
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 22 July 2022
Sec. Stroke
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.941668
This article is part of the Research TopicSmall Vessel Disease: From Diagnosis to Organized Management PathwaysView all 6 articles
Objective: Cerebral small vessel disease (CSVD) is associated with gait and balance deficits in older adults. However, the effect of CSVD-related brain injury on post-stroke mobility is unknown. This study aimed to investigate the association of CSVD with gait and balance impairment after a minor stroke.
Methods: A total of 273 patients with a minor stroke (NIHSS ≤ 5 points) who were hospitalized at the Affiliated Hospital of Qingdao University were enrolled. The manifestations of white matter hyperintensities (WMH), lacunes, enlarged perivascular spaces (EPVS), and cerebral microbleeds (CMB) were statistically analyzed according to magnetic resonance imaging results, and the total burden score of CSVD was calculated. Gait function was assessed by a 6-m walking speed test, and balance function was assessed by the timed-up-and-go (TUG) test. Linear regression analysis was applied to determine the association after adjusting for key variables.
Results: The correlation results showed that in patients with minor stroke, age, sex, smoking history, and the infarct site were associated with gait speed, and age and the infarct site were associated with the TUG test. In the univariate linear regression model, periventricular white matter hyperintensities (PVWMH), deep white matter hyperintensities (DWMH), and the total burden of CSVD were correlated with gait speed, while only PVWMH correlated with the TUG test. After adjusting for confounders, only PVWMH were independent predictors of gait speed (β = −0.089, p < 0.05) and the TUG test (β = 0.517, p < 0.05).
Conclusions: Our study confirmed that CSVD is associated with gait and balance disorders after a minor stroke. PVWMH are independent predictors of gait and balance disorders in patients with minor stroke. These findings should be confirmed in larger prospective studies.
Cerebral small vessel disease (CSVD) is a pathological process in cerebral arterioles, capillaries, and venules caused by a variety of factors. It manifests in magnetic resonance imaging (MRI) as white matter hyperintensities (WMH), microbleeds (CMB), lacunes, and enlarged perivascular spaces (EPVS) (1, 2). CSVD is common in elderly individuals, and gait and balance disorders are the second most common problem in CSVD after cognitive impairment (3, 4). Gait and balance disorders can lead to falls and functional dependence, increasing the risk of hospitalization and death (5, 6). Previous studies have shown that gait and balance disorders are associated with individual MRI features of CSVD, such as WMH and CMB (7–9). Painter et al. (10) first explored and showed a correlation between the total burden of CSVD and gait impairment in 2017.
Minor ischemic stroke is an ischemic stroke in which the symptoms are only mild neurological deficits (11). A prospective cohort study showed that minor ischemic stroke is the most common form of ischemic cerebral artery disease, accounting for up to 35% of cases, and it has a 3-month recurrence rate of 19% (12). A study showed that 25–50% of patients with minor ischemic stroke have varying degrees of disability at 90 days of onset (13). The functional impairment caused by minor stroke is often ignored due to its mild symptoms, short duration, and limited rehabilitation and follow-up treatment. Therefore, its prognosis should be considered. Previous studies have shown that CSVD is associated with worse functional outcomes after stroke (14–16). The association between CSVD and gait and balance function impairment after stroke is unknown. Only one study reported that the total burden of CSVD was not associated with gait and balance after minor stroke (17).
In this study, we obtained objective assessments of gait and body balance using the 6-m walking speed test and the timed-up-and-go (TUG) test to investigate whether individual imaging markers of CSVD and the total burden can independently be used to predict gait and balance impairment in patients with minor stroke.
