consequently, an optimal stimulation protocol is needed to formulate. In addition, factors regarding the PSD such as stroke types, time since stroke, and the duration of depression onset after stroke also need to be considered in the tDCS effects. Homogeneity tests, therefore, need to be conducted before each study, as shown in studies by An et al., Li et al., and Valiengo et al. (43).
FNIRS is a promising noninvasive neuroimaging technique used to measure activation-induced changes in the cerebral hemoglobin concentrations of oxyhemoglobin (ΔHbO) and deoxyhemoglobin (ΔHbR) (55). Greater blood flow can be detected by oxyhemoglobin in veins of activation of brain cortical neurons than inactive ones (56). In Li's study, the authors use fNIRS before and after treatment, and reaction times and relative concentration changes of oxyhemoglobin (Oxy-Hb) in the PFC are assessed using an emotional face sex judgment task and a “1-back” working memory task. They conclude that enhancing aerobic metabolism in the PFC is the main mechanism of tDCS in improving the processing of negative emotions and working memory in patients with PSD. In comparison with the existing neuroimaging techniques involving direct neural activation measurement methods such as functional magnetic resonance imaging (fMRI) and electroencephalography (EEG), fNIRS is more attractive with the advantage of higher spatial resolution and lower susceptibility to the movement artifact (55). Thus, fNIRS technology can be applied in the future to explore the neural mechanism of tDCS improving PSD and even other post-stroke diseases.
Two studies (41, 44) report that antidepressants are used as a concomitant task, and one study records that conventional occupational therapy is conducted during tDCS stimulation. Furthermore, in Bueno's study, although fluoxetine alone did not improve depression in the patient, the combined use of fluoxetine with tDCS led to mood and affective improvement. The American Heart Association/American Stroke Association (AHA/ASA) recommends the pharmacological treatment of PSD with selective serotonin reuptake inhibitors (SSRIs) or tricyclic antidepressants (TCAs), especially for patients in rehabilitation settings (7). According to a factorial, randomized, and controlled trial, tDCS stimulation combines with sertraline increases the efficacy of each treatment (57). Therefore, a combination of tDCS stimulation with other therapies may lead to better clinical outcomes compared with mono-therapy.
Another interesting finding is that it takes a long time for tDCS to reach its maximum effect. This was well-demonstrated in Valiengo et al.'s (42, 43) and Hassan's experiments. As has been discussed earlier, tDCS also has long-lasting aftereffects, which involve a lot of neurotransmitters and pathological processes in the central nervous system, and its effects are associated with synaptic plasticity changes in the brain (50). The findings could guide clinical trials in which tDCS should be used before concomitant therapies to help maximize its aftereffects when more than two interventions are needed to treat the diseases.
Limitations were analyzed in five studies except for Bueno's study. An's and Valiengo's studies had a common limitation, a small sample size. In addition to this, as we all know, RCTs are the most convincing designs to assess the efficacy of new treatments or interventions (58). However, there is very low-certainty evidence from these conclusions because only one RCT research is included. As a result, we can only put forward a weak recommendation in favor of the tDCS for PSD. Therefore, future multi-center, large-sample clinical trials are needed to allow generalization of the results. Furthermore, follow-up of depression and improvement in quality of life in patients with PSD is necessary.
Conclusion
Based on the current evidence, tDCS presents promising results for the treatment of PSD. Moreover, tDCS is also effective in PSD patients with aphasia or CPSP. However, an optimal stimulation protocol is needed to formulate. Thus, the development of robustly controlled, randomized, and high-quality clinical trials to further assess the utility of tDCS as a therapeutic tool for the treatment of PSD survivors is encouraged.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
WH and YN managed the literature searches and analyses and wrote the first draft of the manuscript. YL reviewed and revised the manuscript. WH and XG carried out the data extraction and all the authors were assigned to assess methodological quality. YG analyzed the data and made tables. When it came to any disagreements, YL was consulted to cope with inconsistencies. All authors contributed to and approved the final manuscript.
