
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 01 September 2022
Sec. Pediatric Neurology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.948727
 Yongheng Zhao1,2†
Yongheng Zhao1,2† Jun Li1†
Jun Li1† Liang Gao1,2
Liang Gao1,2 Xiaofan Yang1
Xiaofan Yang1 Haiqing Zhao1,2
Haiqing Zhao1,2 Yumei Li1,2
Yumei Li1,2 Li Su1,2
Li Su1,2 Xiaoyu Zhao1,2
Xiaoyu Zhao1,2 Hao Ding1,2
Hao Ding1,2 Baomin Li1*
Baomin Li1*This study aimed to discuss clinical characteristics, therapy, and antibody prevalence in epilepsy (APE) score for short-term, frequent epileptic seizures in children who are autoimmune-antibody negative and respond well to immunotherapy. The clinical characteristics, imaging manifestations, electrophysiology, and effective treatment plan of 9 children who met the above criteria were retrospectively analyzed in the Pediatric Neurology Department of Qilu Hospital at Shandong University from June 2019 to December 2021. All 9 patients (6 boys, 3 girls; aged 13 months−11 years and 5 months, median 3.5 years) had acute-onset seizures within 3 months. All had previous normal growth/development with no family history of disease. Seizure types were focal motor seizures (6), generalized tonic-clonic seizures (2), and generalized secondary-to-focal (1); occurred >10 times/day; and lasted <1 min/episode. Formal treatment with ≥2 types of antiseizure medicine (ASM) achieved an unsatisfactory effect. Cranial magnetic resonance imaging showed an abnormal result in 1 case. The APE score was ≥4 in 3 cases and <4 in 6 cases. All patients experienced symptomatic relief with immunotherapy; subsequently, 8 patients were free of recurrence and 1 had significantly reduced seizure frequency. Autoimmune antibody screening is recommended for children who were previously well and have acute-onset epilepsy; high frequency, short-duration seizures; no good response to 2 types of ASM; and other etiologic factors excluded, even with APE score <4. Even with negative autoimmune antibody results, the possibility of autoimmune epilepsy should be considered for urgent initiation of immunotherapy, which can achieve good results.
The correlation between epilepsy and immunity is well established. Similar to West syndrome, Landau–Kleffner syndrome, and other diseases, immunotherapy is the first-line treatment for autoimmune epilepsy. Autoimmune epilepsy (AE), first proposed by Levite in 2002 (1), describes epilepsy in which a series of immune cells and antibodies are associated. Until 2017, the International League Against Epilepsy (ILAE) formally listed the immune factor as 1 of the 6 etiologic factors of epilepsy, and formally proposed the definition of autoimmune epilepsy: Epilepsy is caused by immune dysfunction, and the key symptom of the immune dysfunction is a seizure episode (2). Research reported that the HMGB1/cxcl12-mediated immunity and T helper cell 17 activation may play a key role in the pathogenesis of AE (3).
In recent years, the popularization of autoimmune antibody screening has consistently improved the diagnostic rate of autoimmune encephalitis (4). As the antibody of autoimmune encephalitis is the same as that of AE, and both can cause epileptic seizures and cognitive mental disorder, the tendency is to misuse the term “autoimmune epilepsy.” Recent studies on patients with encephalitis recovering from treatment reported that the epileptic seizures in autoimmune encephalitis were gradually reduced, the incidence of long term epileptic seizure was low, and the difference between seizure and epilepsy was significant (5, 6).
In 2020, the ILAE clearly differentiated the conceptual difference between “acute symptomatic seizures secondary to autoimmune encephalitis” and “autoimmune-associated epilepsy” (7). The former is in the process of autoimmune encephalitis in the occurrence of epilepsy, through immune targeted treatment, the main symptoms of autoimmune encephalitis is relieved at the same time, the seizures can be completely controlled, and in most cases, can be completely out of use antiseizure medicines (ASMs) finally. AE is also different from autoimmune-associated epilepsy. Autoimmune-associated epilepsy refers to chronic epilepsy secondary to autoimmune brain disease, where some immune-mediated attack can become a chronic brain disease in patients. Although ASMs and immunotherapy are invalid, there is evidence that cytotoxic T cells play an important role in pathogenic mechanism; it leads to the death of neurons, and the nervous system autoantibodies are more of an immune response by-product that do not have a direct pathogenic effect. Therefore, the etiology of autoimmune-associated epilepsy emphasizes the pathogenic role of brain structural factors, whereas, AE only emphasizes that immune factors play a leading role in its etiology and does not include structural factors. Although the therapeutic effect of ASMs is not good for AE, the effect of immunotherapy is better. At present, there is still no clear diagnostic criteria for AE and the negative results of serum and cerebrospinal fluid antibodies do not exclude its possibility. This study retrospectively reviewed 9 children with epileptic seizures who had short-term recurrent seizures with autoimmune antibody negativity and responded well to immunotherapy. The patients were analyzed for clinical manifestations, auxiliary examination results, the effect of immunotherapy and APE score (8) (Table 1).