The inpatient data of patients with acute stroke in the Affiliated Hospital of Qingdao University were collected. Inclusion criteria were as follows: patients with cerebral infarction treated within 7 days of onset (NIHSS ≤ 5); age range from 18 to 80; able to walk at least 10 m independently; complete imaging and clinical data; and informed consent of the patient. Exclusion criteria: clinical diagnosis of movement disorders such as Parkinson's disease; clear history of cerebral hemorrhage or subarachnoid hemorrhage; mental abnormality (including diagnosed depression, anxiety, or mental illness; long-term use of anti-anxiety, anti-depression, and other psychotropic drugs); other causes of WMH (such as multiple sclerosis, toxic encephalopathy, and infections); whether MRI contraindications existed; generally poor condition, complicating serious heart, liver, and lung dysfunction; associated with intracranial tumors, craniocerebral trauma; and other serious central nervous system diseases. NIHSS was performed on eligible patients after admission. Demographic data (age, sex) and vascular risk factors (hypertension, diabetes, hyperlipidemia, smoking history, and drinking history) were collected.
We obtained written consent from each patient, and the Ethics Review Committee of the Affiliated Hospital of Qingdao University approved the study protocol.
Gait speed was assessed by the 6-m walk speed test, a measure of motor function commonly used in clinical trials. Participants were asked to walk at a comfortable walking pace. They were asked to start walking 2 m from the start sign and stop 2 m behind the end sign to measure steady-state walking speed. Trunk balance was measured by the TUG test, a reliable, valid, and easily administered clinical tool that correlates well with the Berg Balance Scale (18) and mostly assesses functional activity after stroke (19). The test recorded the time it takes for the subject to rise to complete a 3-m walk, return to the armchair, and then resit; the longer the time, the worse the balance (20).
A single HD 3.0T strong superconducting MRI scanner (GE, USA) was used to perform an MRI of the head, including T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), fluid-attenuated flip recovery sequence (FLAIR), diffusion-weighted imaging (DWI), and magnetic susceptibility-weighted imaging (SWI). The MRI images were read and evaluated by two neurologists. Each read the images independently, and the final report was based on a consensus between the two. The radiologist made the final judgment in case of inconsistent assessment.
In this study, WMH, EPVS, CMB, and lacunes were scored using the Standards for Reporting Vascular Changes on Neuroimaging (STRIVE) (21). Lacunes were defined as ovoid lesions with a high signal around a middle-low signal on FLAIR images, 3–15 mm in diameter. WMH were high signal lesions on FLAIR and T2-weighted images, divided into two parts, periventricular white matter hyperintensities (PVWMH) and deep white matter hyperintensities (DWMH), evaluated using the Fazekas scale (0–3 points) (22). EPVS were lesions located in the basal ganglia and semioval center with the same signal intensity of cerebrospinal fluid on MRI and <3 mm in diameter. A score of 0 was assigned if EPVS = 0, 1 if EPVS ≤ 10, 2 if EPVS = 11–20, 3 if EPVS = 21–40, and 4 if EPVS ≥ 40 (23). CMB were defined as homogeneous round lesions (<10 mm) with low signals on SWI images. Total CSVD burden (0–4 points): 1 point for ≥1 lacunes; 1 point for DWMH score of 2 or 3 or PVWMH score of 3; 1 point for moderate to severe EPVS (2–4 points); and 1 point for CMB ≥1 (24).
We used R studio (3.6.1) for statistical processing. Pearson correlation test and Spearman correlation test were used to evaluate the correlation between demographic data, vascular risk factors (such as gender, age, smoking, etc.,), NIHSS score, infarct location and CSVD, and walking speed and balance. Variables in the CSVD (including individual markers and total burden) that were significantly associated with gait and balance were subjected to linear regression analysis. Variables (except CSVD) that were statistically significant in the above correlation analysis were extracted as variables to be controlled for multiple hierarchy regression analysis. Multiple hierarchy linear regression was used to assess the independent prediction ability of individual radiographic markers and the total burden of CSVD. The statistical significance level was set to 0.05.