Funding
This work was supported by the National Innovative Key Talents Program of Traditional Chinese Medicine (National Administration of Traditional Chinese Medicine [2019] No. 128) and the Dalian Science and Technology Innovation Think Tank Project (USTF [2020] No. 3).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.955209/full#supplementary-material
References
1. Jones M, Corcoran A, Jorge RE. The psychopharmacology of brain vascular disease/poststroke depression. Handbook Clin Neurol. (2019) 165:229–41. doi: 10.1016/B978-0-444-64012-3.00013-7
2. Jørgensen TS, Wium-Andersen IK, Wium-Andersen MK, Jørgensen MB, Prescott E, Maartensson S, et al. Incidence of depression after stroke, and associated risk factors and mortality outcomes, in a large cohort of danish patients. JAMA Psychiatry. (2016) 73:1032–40. doi: 10.1001/jamapsychiatry.2016.1932
3. Cai W, Mueller C, Li YJ, Shen WD, Stewart R. Post stroke depression and risk of stroke recurrence and mortality: A systematic review and meta-analysis. Ageing Res Rev. (2019) 50:102–9. doi: 10.1016/j.arr.2019.01.013
4. Bartoli F, Pompili M, Lillia N, Crocamo C, Salemi G, Clerici M, et al. Rates and correlates of suicidal ideation among stroke survivors: a meta-analysis. J Neurol Neurosurg Psychiatry. (2017) 88:498–504. doi: 10.1136/jnnp-2017-315660
5. Loubinoux I, Kronenberg G, Endres M, Schumann-Bard P, Freret T, Filipkowski RK, et al. Post-stroke depression: mechanisms, translation and therapy. J Cell Mol Med. (2012) 16:1961–9. doi: 10.1111/j.1582-4934.2012.01555.x
6. Robinson RG, Jorge RE. Post-Stroke Depression: A Review. Am J Psychiatry. (2016) 173:221–31. doi: 10.1176/appi.ajp.2015.15030363
7. Villa RF, Ferrari F, Moretti A. Post-stroke depression: mechanisms and pharmacological treatment. Pharmacol Therap. (2018) 184:131–44. doi: 10.1016/j.pharmthera.2017.11.005
8. Jiang W, Gong L, Liu F, Ren Y, Mu J. Alteration of gut microbiome and correlated lipid metabolism in post-stroke depression. Front Cell Infect Microb. (2021) 11:663967. doi: 10.3389/fcimb.2021.663967
9. Hackett ML, Köhler S, O'Brien JT, Mead GE. Neuropsychiatric outcomes of stroke. Lancet Neurol. (2014) 13:525–34. doi: 10.1016/S1474-4422(14)70016-X
10. Wang SB, Wang YY, Zhang QE, Wu SL, Ng CH, Ungvari GS, et al. Cognitive behavioral therapy for post-stroke depression: a meta-analysis. J Affect Disord. (2018) 235:589–96. doi: 10.1016/j.jad.2018.04.011
11. Cheng C, Liu X, Fan W, Bai X, Liu Z. Comprehensive rehabilitation training decreases cognitive impairment, anxiety, and depression in poststroke patients: a randomized, controlled study. J Stroke Cerebrovasc Dis. (2018) 27:2613–22. doi: 10.1016/j.jstrokecerebrovasdis.2018.05.038
12. Castilla-Guerra L, Fernandez Moreno M, Esparrago-Llorca G, Colmenero-Camacho MA. Pharmacological management of post-stroke depression. Exp Rev Neurotherap. (2020) 20:157–66. doi: 10.1080/14737175.2020.1707666
13. Luo M, Duan Z, Song X, Liu C, Li R, Su K, et al. Effects of optimized acupuncture and moxibustion treatment on depressive symptoms and executive functions in patients with post-stroke depression: study protocol for a randomized controlled trial. Front Neurol. (2022) 13:833696. doi: 10.3389/fneur.2022.833696
14. Guo J, Wang J, Sun W, Liu X. The advances of post-stroke depression: 2021 update. J Neurol. (2022) 269:1236–49. doi: 10.1007/s00415-021-10597-4