The study included 9 children considered to have AE. All patients were hospitalized in the Department of Pediatrics, Qilu Hospital, Shandong University, from June 2019 to December 2021. The inclusion criteria were as follows: (1) course of disease <3 months; (2) epileptic seizures occurring with high frequency, with an average daily seizure frequency of >10 times and each seizure duration being relatively short—usually <1 min; (3) negative serum + cerebrospinal fluid (CSF) neural autoimmune antibody examination, including the antibodies NMDA-R-Ab, LGI1-Ab, CASPR2-Ab, GABAB-R-Ab, GAD65-Ab, AMPA1-R-Ab, AMPA2-R-Ab, and anti-YO antibody; (4) development of mental motor was normal before onset, no family disease history, no history of epilepsy caused by tumor, cerebrovascular disease, encephalitis, trauma, or metabolic disease and others; (5) absence of definite radiographic abnormalities; and (6) no good response to ≥2 types of ASM and later acceptance of systemic immunotherapy. Patients were 6 boys and 3 girls, age 13 months−11 years and 5 months (median 3.5 years).
Patients were assessed for their clinical background, including medical history, symptoms, physical signs, and CSF analysis; imaging results; electrophysiology; serum antibody concentrations; and auxiliary examinations, including CSF autoimmune antibody testing; APE score; effective treatment plan; follow-up status; and prognosis. Each child was followed up for 1, 3, and 6 months after immunotherapy. All patients were followed up for at least 6 months.
Among the nine patients, seizure types were focal motor seizures (6 patients, 66.7%), generalized tonic-clonic seizures (two patients, 22.2%), and generalized secondary-to-focal (1 patient, 11.1%), and 1 patient (11.1%) had special manifestations, such as paroxysmal limb pain and panic. The epileptic seizure emerged with high frequency, with the average daily seizure frequency >10 times, and the duration of each seizure was relatively short, usually <1 min. The use of ≥2 types of ASM did not provide a satisfactory treatment effect.
All nine patients underwent CSF examination and cytology in addition to routine, biochemical, immune, and culture examinations, with no abnormalities reported. Examination of serum + CSF neural autoimmune encephalitis antibody was negative, which included NMDA-R-Ab, LGI1-Ab, CASPR2-Ab, GABAB-R-Ab, GAD65-Ab, AMPA1-R-Ab, and AMPA2-R-Ab. In addition, demyelinating antibody (anti-MOG antibody IgG, anti-AQP4 antibody IgG, anti-MBP antibody IgG, anti-amphiphysin antibody IgG, anti-Hu antibody IgG, anti-Ri antibody IgG, anti-SOX1 antibody IgG) was negative.
Cranial magnetic resonance imaging (MRI) showed an abnormal result in 1 case; results were normal for the other eight cases (Figure 1). All nine patients demonstrated an electroencephalograph (EEG) abnormality (Figure 2). The specific examination results are shown in Table 2.
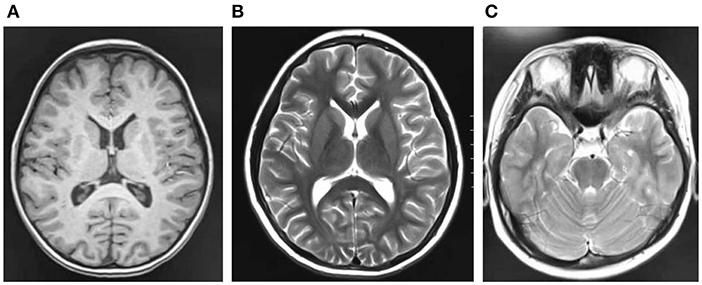
Figure 1. (A–C) Are craniocerebral magnetic resonance imaging of some children, and the results are negative.