A total of 273 patients were enrolled during the study, including 170 men (62%) and 103 women (38%); age averaged 63.3 ± 9.2 years; gait speed averaged 1.02 ± 0.21 m/s; and TUG averaged 13.44 ± 3.44 s. Details are shown in Table 1.
In terms of demographic and vascular risk factors, correlation analysis showed that gait speed was significantly slower in elderly, female, and smoking patients, but only older age was associated with longer TUG duration. Regarding the NIHSS score and the infarct site, correlation analysis showed that stroke severity (NIHSS) was not related to gait speed or length of TUG test time. Infarcts at the basal ganglia and radial coronary sites were associated with slower gait speed and longer TUG test duration.
Univariate correlation analysis of CSVD imaging markers with gait and balance disturbances showed that patients with higher PVWMH scores, DWMH scores, and total CSVD burden had slower gait speed. Subjects with higher PVWMH scores spent more time on the TUG test (Table 2).
A univariate linear regression model was used to evaluate the individual contribution of CSVD markers to gait and balance performance. PVWMH scores (β = −0.107, P = 0.000), DWMH scores (β = −0.043, P = 0.000), and the total CSVD burden (β = −0.030, P = 0.027) significantly predicted gait speed. PVWMH scores (β = 0.708, P = 0.004) predicted trunk balance (Table 3).
To determine the independent predictive power of CSVD for gait and balance after including demographics, vascular risk factors, infarct location, and NIHSS, a multiple hierarchical linear regression model was used for statistical analysis. Only the variables associated with gait and balance in the above statistical analysis were included in the model. For CSVD and gait speed, we included PWMH, DWMH, and CSVD total burden as coexisting predictors in the model. Model 1 only adjusted for demographic factors (age, sex), and the results showed that only PVWMH could independently predict gait speed (β = −0.090, P < 0.001). We added vascular risk factors (smoking) to Model 2 and showed that PVWMH independently still predicted gait speed (β = −0.086, P < 0.001). In Model 3, we included infarct sites based on Model 2, and the association remained significant (β = −0.089, P < 0.001) (Table 4) Regarding CSVD and body balance, we included PVWMH score and age in Model 1, and the results showed that PVWMH significantly independently predicted gait speed (β = 0.492, P = 0.041). In Model 2, we added infarct sites and found that PVWMH were still independent predictors of TUG (β = 0.517, P = 0.022) (Table 5).
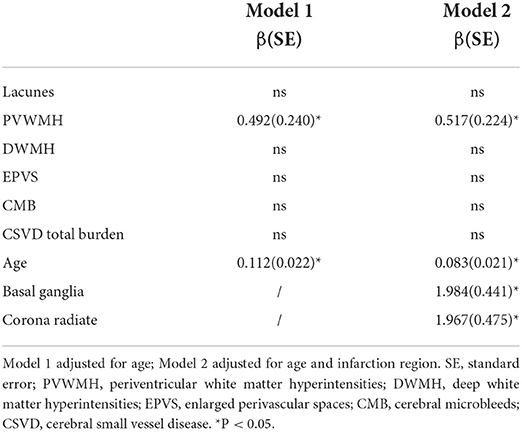
Table 5. Independent predictive analysis of timed-up-and-go test by CSVD in patients with minor stroke.