15. Chen B, Zhao M, Chen B, Yang Z, Yu X, Lin X, et al. (2020). Effectiveness and safety of acupuncture in post-stroke depression (PSD): Protocol for a Bayesian analysis. Medicine 99:e18969. doi: 10.1097/MD.0000000000018969
16. Hou Y, Zhang N, Hao J, Wang X, Wen Z, Luo D, et al. Acupuncture plus rehabilitation for post-stroke depression: A protocol for systematic review and meta-analysis. Medicine. (2020) 99:e21078. doi: 10.1097/MD.0000000000021078
17. Begemann MJ, Brand BA, Curčić-Blake B, Aleman A, Sommer IE. Efficacy of non-invasive brain stimulation on cognitive functioning in brain disorders: a meta-analysis. Psychol Med. (2020) 50:2465–86. doi: 10.1017/S0033291720003670
18. Baeken C, Brem AK, Arns M, Brunoni AR, Filipčić I, Ganho-Ávila A, et al. Repetitive transcranial magnetic stimulation treatment for depressive disorders: current knowledge and future directions. Curr Opin Psychiatry. (2019) 32:409–15. doi: 10.1097/YCO.0000000000000533
19. Razza LB, Palumbo P, Moffa AH, Carvalho AF, Solmi M, Loo CK, et al. A systematic review and meta-analysis on the effects of transcranial direct current stimulation in depressive episodes. Depress Anxiety. (2020) 37:594–608. doi: 10.1002/da.23004
20. Hara T, Shanmugalingam A, McIntyre A, Burhan AM. The effect of Non-Invasive Brain Stimulation (NIBS) on attention and memory function in stroke rehabilitation patients: a systematic review and meta-analysis. Diagnostics (Basel, Switzerland). (2021) 11:227. doi: 10.3390/diagnostics11020227
21. Li Y, Li K, Feng R, Li Y, Li Y, Luo H, et al. Mechanisms of repetitive transcranial magnetic stimulation on post-stroke depression: a resting-state functional magnetic resonance imaging study. Brain Topogr. (2022) 35:363–74. doi: 10.1007/s10548-022-00894-0
22. Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. (2000) 527:633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x
23. Valiengo LC, Benseñor IM, Lotufo PA, Fraguas R, Brunoni AR. Transcranial direct current stimulation and repetitive transcranial magnetic stimulation in consultation-liaison psychiatry. Braz J Med Biol Res. (2013) 46:815–23. doi: 10.1590/1414-431X20133115
24. Kumar S, Wagner CW, Frayne C, Zhu L, Selim M, Feng W, et al. Noninvasive brain stimulation may improve stroke-related dysphagia: a pilot study. Stroke. (2011) 42:1035–40. doi: 10.1161/STROKEAHA.110.602128
25. Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke. (2010) 41:1229–36. doi: 10.1161/STROKEAHA.109.576785
26. Choi CH, Iordanishvili E, Shah NJ, Binkofski F. Magnetic resonance spectroscopy with transcranial direct current stimulation to explore the underlying biochemical and physiological mechanism of the human brain: A systematic review. Human Brain Mapp. (2021) 42:2642–71. doi: 10.1002/hbm.25388
27. Stagg CJ, Antal A, Nitsche MA. Physiology of transcranial direct current stimulation. J ECT. (2018) 34:144–52. doi: 10.1097/YCT.0000000000000510
28. Friston KJ. Functional and effective connectivity: a review. Brain Connect. (2011) 1:13–36. doi: 10.1089/brain.2011.0008
29. Amadi U, Ilie A, Johansen-Berg H, Stagg CJ. Polarity-specific effects of motor transcranial direct current stimulation on fMRI resting state networks. Neuroimage. (2014) 88:155–61. doi: 10.1016/j.neuroimage.2013.11.037
30. Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. (2002) 125:2238–47. doi: 10.1093/brain/awf238
31. Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. (2003) 553:293–301. doi: 10.1113/jphysiol.2003.049916
32. Kronberg G, Bridi M, Abel T, Bikson M, Parra LC. Direct current stimulation modulates LTP and LTD: activity dependence and dendritic effects. Brain Stimul. (2017) 10:51–8. doi: 10.1016/j.brs.2016.10.001
33. Chan M, Yau S, Han Y. The neurobiology of prefrontal transcranial direct current stimulation (tDCS) in promoting brain plasticity: A systematic review and meta-analyses of human and rodent studies. Neurosci Biobehav Rev. (2021) 125:392–416. doi: 10.1016/j.neubiorev.2021.02.035
34. Ardolino G, Bossi B, Barbieri S, Priori A. Non-synaptic mechanisms underlie the after-effects of cathodal transcutaneous direct current stimulation of the human brain. J Physiol. (2005) 568:653–63. doi: 10.1113/jphysiol.2005.088310
35. Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. (2001) 57:1899–901. doi: 10.1212/WNL.57.10.1899
36. Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. (2014) 14:579. doi: 10.1186/s12913-014-0579-0
37. Brown D. A Review of the PubMed PICO Tool: Using Evidence-Based Practice in Health Education. Health Promot Pract. (2020) 21:496–8. doi: 10.1177/1524839919893361
38. Bucur M, Papagno C. A systematic review of noninvasive brain stimulation for post-stroke depression. J Affect Disord. (2018) 238, 69–78. doi: 10.1016/j.jad.2018.05.026
39. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
40. An TG, Kim SH, Kim KU. Effect of transcranial direct current stimulation of stroke patients on depression and quality of life. J Phys Ther Sci. (2017) 29:505–7. doi: 10.1589/jpts.29.505
41. Bueno VF, Brunoni AR, Boggio PS, Bensenor IM, Fregni F. Mood and cognitive effects of transcranial direct current stimulation in post-stroke depression. Neurocase. (2011) 17:318–22. doi: 10.1080/13554794.2010.509319
42. Valiengo L, Casati R, Bolognini N, Lotufo PA, Benseñor IM, Goulart AC, et al. Transcranial direct current stimulation for the treatment of post-stroke depression in aphasic patients: a case series. Neurocase. (2016) 22:225–8. doi: 10.1080/13554794.2015.1130231
43. Valiengo LC, Goulart AC, de Oliveira JF, Benseñor IM, Lotufo PA. Transcranial direct current stimulation for the treatment of post-stroke depression: results from a randomised, sham-controlled, double-blinded trial. J Neurol Neurosurg Psychiatry. (2017) 88:170–5. doi: 10.1136/jnnp-2016-314075
44. Li H, Zhu N, Klomparens EA, Xu S, Wang M, Wang Q, et al. Application of functional near-infrared spectroscopy to explore the neural mechanism of transcranial direct current stimulation for post-stroke depression. Neurol Res. (2019) 41:714–21. doi: 10.1080/01616412.2019.1612539
45. Hassan AB, Danazumi MS, Abdullahi A, Yakasai AM. Effect of transcranial direct current stimulation (tDCS) delivered via dorsolateral prefrontal cortex on central post-stroke pain and depression: a case report. Physiother Theory pract. (2022) 38:1799–1806. doi: 10.1080/09593985.2021.1891591
46. Medeiros GC, Roy D, Kontos N, Beach SR. Post-stroke depression: A 2020 updated review. Gener Hosp Psychiatry. (2020) 66:70–80. doi: 10.1016/j.genhosppsych.2020.06.011