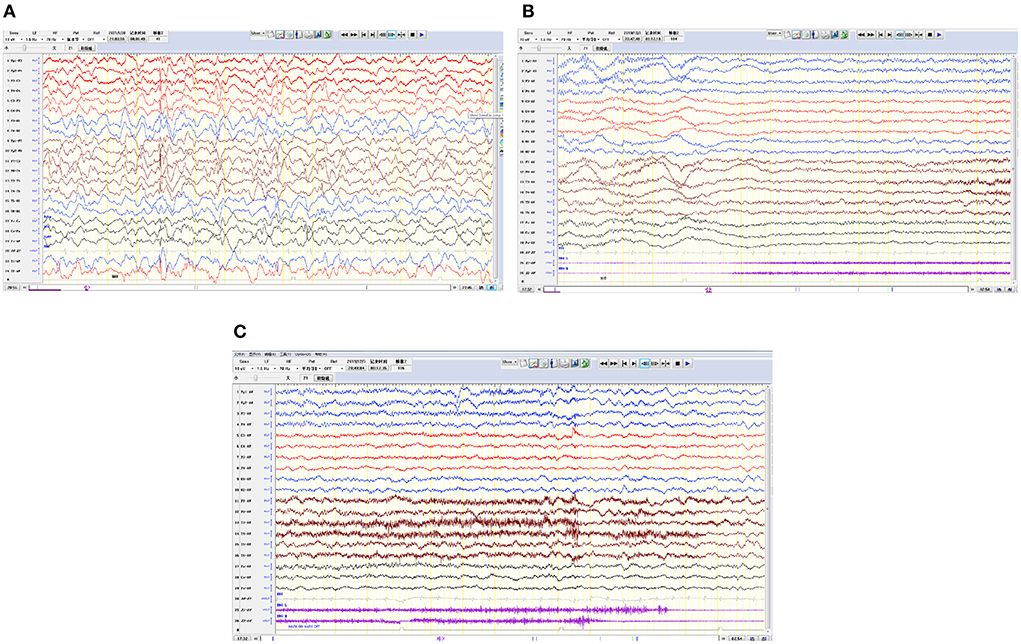
Figure 2. Electroencephalograms for 3 pediatric patients. (A) The right hemisphere is dominated by multifocal spike waves and slow spike waves. (B,C) Generalized decreased voltage. In the bilateral frontal pole and frontal and anterior temporal regions, a fast wave is actively released with a low amplitude, lasting for 20 s, and in the late stage of onset, 1–3 Hz δ is actively released in the temporal region and accompanied by a large amount of electromyography pseudo error.
All nine patients had already tried ≥2 types of ASMs, with poor therapeutic effects—their seizures were not reduced. One month after the first immunotherapy, epileptic seizure did not occur in 4 children, and occasional epileptic seizure occurred in 1. The other four children had obvious decrease in epileptic seizure frequency, and while three of them were administered a second course of immunotherapy, one declined further treatment for personal reasons.
At 3 months after the first immunotherapy, eight patients no longer experienced epileptic seizures. Among these eight patients, three had undergone a second course of immunotherapy 1 month after the first immunotherapy. The one patient who did not accept the second course of immunotherapy experienced reduced epileptic seizure frequency, shorter duration, and reduced intensity of the seizure episodes, and an objectively improved mental status and cognitive function.
At 6 months after the first immunotherapy, eight patients continued to experience freedom from seizures. The other one patient occasionally experienced epileptic seizure, with greatly reduced frequency and duration, and significantly improved cognition and quality of life.
Among the nine patients in our current study, seven were only treated with IVIG, and the remaining two were treated with IVIG combined with IVMP, which all had significant effects. Detailed use of immunotherapy is displayed in Table 2.
The APE score was ≥4 in 3 cases and <4 in six cases; however, the score was not correlated with the treatment effect.
Epilepsy is a common chronic brain disease caused by multiple etiologic factors that result in the abnormal discharge of brain neurons and lead to the occurrence of clinical symptoms. Recently, increasingly more studies are focused on the etiology of epilepsy. Until 2017, ILAE officially listed immune factors as 1 of the 6 etiologies of epilepsy (2): structural, genetic, infectious, metabolic, immune, and unknown. The increased diagnosis of autoimmune encephalitis—which has the same anti-neural antibody as autoimmune-associated epilepsy and, in most cases, includes epileptic seizures, resistance to ASM, and other similar characteristics—has led to the widespread misuse of the diagnosis of AE and to the excessive use of ASMs (7, 9).