Previous studies have shown that age-related white matter lesions in the periventricular and deep frontal lobes are associated with falls, and that deep frontal white matter lesions are associated with balance deficits in a cohort of older adults with a history of falls (7). In patients with subcortical vascular cognitive impairment, PVWMH were associated with gait scores (9). Regional brain white matter injury is associated with gait and balance deficits in patients with Alzheimer's disease (25). A follow-up of up to 13 years in a longitudinal study showed that increased and progressive total WMH and PVWMH load were associated with decreased gait function in cognitively normal adults (26). In community-dwelling older adults, WMH and the total CSVD burden are major drivers of gait impairment but are not associated with balance impairment (10). In older patients with CSVD, CMB in temporal, frontal, and basal ganglia regions was associated independently of other CSVD markers with gait and balance impairment (8). In community-dwelling patients without neurological diseases, total CSVD burden, WMH, CMB, and lacunes were independently associated with gait and balance impairment (27). Recently, a prospective study included 200 patients with minor stroke (NIHSS ≤ 7 points) and divided them into lacunar and non-lacunar stroke groups based on MRI findings. The researchers objectively measured patients' gait balance impairment by the TUG test 3 years after stroke, subjectively assessed by the Stroke Impact Scale activity domain score, and tested the relationship between the total CSVD burden scale and functional stroke outcomes in a secondary analysis. The results showed that in the overall minor stroke population, total CSVD burden was not associated with TUG, Stroke Impact Scale scores, or Modified Rankin Scale scores 3 years after stroke; in patients with non-lacunar stroke, total CSVD burden was only associated with subjective activity impairment (17). However, the study sample size was small, and selection bias during clinical follow-up may be large.
Selection bias in cohort studies refers to the bias in the study that results from improper selection of study subjects. Loss to follow-up bias is a bias caused by study subjects dropping out of the cohort for various reasons. Loss to follow-up bias is also essentially selection bias (28). The study had a long follow-up period, so it is possible that patients with significant disabilities did not participate in the clinical follow-up. The study had a missed follow-up rate of >20%, so the loss to follow-up bias was large (17). To control for missing visit bias, researchers should inquire whether the missing person has died and the cause of death, and compare information on certain characteristics obtained at the baseline survey between the missing and non-missing subjects. However, the best way to control for loss to follow-up bias is to reduce missing subjects as much as possible and to improve the compliance of study subjects (29). This is a cross-sectional study, and there may be a non-response bias in selection bias. Non-response bias refers to bias due to the unwillingness of survey respondents to cooperate or their inability to participate in the survey for other reasons (28). The control for this bias should focus on improving the compliance and acceptance rate of the study subjects (30).
Minor stroke is common, and a growing number of epidemiological studies suggest that patients with minor stroke have some degree of physical and cognitive dysfunction, leading to a reduced quality of life (31, 32). Our study found that the presence of PVWMH is the most important predictor of gait and balance disorders in patients with minor stroke. PVWMH, DWMH, and the total CSVD burden were correlated with gait speed in the regression model, but only PVWMH explained the changes in TUG. After adjusting for the effects of demographics, vascular risk factors, NIHSS, and infarct sites on gait and balance, PVWMH significantly predicted gait speed and TUG time, independent of other radiographic markers and overall CSVD burden. The severity of gait and balance disorders was associated with infarct sites but not with stroke severity (NIHSS). After excluding the effect of cerebral infarction in this study, PVWMH remained an independent predictor of gait and balance and was independent of other CSVD markers.
The present study demonstrated the effect of PVWMH on gait and balance deficits in subjects, which is in general agreement with previous studies (9, 26). Unlike previous studies, the present study did not show the independent predictive power of lacunes (27) and CMB (8) on gait deficits. Although the total CSVD burden may better reflect the overall effect of CSVD on the whole brain, (33, 34) the present study did not show an independent effect of total CSVD burden on gait and balance. Possible reasons for the dissimilar results are as follows. First, the population included in our study were patients with acute ischemic minor stroke, and not the community population included in most studies. We had to take into account the limitation of mobility caused by the infarctions, which made CSVD less sensitive to the effects of gait and balance. Second, the subjects included in our study were relatively young, with an average age of 63.3 years. Third, we assessed the burden of CSVD with a visual rating scale and mobility impairment with a 6-m walking test and a TUG test. In addition, the present study showed that in the acute phase of cerebral infarction, only infarcts in the basal ganglia and radial crown sites were associated with gait and balance, while failing to confirm the idea that sites such as the cerebellum and frontoparietal lobe are involved in motor and ataxic regulation (35). This may be related to the small sample size included in the study.