47. Silva F, Mac-Kay A, Chao JC, Santos M, Gagliadi RJ. Transcranial direct current stimulation: a study on naming performance in aphasic individuals. Estimulação transcraniana por corrente contínua: estudo sobre respostas em tarefas de nomeação em afásicos CoDAS. (2018) 30:e20170242. doi: 10.1590/2317-1782/20182017242
48. Darkow R, Martin A, Würtz A, Flöel A, Meinzer M. Transcranial direct current stimulation effects on neural processing in post-stroke aphasia. Human Brain Mapp. (2017) 38:1518–31. doi: 10.1002/hbm.23469
49. Valero-Cabré A, Amengual JL, Stengel C, Pascual-Leone A, Coubard OA. Transcranial magnetic stimulation in basic and clinical neuroscience: A comprehensive review of fundamental principles and novel insights. Neurosci Biobehav Rev. (2017) 83:381–404. doi: 10.1016/j.neubiorev.2017.10.006
50. Fregni F, El-Hagrassy MM, Pacheco-Barrios K, Carvalho S, Leite J, Simis M, et al. Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation in neurological and psychiatric disorders. Int J Neuropsychopharmacol. (2021) 24:256–313. doi: 10.1093/ijnp/pyaa051
51. Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. (2006) 117:845–50. doi: 10.1016/j.clinph.2005.12.003
52. Elder GJ, Taylor JP. Transcranial magnetic stimulation and transcranial direct current stimulation: treatments for cognitive and neuropsychiatric symptoms in the neurodegenerative dementias?. Alzheimer's Res Ther. (2014) 6:74. doi: 10.1186/s13195-014-0074-1
53. Brunoni AR, Moffa AH, Fregni F, Palm U, Padberg F, Blumberger DM, et al. Transcranial direct current stimulation for acute major depressive episodes: meta-analysis of individual patient data. Br J Psychiatry. (2016) 208:522–31. doi: 10.1192/bjp.bp.115.164715
54. Charvet LE, Shaw MT, Bikson M, Woods AJ, Knotkova H. Supervised transcranial direct current stimulation (tDCS) at home: A guide for clinical research and practice. Brain Stimul. (2020) 13:686–93. doi: 10.1016/j.brs.2020.02.011
55. Yang D, Huang R, Yoo SH, Shin MJ, Yoon JA, Shin YI, et al. Detection of mild cognitive impairment using convolutional neural network: temporal-feature maps of functional near-infrared spectroscopy. Front Aging Neurosci. (2020) 12:141. doi: 10.3389/fnagi.2020.00141
56. Yang D, Shin YI, Hong KS. Systemic Review on Transcranial Electrical Stimulation Parameters and EEG/fNIRS Features for Brain Diseases. Front Neurosci. (2021) 15:629323. doi: 10.3389/fnins.2021.629323
57. Brunoni AR, Valiengo L, Baccaro A, Zanão TA, de Oliveira JF, Goulart IM, et al. The sertraline vs. electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry. (2013) 70:383–91. doi: 10.1001/2013.jamapsychiatry.32
Keywords: stroke, depression, mechanism, transcranial direct current stimulation, post-stroke depression (PSD)
Citation: Hao W, Liu Y, Gao Y, Gong X and Ning Y (2023) Transcranial direct current stimulation for the treatment of post-stroke depression: A systematic review. Front. Neurol. 13:955209. doi: 10.3389/fneur.2022.955209
Received: 28 June 2022; Accepted: 22 December 2022;
Published: 18 January 2023.
Edited by:
Andreas R. Luft, University of Zurich, SwitzerlandReviewed by:
Xiao Qin Duan, Jilin University, ChinaLais Boralli Razza, University of São Paulo, Brazil
Copyright © 2023 Hao, Liu, Gao, Gong and Ning. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Liu,  ZnV3YTUyMDA4QDEyNi5jb20=
ZnV3YTUyMDA4QDEyNi5jb20=
 Wenjian Hao
Wenjian Hao Yong Liu1,2*
Yong Liu1,2*