In July 2020, ILAE made a clear distinction between the two concepts in Epilepsia magazine, describing “acute symptomatic seizures secondary to autoimmune encephalitis” and “autoimmune-associated epilepsy” (7). In fact, the epileptic seizure in autoimmune encephalitis did not respond well to the treatment with multiple ASMs. Most pediatric patients can control the epileptic seizure after achieving control of autoimmune encephalitis, and do not need to take ASMs for a long term, and the possibility of developing epilepsy is relatively small (5, 10, 11). AE is increasingly recognized as a potentially reversible cause of frequent medically refractory seizures and cognitive decline. At present, there is no clear diagnostic standard for AE. Previously, it was generally believed that any disease accompanied by seizures and autoantibodies was AE (12). Therefore, most autoimmune encephalitis is usually classified as AE, regardless of the disease causing the seizure, antibody type, or the definition of epilepsy used (13). This widely used classification is inaccurate and has led to the development of an APE scoring system based on the same clinical perspectives and diagnostic criteria as antibody-associated encephalitis, leading to important selection biases (8). Some scholars believe that the diagnostic criteria of AE can be determined according to APE score, neurospecific antibody, and immunotherapy test (14, 15) (Figure 3). The APE score can be very high in children with autoimmune encephalitis and even epilepsy caused by common encephalitis and abnormal CSF and cranial MRI. At present, there is a great controversy over the use of APE score, and some scholars have proposed that fluorodeoxyglucose (FDG)-positron emission tomography (PET) examination is of great value to supplement the APE score, especially in pediatric patients with negative MRI (16).
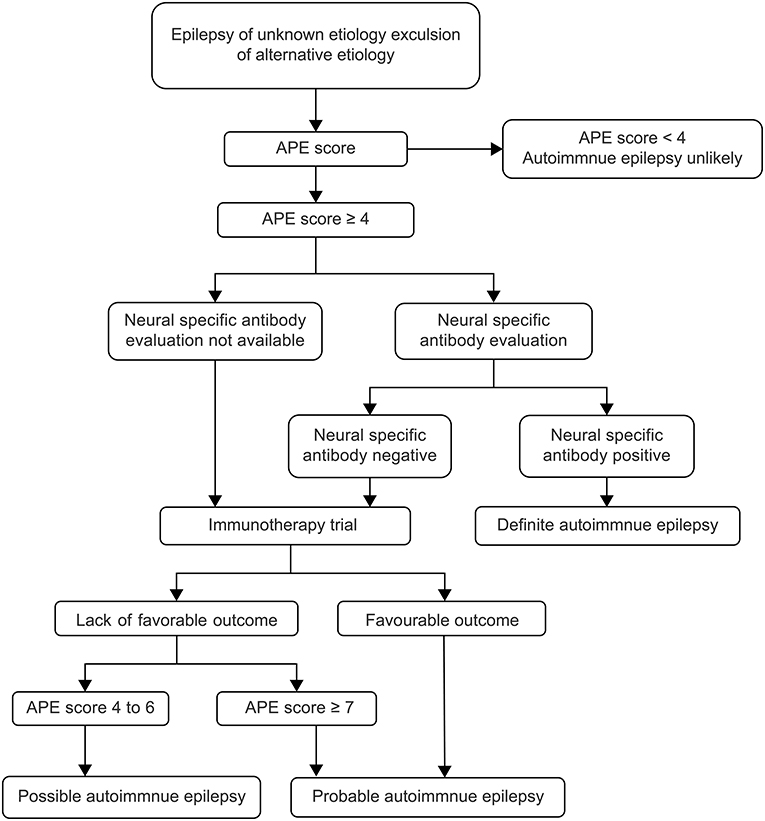
Figure 3. Flow diagram of diagnosis and therapy (15). APE, antibody prevalence in epilepsy.