This is the first study to investigate the effects of individual imaging markers of CSVD on gait and balance disorders in patients with minor stroke, and only one study mentioned above reported an association between overall CSVD burden and gait (17). PVWMH had significant independent predictive power after considering the effects of age, sex, vascular risk factors, stroke severity, and infarct location on gait and balance disorders. Gait is a highly cognitive process, and the poor gait performance caused by CSVD is partly mediated by cognitive function (36). PVWMH are more likely to cause cognitive damage because the periventricular white matter contains a large concentration of neurons and fibers related to learning, memory, and cognition. The white matter bundles passing through the periventricular area are denser (37). PVWMH can also directly damage motor pathways, leading to decreased gait and trunk stability (38, 39).
Our study had some limitations. First, our sample size was relatively small for stroke, a common disease. Large-scale studies are needed to determine the association between CSVD and gait and balance disorders in patients with minor stroke. Second, although we adjusted the results for stroke, the infarct sites are bound to have an impact on the clinical manifestations caused by CSVD in the acute stage of stroke. Third, our study was cross-sectional, and a causal relationship between CSVD and gait and balance disorders cannot be confirmed.
In summary, this study is the first to examine the association of individual markers and the total burden of CSVD with gait and balance impairment in patients with minor stroke. The findings show that PVWMH are independently associated with gait speed and trunk balance in patients with minor stroke. The PVWMH score is a possible independent marker to identify minor stroke patients at risk for gait and balance disorders. These findings should be confirmed in future large studies. It is suggested that in clinical work, we should focus on prevention strategies directed against the progression of WMH, which may provide useful assistance in the prevention and treatment of gait and balance dysfunction in patients with minor stroke.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Review Committee of the Affiliated Hospital of Qingdao University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
RZ planned the study. CS, XY, and SW analyzed the data and edited the manuscript. CS wrote the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.941668/full#supplementary-material
1. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. (2010) 9:689–701. doi: 10.1016/S1474-4422(10)70104-6
2. Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. (2013) 12:483–97. doi: 10.1016/S1474-4422(13)70060-7
3. de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The rotterdam scan study. J Neurol Neurosurg Psychiatry. (2001) 70:9–14. doi: 10.1136/jnnp.70.1.9
4. Okroglic S, Widmann CN, Urbach H, Scheltens P, Heneka MT. Clinical symptoms and risk factors in cerebral microangiopathy patients. PLoS ONE. (2013) 8:e53455. doi: 10.1371/journal.pone.0053455
5. van der Holst HM, van Uden IW, Tuladhar AM, de Laat KF, van Norden AG, Norris DG, et al. Factors associated with 8-year mortality in older patients with cerebral small vessel disease: the radboud university nijmegen diffusion tensor and magnetic resonance cohort (RUN DMC) study. JAMA Neurol. (2016) 73:402–9. doi: 10.1001/jamaneurol.2015.4560
6. Bower K, Thilarajah S, Pua YH, Williams G, Tan D, Mentiplay B, et al. Dynamic balance and instrumented gait variables are independent predictors of falls following stroke. J Neuroeng Rehabil. (2019) 16:3. doi: 10.1186/s12984-018-0478-4
7. Blahak C, Baezner H, Pantoni L, Poggesi A, Chabriat H, Erkinjuntti T, et al. Deep frontal and periventricular age related white matter changes but not basal ganglia and infratentorial hyperintensities are associated with falls: cross sectional results from the LADIS study. J Neurol Neurosurg Psychiatry. (2009) 80:608–13. doi: 10.1136/jnnp.2008.154633
8. de Laat KF, van den Berg HA, van Norden AG, Gons RA, Olde Rikkert MG, de Leeuw FE. Microbleeds are independently related to gait disturbances in elderly individuals with cerebral small vessel disease. Stroke. (2011) 42:494–7. doi: 10.1161/STROKEAHA.110.596122
9. Kim YJ, Kwon HK, Lee JM, Cho H, Kim HJ, Park HK, et al. Gray and white matter changes linking cerebral small vessel disease to gait disturbances. Neurology. (2016) 86:1199–207. doi: 10.1212/WNL.0000000000002516
10. Pinter D, Ritchie SJ, Doubal F, Gattringer T, Morris Z, Bastin ME, et al. Impact of small vessel disease in the brain on gait and balance. Sci Rep. (2017) 7:41637. doi: 10.1038/srep41637
11. Fischer U, Baumgartner A, Arnold M, Nedeltchev K, Gralla J, De Marchis GM, et al. What is a minor stroke? Stroke. (2010) 41:661–6. doi: 10.1161/STROKEAHA.109.572883
12. Ju Y, Zhao XQ, Wang CX, Wang YL, Liu GF, Wang YJ. Neurological deterioration in the acute phase of minor ischemic stroke is an independent predictor of poor outcomes at 1 year: results from the China National Stroke Registry (CNSR). Chin Med J. (2013) 126:3411–6.