Other studies have shown that cytokines or chemokines can be used as alternative biomarkers for diagnosing suspected AE (sAE) (3). Prominent changes in several immune and inflammatory pathways, such as IL-1R1/TLR4, COX-2, TNF-a, and chemokines have been reported within the epileptogenic lesions in clinical studies. Specific drugs or interventions targeting them may decrease established seizures or play a role in antiepileptogenesis effects (17). Currently, inflammation is believed to contribute to the occurrence of epilepsy, and epilepsy seizures have pro-inflammatory activities. AE is one of the common causes of status epilepticus (SE), which damages the blood–brain barrier. Activation of astrocytes, microglia, reactive astrocytes, and other inflammatory mediators, through various pathways, ultimately lead to the influx of calcium ions in neurons, reduce the threshold of epileptic seizures, thus aggravating the onset of SE. Therefore, activation of the immune system can be both the cause and result of seizures (18). The diagnosis of AE is very important—the earlier the diagnosis, the better the prognosis (19). At present, the diagnosis of AE greatly relies on antibody detection and immunotherapy response. When antibody detection is difficult, imaging is particularly important. About 40% of pediatric brain MRI results showed no abnormalities. The abnormal MRI signals are typically in 1 or both medial temporal lobes, and some AE involve structures other than temporal lobes, including basal ganglia, non-limbic cortex, cerebellum, brainstem, and white matter (20). In addition to brain F-FDG-PET examination, whole-body F-FDG-PET can help identify potential tumors and detect metastasis. Studies have found that brain MRI is not always related to brain F-FDG-PET, so these 2 examinations can complement each other (21, 22). Both MRI and PET are valuable tools in identifying and evaluating the progression of autoimmune-related epilepsy. Because F-FDG-PET is usually more sensitive to the brain lesions of AE, it is recommended to perform F-FDG-PET early when the diagnosis of AE is suspected clinically, especially for cases in which MRI is normal or cannot be judged (23). AE on EEG is characterized by multiple focal seizures with repeated sharp slow wave discharge (24).
At present, there are still no clear diagnostic criteria for AE. After a wide literature review and based on with our experience with the children admitted to our hospital who have AE, we believe that AE should be considered in these cases: first, for children with a positive serum or CSF antibody test, after excluding autoimmune encephalitis and with epilepsy seizure as the only manifestation, AE can be considered as a diagnosis; and second, for children with a negative antibody test, the need to meet the following conditions can also be considered to diagnose AE: (1) normal growth and development in the past, sudden-onset of frequent seizures within the past 3 months, short seizure duration, usually <1 min; (2) standard treatment with ≥2 ASMs is not effective; (3) exclusion of intracranial infection, cerebrovascular disease, tumor and other diseases with clear etiology; (4) brain MRI is negative; and (5) after immunotherapy, the frequency of seizures decrease significantly.
In terms of treatment, the addition of immunotherapy can better control seizures than the use of ASMs alone (25). The first-line treatment of AE in children is hormone, human immunoglobulin, and TPE, among which TPE is rarely used in pediatric patients versus adults, especially in the case of restlessness or autonomic nervous instability (14, 26–28). Depending on the response to treatment and the severity of the clinical presentation, combination therapy, usually high-dose intravenous methylprednisolone (IVMP) shock and intravenous immunoglobulin (IVIG), or second-line therapy (rituximab or cyclophosphamide), may be considered. Among the 9 patients in our current study, 7 were only treated with IVIG, and the remaining 2 were treated with IVIG combined with glucocorticoid, all of which had significant effects. According to the different clinical manifestations among pediatric patients, ASMs are still needed to reduce the frequency of epileptic seizures or the duration of epilepsy while waiting for immunotherapy to take effect (29). At present, the latest study indicates that neuroinflammation is a potential target for the treatment of epilepsy, especially for children with drug-resistant and refractory status epilepsy. The experience of anti-cytokine agents and lymphocyte-targeting drugs for the treatment of epilepsy mainly comes from case reports or series. The use of anti-IL-1, anti-IL-6, and anti-CD20 agents in patients with drug-resistant and refractory epileptic status has shown good therapeutic results. Current experience with TNF inhibitors is limited to Rasmussen encephalitis (30). Moreover, our study had some limitations, 1 of which is that the number of cases in this study was small.
Although there is currently no effective pediatric scale to identify the population in need of antibody testing, screening for AE is necessary to identify patients who may be eligible for immunotherapy and to avoid overuse of ASM and excessive psychological stress for parents. Since not all antibodies have been discovered and reported, and not all discovered antibodies can be detected, negative MRI results of antibodies, cerebrospinal fluid, and brain do not exclude the possibility of AE (31, 32). For newly emerged seizures of short duration and high frequency with poor response to ASMs, and the exclusion of other clear causes in pediatric patients, the possibility of AE should be considered, with the relevant antibody screening carried out as soon as possible. Even if results are negative, experimental immunotherapy should be carried out at the earliest.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Qilu Hospital of Shangdong University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
YZ, JL, and BL were responsible for the original concept, overall design of the research, and wrote and revised the manuscript. YZ, JL, XY, and BL analyzed the EEG results and diagnosed the patients. LG, HZ, YL, LS, XZ, and HD collected the clinical data and statistical analysis. All authors read and approved the final manuscript.