13. Leira EC, Ludwig BR, Gurol ME, Torner JC, Adams HPJr. The types of neurological deficits might not justify withholding treatment in patients with low total National Institutes of Health Stroke Scale scores. Stroke. (2012) 43:782–6. doi: 10.1161/STROKEAHA.111.620674
14. Leonards CO, Ipsen N, Malzahn U, Fiebach JB, Endres M, Ebinger M. White matter lesion severity in mild acute ischemic stroke patients and functional outcome after 1 year. Stroke. (2012) 43:3046–51. doi: 10.1161/STROKEAHA.111.646554
15. group ISTc. Association between brain imaging signs, early and late outcomes, and response to intravenous alteplase after acute ischaemic stroke in the third International Stroke Trial (IST-3): secondary analysis of a randomised controlled trial. Lancet Neurol. (2015) 14:485–96. doi: 10.1016/S1474-4422(15)00012-5
16. Kim BJ, Lee SH. Prognostic impact of cerebral small vessel disease on stroke outcome. J Stroke. (2015) 17:101–10. doi: 10.5853/jos.2015.17.2.101
17. Loos CM, McHutchison C, Cvoro V, Makin SD, Staals J, Chappell F, et al. The relation between total cerebral small vessel disease burden and gait impairment in patients with minor stroke. Int J Stroke. (2018) 13:518–24. doi: 10.1177/1747493017730780
18. Podsiadlo D, Richardson S. The timed “Up and Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. (1991) 39:142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x
19. Chan PP, Si Tou JI, Tse MM, Ng SS. Reliability and validity of the timed up and go test with a motor task in people with chronic stroke. Arch Phys Med Rehabil. (2017) 98:2213–20. doi: 10.1016/j.apmr.2017.03.008
20. Wu YZ, Lin JY, Wu PL, Kuo YF. Effects of a hybrid intervention combining exergaming and physical therapy among older adults in a long-term care facility. Geriatr Gerontol Int. (2019) 19:147–52. doi: 10.1111/ggi.13575
21. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. (2013) 12:822–38. doi: 10.1016/S1474-4422(13)70124-8
22. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. (1987) 149:351–6. doi: 10.2214/ajr.149.2.351
23. Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke. (2010) 41:450–4. doi: 10.1161/STROKEAHA.109.564914
24. Staals J, Makin SD, Doubal FN, Dennis MS, Wardlaw JM. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology. (2014) 83:1228–34. doi: 10.1212/WNL.0000000000000837
25. Ogama N, Sakurai T, Shimizu A, Toba K. Regional white matter lesions predict falls in patients with amnestic mild cognitive impairment and Alzheimer's disease. J Am Med Dir Assoc. (2014) 15:36–41. doi: 10.1016/j.jamda.2013.11.004
26. Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology. (2008) 71:108–13. doi: 10.1212/01.wnl.0000316799.86917.37
27. Li P, Wang Y, Jiang Y, Zhang K, Yang Q, Yuan Z, et al. Cerebral small vessel disease is associated with gait disturbance among community-dwelling elderly individuals: the Taizhou imaging study. Aging. (2020) 12:2814–24. doi: 10.18632/aging.102779