This study was supported by the Foundation of National Key Research and Development Program of China (NO. 2016YFC1306202).
We thank the patients and their parents for participating in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
2. Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia. (2017) 58:512–21. doi: 10.1111/epi.13709
3. Han Y, Yang L, Liu X, Feng Y, Pang Z, Lin Y. HMGB1/CXCL12-Mediated immunity and Th17 cells might underlie highly suspected autoimmune epilepsy in elderly individuals. Neuropsychiatr Dis Treat. (2020) 16:1285–93. doi: 10.2147/NDT.S242766
4. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. (2016)15:391–404. doi: 10.1016/S1474-4422(15)00401-9
5. Geis C, Planagumà J, Carreño M, Graus F, Dalmau J. Autoimmune seizures and epilepsy. J Clin Invest. (2019) 129:926–40. doi: 10.1172/JCI125178
6. Huang Q, Ma M, Wei X, Liao Y, Qi H, Wu Y, et al. Characteristics of seizure and antiepileptic drug utilization in outpatients with autoimmune encephalitis. Front Neurol. (2019) 9:1136. doi: 10.3389/fneur.2018.01136
7. Steriade C, Britton J, Dale RC, Gadoth A, Irani SR, Linnoila J, et al. Acute symptomatic seizures secondary to autoimmune encephalitis and autoimmune-associated epilepsy: conceptual definitions. Epilepsia. (2020) 61:1341–51. doi: 10.1111/epi.16571
8. Dubey D, Singh J, Britton JW, Pittock SJ, Flanagan EP, Lennon VA, et al. Predictive models in the diagnosis and treatment of autoimmune epilepsy. Epilepsia. (2017) 58:1181–9. doi: 10.1111/epi.13797
9. Nian Y, Qing D. Correct understanding of the relationship between autoimmune epilepsy and autoimmune encephalitis. Chin J Neurol. (2020) 2:152–3. doi: 10.3760/cma.j.issn.1006-7876.2020.02.015
10. Liu X, Yan B, Wang R, Li C, Chen C, Zhou D, et al. Seizure outcomes in patients with anti-NMDAR encephalitis: a follow-up study. Epilepsia. (2017) 58:2104–11. doi: 10.1111/epi.13929
11. Vogrig A, Joubert B, André-Obadia N, Gigli GL, Rheims S, Honnorat J. Seizure specificities in patients with antibody-mediated autoimmune encephalitis. Epilepsia. (2019) 60:1508–25. doi: 10.1111/epi.16282
12. Bien CG, Holtkamp M. “Autoimmune epilepsy”: encephalitis with autoantibodies for epileptologists. Epilepsy Curr. (2017) 17:134–41. doi: 10.5698/1535-7511.17.3.134
13. Dubey D, Samudra N, Gupta P, Agostini M, Ding K, Van Ness PC, et al. Retrospective case series of the clinical features, management and outcomes of patients with autoimmune epilepsy. Seizure Eur J Epilepsy. (2015) 29:143–7. doi: 10.1016/j.seizure.2015.04.007
14. Toledano M, Britton JW, McKeon A, Shin C, Lennon VA, Quek AM, et al. Utility of an immunotherapy trial in evaluating patients with presumed autoimmune epilepsy. Neurology. (2014) 82:1578–86. doi: 10.1212/WNL.0000000000000383
15. Dubey D, Pittock SJ, McKeon A. Antibody prevalence in epilepsy and encephalopathy score: increased specificity and applicability. Epilepsia. (2019) 60:367–9. doi: 10.1111/epi.14649
16. Tripathi M, Thankarajan ARS, Ihtisham K, Garg A, Vibha D, Singh R, et al. Metabolic scoring in autoimmune epilepsy—should APE scores be modified? Acta Neurol Scand. (2021) 143:13–8. doi: 10.1111/ane.13346
17. van Vliet EA, Aronica E, Vezzani A, Ravizza T. Review: Neuroinflammatory pathways as treatment targets and biomarker candidates in epilepsy: emerging evidence from preclinical and clinical studies. Neuropathology Appl Neurobiol. (2018) 44:91–111. doi: 10.1111/nan.12444
18. de Vries EE, van den Munckhof B, Braun KP, van Royen-Kerkhof A, de Jager W, Jansen FE. Inflammatory mediators in human epilepsy: a systematic review and meta-analysis. Neurosci Biobehav Rev. (2016) 63:177–90. doi: 10.1016/j.neubiorev.2016.02.007
19. Chang Z, Mian W, Chen Z, et al. Correlation between admission diagnosis and outcome of immunotherapy in patients with autoimmune epilepsy. J Central S Univ. (2019) 44:885–91.
20. Guerin J, Watson RE, Carr CM, Liebo GB, Kotsenas AL. Autoimmune epilepsy: findings on MRI and FDG-PET. Br J Radiol. (2019) 92:20170869. doi: 10.1259/bjr.20170869
21. Ances BM, Vitaliani R, Taylor RA, Liebeskind DS, Voloschin A, Houghton DJ, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. (2005) 128:1764–77. doi: 10.1093/brain/awh526
22. Basu S, Alavi A. Role of FDG-PET in the clinical management of paraneoplastic neurological syndrome: detection of the underlying malignancy and the brain PET-MRI correlates. Mol Imaging Biol. (2008) 10:131–7. doi: 10.1007/s11307-008-0134-7
23. Gillinder L, Papacostas J, Dionisio S. Electroclinical insights into autoimmune epilepsy. J Neuroimmunol. (2019) 330:44–7. doi: 10.1016/j.jneuroim.2019.02.005
24. Solnes LB, Jones KM, Rowe SP, Pattanayak P, Nalluri A, Venkatesan A, et al. Diagnostic value of 18F-FDG PET/CT versus MRI in the setting of antibody-specific autoimmune encephalitis. J Nucl Med. (2017) 58:1307–13. doi: 10.2967/jnumed.116.184333
25. de Bruijn MAAM, van Sonderen A, van Coevorden-Hameete MH, Bastiaansen AEM, Schreurs MWJ, Rouhl RPW, et al. Evaluation of seizure treatment in anti-LGI1, anti-NMDAR, and anti-GABABR encephalitis. Neurology. (2019) 92:e2185–e96. doi: 10.1212/WNL.0000000000007475
26. Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. (2011) 10:63–74. doi: 10.1016/S1474-4422(10)70253-2
27. Suleiman J, Dale RC. The recognition and treatment of autoimmune epilepsy in children. Dev Med Child Neurol. (2015) 57:431–40. doi: 10.1111/dmcn.12647
28. Leary LD. Autoimmune epilepsy in children: unraveling the mystery. Pediatr Neurol. (2020) 112:73–7. doi: 10.1016/j.pediatrneurol.2020.03.016
29. Gaspard N. Putting a band-aid on a broken leg: antiseizure medications are inferior to immune therapies in autoimmune epilepsy. Epilepsy Curr. (2019) 19:302–4. doi: 10.1177/1535759719868690
30. Costagliola G, Depietri G, Michev A, Riva A, Foiadelli T, Savasta S, et al. Targeting inflammatory mediators in epilepsy: a systematic review of its molecular basis and clinical applications. Front Neurol. (2022) 13:741244. doi: 10.3389/fneur.2022.741244
31. Tiwana HK, Acharya V, Acharya J. S59. Response to immunotherapy in antibody-negative autoimmune epilepsy. Clin Neurophysiol. (2018) 129:e163–e4. doi: 10.1016/j.clinph.2018.04.419
Keywords: autoimmune epilepsy, autoimmune antibody negativity, immunotherapy for epilepsy, antibody prevalence in epilepsy (APE) score, autoimmune encephalitis
Citation: Zhao Y, Li J, Gao L, Yang X, Zhao H, Li Y, Su L, Zhao X, Ding H and Li B (2022) The value of immunotherapy in children with initial short-term frequent seizures. Front. Neurol. 13:948727. doi: 10.3389/fneur.2022.948727
Received: 08 June 2022; Accepted: 01 August 2022;
Published: 01 September 2022.
Edited by:
Pasquale Striano, Giannina Gaslini Institute (IRCCS), ItalyReviewed by:
Giorgio Costagliola, University of Pisa, ItalyCopyright © 2022 Zhao, Li, Gao, Yang, Zhao, Li, Su, Zhao, Ding and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baomin Li, MTk4OTYyMDAwNjkzQHNkdS5lZHUuY24=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.