28. Delgado-Rodriguez M, Llorca J. Bias. J Epidemiol Community Health. (2004) 58:635–41. doi: 10.1136/jech.2003.008466
29. Nohr EA, Liew Z. How to investigate and adjust for selection bias in cohort studies. Acta Obstet Gynecol Scand. (2018) 97:407–16. doi: 10.1111/aogs.13319
30. Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet. (2002) 359:248–52. doi: 10.1016/S0140-6736(02)07451-2
31. Wang YL, Pan YS, Zhao XQ, Wang D, Johnston SC, Liu LP, et al. Recurrent stroke was associated with poor quality of life in patients with transient ischemic attack or minor stroke: finding from the CHANCE trial. CNS Neurosci Ther. (2014) 20:1029–35. doi: 10.1111/cns.12329
32. van Rooij FG, Kessels RP, Richard E, De Leeuw FE, van Dijk EJ. Cognitive impairment in transient ischemic attack patients: a systematic review. Cerebrovasc Dis. (2016) 42:1–9. doi: 10.1159/000444282
33. Klarenbeek P, van Oostenbrugge RJ, Rouhl RP, Knottnerus IL, Staals J. Ambulatory blood pressure in patients with lacunar stroke: association with total MRI burden of cerebral small vessel disease. Stroke. (2013) 44:2995–9. doi: 10.1161/STROKEAHA.113.002545
34. Staals J, Booth T, Morris Z, Bastin ME, Gow AJ, Corley J, et al. Total MRI load of cerebral small vessel disease and cognitive ability in older people. Neurobiol Aging. (2015) 36:2806–11. doi: 10.1016/j.neurobiolaging.2015.06.024
35. Lo OY, Halko MA, Zhou J, Harrison R, Lipsitz LA, Manor B. Gait speed and gait variability are associated with different functional brain networks. Front Aging Neurosci. (2017) 9:390. doi: 10.3389/fnagi.2017.00390
36. Cai M, Jacob MA, Norris DG, Duering M, de Leeuw FE, Tuladhar AM. Cognition mediates the relation between structural network efficiency and gait in small vessel disease. Neuroimage Clin. (2021) 30:102667. doi: 10.1016/j.nicl.2021.102667
37. Onteddu SR, Goddeau RPJr, Minaeian A, Henninger N. Clinical impact of leukoaraiosis burden and chronological age on neurological deficit recovery and 90-day outcome after minor ischemic stroke. J Neurol Sci. (2015) 359:418–23. doi: 10.1016/j.jns.2015.10.005
38. Srikanth V, Phan TG, Chen J, Beare R, Stapleton JM, Reutens DC. The location of white matter lesions and gait–a voxel-based study. Ann Neurol. (2010) 67:265–9. doi: 10.1002/ana.21826
Keywords: cerebral small vessel disease, minor stroke, total cerebral small vessel disease burden, gait speed, balance, timed-up-and-go
Citation: Su C, Yang X, Wei S and Zhao R (2022) Periventricular white matter hyperintensities are associated with gait and balance in patients with minor stroke. Front. Neurol. 13:941668. doi: 10.3389/fneur.2022.941668
Received: 11 May 2022; Accepted: 27 June 2022;
Published: 22 July 2022.
Edited by:
Aristeidis H. Katsanos, McMaster University, CanadaReviewed by:
Ma Yuanyuan, Fudan University, ChinaCopyright © 2022 Su, Yang, Wei and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renliang Zhao, emhyZW5saWFuZ0AxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.